
42
Drying of Enzymes
Ana M.R. Pilosof and Virginia E. Sa´nchez
CONTENTS
42.1 Introduc tion ........ ............... .......... .......... ............... .......... ............. ............... .......... .......... ............... ...... 981
42.2 Spray Drying .......... ............... .......... .......... ............... .......... .......... ............... .......... .......... ............... ...... 982
42.2. 1 Dryin g-Chamber Layou ts ..... .......... ............. ............... .......... ............. ............... .......... .......... .. 982
42.2. 2 Solute- Induced Protect ion in Spr ay Dryi ng .......... .......... ............... .......... .......... ............... ...... 985
42.3. 1 Granul ation by Fas t Mixer Sy stems ............. ............... .......... ............. ............... .......... .......... .. 988
42.3. 2 Granul ation by the Prilling Process ............. ............... .......... ............. ............... .......... .......... .. 988
42.3. 3 Granul ation by Extrusi on Pro cess ..... .......... ............... .......... ............. ............... .......... .......... .. 989
42.3. 4 Granul ation by Spr ay Coat ing of Core Par ticles ..... .......... ............... .......... .......... ............... ... 989
42.1 INTRODUCTION
Enzymes are protein ca talysts of high molec ular
weight, which are produ ced not only by plants and
anima ls but also mainly by micr oorganis ms as a resul t
of fermen tation pro cesses. Enzy mes fall into tw o cat-
egorie s: (1) bulk indust rial enzymes , whi ch mainly
include pro teases for deterg ents, amylases for textile
desizing and starch hydrolysis, pectinases for fruit-juice
clarificati on, and proteas es for the leather industry
(
); an d (2) analytical enzymes .
In general, enzymes have a disadvantage in that
they ar e deactivated due t o heat-induced structural
changes or, in the c as e of proteolytic enzymes, due
to decom position by t hemselves. It is therefore de-
sirable to distribute and use e nzyme preparations in
the f orm of s olids , such as powders and granules,
instead of l iquids. A lthough drying itself is a valu-
able tool in the improvement of the e nzyme s torage
st ability, the process step itself often causes a sub-
stantial loss of activity and the final product is still
susceptible to inactivation.
Suc h solid enzyme pr eparations are co nventio n-
ally produce d by means of freez e drying or spray
drying. As freeze-dryi ng process is unsuit able for
large-sca le produ ctions, spray drying is used a s the
most fitted process for the indust rial mass produ ction
of soli d enzyme pr eparations. Especial ly in the case of
solid enzyme preparat ions to be used in deterg ents,
spray- drying pro cess is most frequent ly used.
Anal ytical enzymes are invari ably dr ied in smal l
quantities by freez e drying or by spray drying using
low tempe ratures. The spray drying of pa ncreat in, for
exampl e, must not have inlet drying tempe ratures
above 95 8 C [1].
The design of a proper drying process should guar-
antee a high level of acti ve en zyme. Genera lly, enzyme
activit y after drying is a functi on of the composi tion of
the initial liquid to be dehydrated, the process param-
eters, and the physicochemical characteristics of the
enzyme [2], so that drying of each enzyme product
should be considered on an individual basis.
Relevant properties of dried enzymes are listed in
. By modif ying the spray- drying process , it
is possible to alter and control the properties that are
mentioned earlier for spray-dried enzymes.
A major concern in spray drying of enzymes is the
retention of their activities, whereas this complication
is not seen in the case of purely chemical systems.
Therefore, the enzyme activity retention must be
close to 100% in the spray-drying operation; and
moreover, the shelf life of the dried enzyme products
must be excellent, i.e., enzyme activity must be
retained for long-time storage.
The formation of dust during handling of enzyme
preparations in finely divided solid forms is also a
problem. The dust of enzyme preparations incorpor-
ated in detergents and washing compositions could be
dangerous to the health of the workers in detergent
factories and for the end users.
ß
2006 by Taylor & Francis Group, LLC.
Ther e a re relative ly few studi es on the drying of
enzymes in the c hemical an d biologi cal literatu re.
This could partly be due to the indust rial nature of
the subject with the concomitan t proprie tary know-
ledge an d confiden tiality agreem ents abo ut prod ucts
and specific process parame ters . However, a large
number of patent s exist in this field. Selected patent s
where drying plays the dominan t role in the manufa c-
ture of enzyme produ cts are lis ted in
42.2 SPRAY DRYING
Spray drying is a co nvective drying techni que that
uses hot air to trans fer the heat an d remove the
evaporat ed water. It is a short-ti me pro cess in the
range of few seconds; and if proce ssing co nditions
are optim ized and stabili zers are added, it is suitab le
even for heat-sensi tive enzymes . The pro cess may
be su mmarized in three pha ses: (1) spray form ation,
(2) drying, an d (3) air–powder separat ion.
A wi de range of feed and drying propert ies are
success fully han dled to pro duce the powder with de -
sired qualities . The wi de ap plication of spray drying
has been due to the flex ibility of the system and the
developm ent of different drying- chamber designs that
combine with rotar y or nozzle atomizers to hand le
particular products in co ntinuous operation s.
If the desired powder specifica tion cann ot be
achieve d in the single-s tage process , multistage ope r-
ations are necessa ry. By combini ng atomizat ion, flui d-
ization, and agglom eration technol ogies in advanced
spray-d ryer designs , it is possible to meet the quality
specifica tions of the end pro duct, enzyme, within a
safe, hygieni c, and environm ental ly fri endly process .
W hen the spray- drying operati on is cocurrent , i.e.,
hot air intr oduced into the dryer close to the atomi z-
ing de vice, there is less danger of overh eating as the
evaporat ion rates are high (34 to 160 kg/h/m
2
of
particle area) [3]. Thus, co current drying ch ambers are
preferred to minimiz e heat deactivati on of en zymes
during the pro cess.
In the case of enzymes that are particu larly sensi -
tive to oxidation , it is preferable to use inert gas, such
as nitr ogen, during spraying and drying [4].
42.2.1 D
RYING
-C
HAMBER
L
AYOUTS
Several drying- chamber designs may be used to atta in
the desired powd er specifica tions. Standard co current
TABLE 42.1
Major Indust rial Applicati ons of Industria l Enzyme s
Application Enzyme Source
Detergents Protease Bacillus
Amylase Bacillus
Lipase Humicola,
Pseudomonas
Cellulase Bacillus, Humicola
Starch industry Amylase Bacillus
Glucoamylase Aspergillus
Glucose isomerase Bacillus, Streptomyces
Dairy Protease Rhizomucor
Lipase Aspergillus
Lactase Klyveromyces,
Aspergillus
Sulfhydryloxidase Aspergillus
Wine and Juice Pectinase Calf Stomach
Cellulase Aspergillus
Cellobiase Aspergillus,
Trichoderma
Glucose oxidase Aspergillus
Polyphenol oxidase Aspergillus
Distilling industry Amylase Trametes
Glucoamylase Aspergillus
Brewery b-glucanase Aspergillus
Acetoacetate
decarboxylase
Aspergillus, Bacillus
Bakery Amylase Bacillus
Protease
Aspergillus, Bacillus
Glucose oxidase
Aspergillus
Textiles
Amylase
Aspergillus
Cellulase
Bacillus
Catalase
Trichoderma,
Humicola
Animal feed
Phytase
Aspergillus
Cellulase
Aspergillus
Plant cell
wall–degrading
enzyme
Trichoderma,
Humicola,
Aspergillus
Pulp and paper
Xylanase
Aspergillus
Leather
Protease
Trichoderma, Bacillus
Tea
Tannase
Aspergillus
Source: From Oxenboll, K., Aspergillus enzymes and industrial
uses, in The Genus Aspergillus, Powell, K. ed., Plenum Press, New
York, 1994, 147–153.
TABLE 42.2
Quality Parameters for Dried Enzyme Products
Retention of activity
Dust properties
Solubility and dispersibility
Enzyme stability per se
Enzyme stability in detergent
Flow properties
Enzymes protein purity
Mean particle size and particle size distribution
Homogeneity
Bulk density
Color
Odor
ß
2006 by Taylor & Francis Group, LLC.
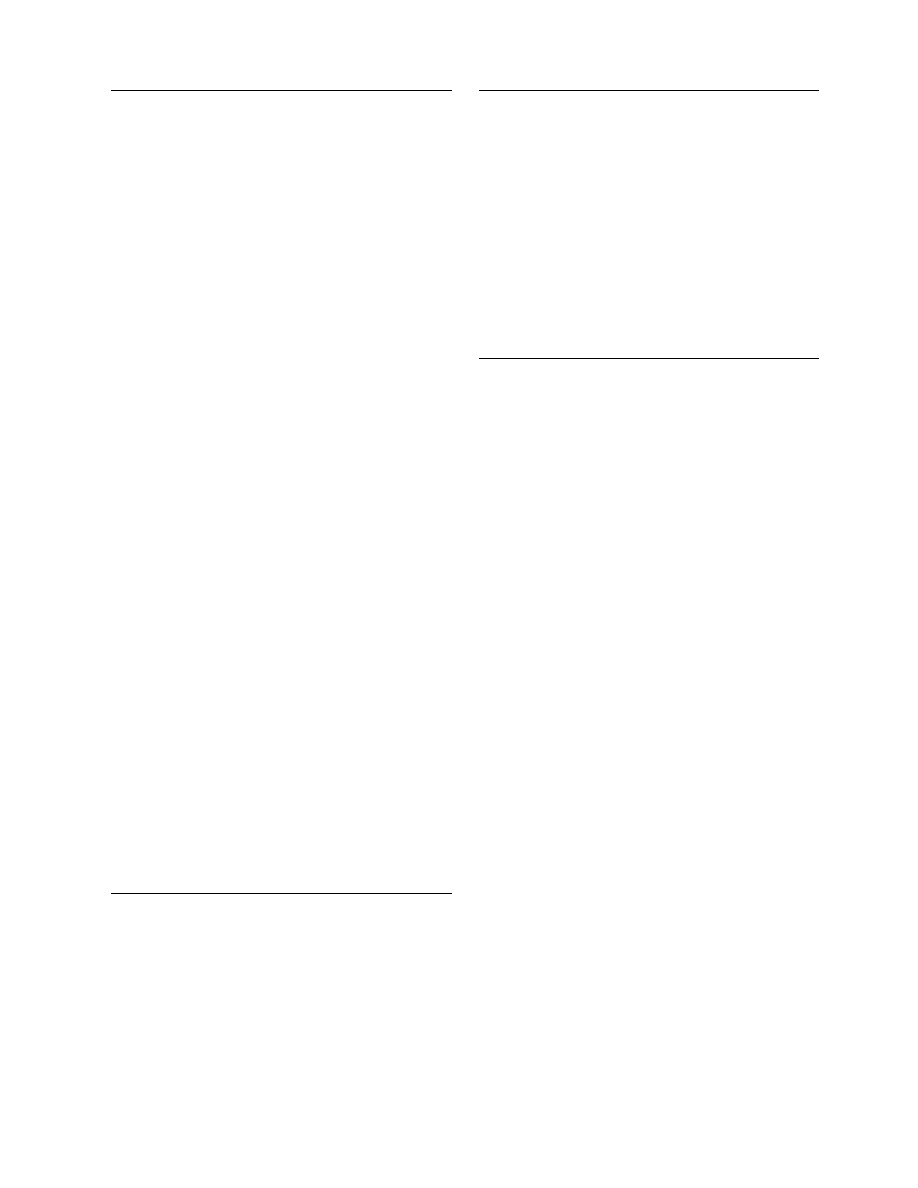
conical chambers (Figur e 42.1a) with eithe r rotary
atomizer or noz zles enable both fine - and coarse-
particled powder s to be pro duced. This layout is
used for therm ostable enzyme pr oducts wher e fair ly
high outlet air tempe ratures may be used. For a sin-
gle-stag e spray drying, enzy me activ ity losses may be
significa nt. Tabl e 42.4 shows the effect of some spray-
drying parame ters on the loss of acti vity of severa l
enzymes . Enzy me activit y decreas es wi th increa sing
outlet air temperatures. Low outlet temperatures were
also found to be important for the preservation of
tyrosinase, glucose oxidase, b-galactosidase, alkaline
phosphatase, and lact ate dehydroge nase (LDH ) [5–7] .
Enzy me activit y also decreas es with increa sing
inlet tempe ratur es. Signific ant enzyme inact ivation
occurs when mois ture decreas es below 15 to 20%.
The loss of enzymat ic acti vity ca n be reduced and
even almost co mpletely av oided when a co ntrolled
combinat ion of spray drying and fluid ized-bed dry ing
is used.
Spr ay drier s with integ rated fluid bed represen t
the latest in the spray- dryer design wher eby the com-
pletion of drying is accompl ished in a fluid bed lo-
cated in the lower con e of the chamber. The ope ration
of the fluid bed enables low er outlet tempe ratures to
accompl ish complet ion of drying, leadi ng to lower
powder tempe ratur es an d higher dryer thermal effi-
ciencies. The integ ration of flui d beds into drying
chambers allow s to pro duce, unde r low pro duct tem-
peratur e co nditions, nondus ty, free-flow ing, coa rse
powder s of indivi dual pa rticles or agglom erates.
Cham ber designs are shown in Figure 42.1b and
Figure 42.1c. Figure 42.1b shows a modificat ion of
the standar d layout wher e the fluid bed locat ed in the
base of the drying chamber is of an an nular de sign
enabli ng the exhaust air to be ducted out through the
center of the chamber base.
dimensions, i.e., a short cylindrical side in relation
to an extended cone section, and has a mixed flow
concept with air entering and leaving the top of the
chamber. This chamber is ideal for heat-sensitive,
sticky products and has enabled many products to
TABLE 42.3
Applications of Enzyme Drying
Issue
Patent No.
Patentee
Date
Process for freeze-drying enzymes
US 4 180 917
Rohm and Haas Co., Philadelphia, PA
January 1, 1980
Spray drying with additives
US 4 233 405
Rohm and Haas Co., Philadelphia, PA
November 11, 1980
Enzyme spraying onto a heated
fluidized bed of inert particles
US 4 617 272
Economics Laboratory Inc., St. Paul, MN
October 14, 1986
Disaccharide-stabilized enzyme preparation
EP 0 501 375 A1
KAO Corporation, Tokyo, Japan
July 2, 1992
Spray auxiliary composed of
hydrophobic silicas
US 5 318 903
BASF, Aktiengesellschaft, Germany
June 7, 1994
Process for storage of materials
US 5 928 469
Inhale Therapeutic Systems, San Carlos, CA
July 27, 1999
Enzyme preparation for leather
RU 2 127 311
Sergeevna et al.
March 10, 1999
Salt-stabilized enzyme preparations
US 5 972 669
Gist-brocades, B.V., The Netherlands
October 26, 1999
Microgranular enzyme composition
US 6 120 811
Genencor International Inc., Palo Alto, CA
September 19, 2000
Drying air
(a)
air
Product
(b)
Annular fluid bed
Product
air
(c)
Drying air
Drying air
Drying air
Drying air
Fluid bed
Product
air
FIGURE 42.1 Drying-chamber designs.
ß
2006 by Taylor & Francis Group, LLC.

TABLE 42.4
Effect of Some Spray-Drying Parameters on the Loss of Enzyme Activity
Enzyme
Spray Dryer
Temperature (
˚
C)
Moisture Spray-Dried
Product (%)
Loss of
Activity (%)
Reference
Inlet
Outlet
Bacillus alkaline protease
Conventional spray-drying tower
131.0
73.0
10.2
2.7
British patent 1 360 969
(Alcalase, Novozymes)
with spinning disk as atomizer
131.0
70.0
7.6
13.6
145.0
40.0
26.8
1.5
146.0
57.0
15.3
7.8
Fungal a-amylase prepared
Conventional spray-drying tower
150 + 5
63.0
5.2
19.0
British patent 1 360 969
by reverse osmosis
with spinning disk as atomizer
150 + 5
66.0
8.3
10.0
150 + 5
78.0
4.4
26.0
150 + 5
80.0
2.1
38.0
Fungal amyloglucosidase
Conventional spray-drying tower
150 + 5
90.0
14.0
34.0
British patent 1 360 969
with spinning disk as atomizer
150 + 5
80.0
17.0
20.0
150 + 5
70.0
20.0
10.0
Aspergillus oryzae protease
Small-scale spray drier
160.0
75.0
14.1
72.7
US patent 4 233 405
120–130
70.0
9.4–10.1
28–49
Neutral fungal protease
prepared by ultrafiltration
Small-scale spray drier
154.5
79.5
6.5
21.0
US patent 4 233 405
Bacillus subtilis neutral bacterial
protease prepared by
ultrafiltration
Small-scale spray drier
154.5
76.6
5.07
22.3
US patent 4 233 405
ß
2006
by
Taylor
&
Francis
Group,
LLC.

be s pr a y dr ie d su c c e s s fu l ly fo r t he fir s t ti m e . A l -
th o ug h it ha s be e n de v e lo pe d fo r f oo d a nd da i ry
pr od uc ts , i t is a ls o us e d fo r dy e st uf f s , a g ro c he m i c al s ,
po lymers, and detergents [8].
Thi s system feat ures a rotar y atomi zer or noz zle
located in an air-d ispersed roof. Pr imary drying takes
place in the conven tional man ner, but the partially
dried product, sti ll having significan t mois ture co n-
tent, passes directly into an e stablished flui dized layer .
As it has been found that gen erally the loss in
enzymat ic activit y increa ses when the water co ntent
in the spray- dried enzyme conce ntrate is lowered
(
), it is desirable that the pr oduct leaving
the spray dr yer has a moisture co ntent of not less than
8 to 10%, prefer ably ab out 20%. This resul ts in ex-
tremely low outlet tempe ratur es from the drying sys-
tem combined with controlled second-s tage drying
that takes place wi thin the stat ic flui d bed at a low er
tempe rature (40 to 50 8 C) to ac hieve the desir ed mois -
ture co ntent. The product from the dryer can be
postcool ed or postdried in a vibrating fluid bed.
A novel featu re of the design is the remova l of the
exhaust air from the roof of the drying chamber [8].
Fine mate rials elutriat ed from the fluidized bed are
carried wi thin the airflow and act to powd er the sur-
face of the drying chamber thereby limit ing deposit
formati on. A pro portion of the elutr iated fines co mes
into con tact with the cloud of atomi zed droplets
resulting in agglom eration prior to their entry into
the fluidized layer. The fine mate rials sep arated from
the exhaust air are recycle d to the chamb er for furth er
agglom eration. The process produ ces a dustl ess free-
flowin g powder with a mean particle size ranging
from app roximatel y 100 to 700 mm depe nding upon
the pr oduct characteris tics.
42.2.2 S
OLUTE
-I
NDUCED
P
ROTECTION
IN
S
PRAY
D
RYING
Several methods have been applied in the fie ld of
stabili zing en zymes agains t losse s dur ing drying and
subsequen t storage and hand ling. The bul k of these
applic ations a re concerned wi th the addition of car-
bohyd rates and, mo re specifica lly, sugars , polyols ,
and salt compon ents to the en zyme co ncentra te [9].
Als o known is the inclus ion of compone nts into
the form ulatio n wi th the aim to produce a glassy
produc t at storage temperatur e, thus impr oving en-
zyme stability. It will general ly be prefer red to emp loy
a carrier substa nce, which must be hy drophil ic (eith er
water- solubl e or water- swellab le), that mu st exist in a
glassy amo rphous state wi th a glass-t ransition tem-
peratur e (T
g
) above 20 8 C (a de sirable range is there-
fore between 60 an d 200 8 C) and should be suffici ently
chemi cally inert [10]. How ever, signi ficant enzyme
inactivat ion oc curs also in form ulation s kept wel l
below their glass -transit ion tempe ratur es [9].
Duri ng the final stage s of air drying, the major
stress that must be ov ercome is the remova l of the
enzyme’ s hy dration shell, which, for at least some
labile enzymes , can result in irreversib le inact ivation
upon rehydrat ion [11]. The mechani sm of this level of
protection is different from that occu rring in solution
[12]. It has been suggest ed that sugars can continue to
protect the dried protei n by hy drogen bonding to the
protein at some critical point during dehyd ration
[13,14], thus servi ng as water substitut es when the
hydratio n shell of the enzyme is remove d.
M oreover, most research ers agree that protection
by compoun ds such as lact ose and trehalose depend s
on the form ation of an amorpho us phase with the
protein [15] . The pro teins are mechan ically imm obil-
ized in the glassy, soli d matrix during de hydratio n.
The restriction of trans lational and relaxati on pro -
cesses is thought to prevent pro tein unfoldi ng, and
spatial separat ion be tween the protein molec ules (i.e.,
dilution of protein molec ules within the glassy matrix)
is prop osed to prevent aggrega tion [12] .
Take n toget her, these studi es support the co nclu-
sion that the impor tance of amorpho us be havior of
the pro tein and the ad ditive is that it allows for ef-
fective hyd rogen bondi ng betw een the add itive and
the protein . A glass y additive that doe s not have
the inter action will not protect the pro tein agains t
dehydrat ion damages [12].
Sug ars are an impor tant grou p of glass-f orming
substa nces, whi ch are also good stabilizers during
drying. Among them malto se- an d trehal ose-type
disacc harides are prefer red. Example s of the mal-
tose-type disacc ha rides include maltose, cello biose,
gentio biose, meli biose, an d lacto se, and exampl es of
the trehal ose-type disacc harides include trehal ose,
isotrehalos e, sucrose , isosuc rose, etc. The amount of
the disacc haride to be included in a solid enzyme
preparat ion may vary de pending on the type of the
enzyme used, but generally it is more prefer ably from
10 to 100% by weight of the enzyme [16].
An addition of 0.5% lact ose in a Bacillu s alkaline
proteas e sp ray-drying process is eno ugh to impr ove
the reco very of active enzyme after drying and ther-
mal treat ment at 90 8 C (
). In the same way,
the exhaust air temperature could be increased by
108C reaching severe drying conditions without sig-
nificant loss of proteas e activit y (
). The solid
enzyme preparation obtained in this process was also
excellent in resistance to mechanical pressure [16].
In addition to plain carbohydrates, other polyhy-
droxy compounds can be used, e.g., carbohydrate de-
rivatives and chemically modified carbohydrates (i.e.,
carbohydrates that have undergone chemical reaction
ß
2006 by Taylor & Francis Group, LLC.
to alte r sub stituent s on the carbon back bone of the
molec ule but withou t alterati on of the back bone).
Protein s are also suitab le. T hus albumin can be used
and also hydrolysis products of ge latin, like Byco
A (Croda Colloids Ltd.) that allowed an excellent en-
zyme storage stab ility afte r hard spray-drying co ndition s
of LD H type XI (ex rabbit muscl e) (Tab le 42.7).
Sug ar copolyme rs may be employ ed as glass -
forming su bstance s. Fico ll (Pharm acia
1
) includes co-
polyme rs with molec ular weigh ts between 5000 and
1,000,000, contain ing sucrose resi dues linked through
ether bridges to bifunct ional groups. Suc h group s
may be alkyl ene of two or more carbon atoms but
not normally more than ten carbon atoms . The bi-
functio nal groups serve to conn ect su gar residues
togeth er. Thes e polyme rs may, for exampl e, be made
by reaction of the sugar with a halohyd rin or a bis-
epoxy compoun d [10] . Ficoll 400 DL (Ph armacia) is a
water- soluble cop olymer of sucrose and ep ichloroh y-
drin that has a T
g
of 97 8C. A mixture of Ficoll 400
DL 4% and LDH type XI (ex rabbit muscle) shows
that enzyme activ ity was effecti vely preser ved
through the spray-d rying proc edure and subsequen t
long-term storage. Good stabilization was also reached
for alcohol oxidase: after 30 d, the spray-dried material
retained 90% of its activit y whi le the freez e-dried
material lost all activit y in 20 d (
) .
Anothe r approach is the add ition to the form ula-
tion of one or severa l compo nents that are able to
bind mo isture. This will reduce the water acti vity of
the final preparat ion or temporaril y prevent the inter -
action of water penetra ting from the surround ings
with the enz yme itself .
The use of organic and inorganic salts as a pro -
cessing aid (e.g., to impro ve flowi ng behavior of the
product) or a s a bulking/ standar dizing agen t is wel l
known. Solid enzyme formulat ions wi th impr oved
drying yiel d a nd stora ge are achieve d by prepari ng a
solution co mprising an enzyme an d a water-so luble
inorgani c salt . The presence of salt prior to drying,
while the enzyme is still in so lution, resul ts not only in
a higher yield dur ing dr ying but a lso in an impr oved
storage stabili ty as well as proce ssing stabi lity of the
obtaine d dry enzy me pr eparation s. Addit ion of mag-
nesium sulf ate to different ind ustrial en zymes allowed
a reco very of more than 100% active enzyme after
drying a nd after long-term storage [17] .
Pr eferably, an inorgani c salt of a dival ent cation ,
like z inc or magnes ium sulfate, can be added to the
enzyme so lution. Als o, a combination of salt s as wel l
as a combinat ion of enzymes can be used. The add -
ition of dival ent cati ons is prefer red be cause they
provide the best storage and process ing stabili ty. Sul-
fate is preferred as anion beca use it provides the best
drying y ield (
Dryi ng of a so lution co ntaining the enzyme and the
salt will result in a soli d composi tion that is homo ge-
neous with respect to the distribution of the enzyme
and the salt. The stabilizing effect of the salt increases
with increasing dosage of the salt to the enzyme solu-
tion, until at a certain point further increases in salt
dosage no longer produce further improvement of the
enzyme stability. For this reason, between 5 and 90%
of salt is added to the enzyme solution based on the
weight of the enzyme in solution.
42.3 POWDERED DETERGENT ENZYMES
Enzymes today are key strategic ingredients for wash-
ing and cleaning formulations. Enzymes not only
remove stains but also improve textile fiber properties.
TABLE 42.5
Effect of Lactose Addition on Production
of Stabilized Enzyme Powders
Lactose
Addition (%)
Residual Activity (%)
after Drying
Residual Activity (%)
after Heating 1 h
at 90
˚
C
0.0
83
84
0.5
90
95
1.0
96
96
2.5
96
96
5.0
95
97
Patent reference: EP 0 501 375 A1.
Enzyme: Bacillus alkaline protease K 16 5%
þ calcium chloride
0.2%
þ sodium sulfate 2.5%.
Drying technology: Atomizer-type spray dryer T
in
: 1508C; T
out
:
608C.
TABLE 42.6
Effect of Lactose Addition on Production of
Stabilized Enzyme Powders by Spray Drying with an
Exhaust Air Temperature Variation from 60 to 70
˚
C
Lactose
Addition (%)
Residual Activity (%) after Drying
60
˚
C
65
˚
C
70
˚
C
0
83
79
70
0.5
96
99
96
1.0
94
95
95
Patent reference: EP 0 501 375 A1.
Enzyme: Bacillus alkaline protease K 16 5%
þ calcium chloride
0.2%
þ sodium sulfate 2.5%.
Drying technology: Atomizer-type Spray Dryer T
in
: 1508C.
ß
2006 by Taylor & Francis Group, LLC.

TABLE 42.7
Solute-Induced Protection of Enzymes during Spray Drying and Storage
Enzyme (w/w %)
Brand Name
Additive
(w/w %)
Drying Technology
Temperature (
˚
C)
Inlet–Outlet
Residual
Activity after
Drying (%)
Storage
Residual
Activity
after
Storage (%)
Time
Temperature
(Weeks)
(
˚
C)
1. Fungal phytase (11)
Gist-brocades
MgSO
4
7 H
2
O (24)
Buchi lab-scale spray drier
130–85
—
8
30
146
2. Bacillus alkaline
protease (12)
Genencor
International Inc.
MgSO
4
0 H
2
O (8.5)
Niro STREA-1 lab-scale
fluid-bed coater
80–50
119
6
35
121
3. Trichoderma
b
-glucanase
þ
endoxylanase (25)
Gist-brocades
MgSO
4
7 H
2
O (24)
Glatt WSG-60 fluid-bed coater
80–50
105
þ 120
12
30
111
þ 120
4. Lactate dehydrogenase
type XI (0.005)
Sigma Chemical Co.
Ficoll 400 DL
a
(4)
Drytec pilot-scale spray drier
210–70
82
20
10–35
100
5. Lactate dehydrogenase
type XI (0.005)
Sigma Chemical Co.
Ficoll 400 DL
a
(10)
Lab-Plant SD-04 spray drier
170–75
91
9
10–35
103
6. Lactate dehydrogenase
type XI (0.005)
Sigma Chemical Co.
Byco A
b
(4)
Drytec pilot-scale spray drier
210–70
88
15
25
113
7. Alcohol oxidase (0.00005)
Provesta Enzymes
Ficoll 400 DL
a
(4)
Drytec pilot-scale spray drier
150–70
52
4
35
90
a
Copolymer of sucrose and epichlorohydrin (Pharmacia Reg. Trade Mark).
b
Cold water–soluble protein obtained from gelatin by enzymatic hydrolysis (Croda Colloids Ltd.).
Patent references 1–3: US 5 972 669.
4–7: US 5 928 469.
ß
2006
by
Taylor
&
Francis
Group,
LLC.

Pr ior to the introd uction of co mpacts, the use of
enzymes in detergen ts was limited prim arily to one
class of en zymes— the proteas es. Pro teases catalyze
the hydrolys is of protei n-based soils like bloo d and
grass. Mo st powder and liquid laund ry de tergen ts on
the market today, both low density and compact s,
employ a proteas e [18]. Recently, protein en gineer ing
has been us ed to constr uct deterge nt pr oteases wi th
impro ved stability and perfor mance charact eristic s.
In add ition to protease s, a limited num ber of
brands employ amyl ases. Dete rgent amylases catalyze
hydrolys is of the a -1,4-gl ycosidic linkag es in star ch.
As such they show be nefits on a number of common
food sols like gravies, sauces, pastas , an d baby foods.
Advan ces in genetic and protei n engineer ing have
led to ne w classes of enzymes with novel ben efits for
use in co mpact pro ducts. In 1988, lipase app eared in
one of the first compact powder s to hit the Japane se
market . Sin ce then lipas e has foun d broad applic ation
in the global de tergen t market . Second -generati on lip-
ases with impr oved cleanin g effici ency were developed
in the compact deterg ent market [19].
Pr oducts contai ning up to four different en zymes
—prote ase, amyl ase, lipase, and cell ulase— are now
on the market . The patent literat ure suggests that
even more novel de tergent enzymes are on the way.
As deterg ent enzymes hav e now become co mmod-
ities and are rather low -priced products , full atten tion
is pa id to the produc tion pr ocess that, despit e all the
techni cal demand s, has to be very econo mical.
The very first enzyme products that were intro -
duced on the market in the 1960s wer e powder s, and
using tod ay’s termino logy they contai ned 100% dust.
Today the powd ers ha ve been replac ed by various
types of granula tes. Ho wever, certa in kind s of a g-
glome rates, e.g. , fluidized- bed agglom erates, are still
available. Thes e agglom erate s have an accep table
particle size dist ribut ion but the physica l stre ngth is
normal ly poor .
To protect both deterg ent plant ope rator s and end
users, the enzyme pa rticles have to be coated in such a
way that no ac tive enzyme dust is presen t or released
during hand ling. Besides avoidance of dust, the coat-
ing has to stabi lize the particles agains t abrasive
forces an d protect the gran ule ag ainst chemi cal agents
such as water, hydroperoxi de, and pe racetic acid (the
bleach that migrates in deterg ent formu lations) . On
the other han d, the coati ng shou ld not be sticky be -
cause this woul d result in oversi zed particles during
the coatin g process and would deteriorat e the free-
flowin g prop erties of the particles in the dos ing sys-
tems of deterg ent manufa cturer s [20]. Finally, the
coatin g ha s to dissol ve readily in the was hing liquor ,
even at low tempe ratur es.
Since the introd uction of enzymes into the deter-
gent and other indust rial segme nts, many develop -
ments have been made regardi ng the granu lation
and coating of enzymes to reduce e nzyme dust. How -
ever, in today ’s state of ever-increa sing environm ental
concern a nd height ened awareness of indust rial hy -
giene, there remains a continuing need for low-dust
enzyme granules. The following are the most important
process es to granula te enzymes [20] .
42.3.1 G
RANULATION BY
F
AST
M
IXER
S
YSTEMS
With fast-rotating mixing systems of ‘‘ploughshare’’
type mixers or Schigi type with horizontal or vertical
shafts, equipped with blenders, the high turbulence in
the rotating mixture of ingredients determines the
particle size.
Usually, a premix of dry powders is loaded into the
equipment and the liquid enzyme concentrate is injected
and mixed. After a certain time, depending on the recipe
and moisture content, granules are formed that are
discharged and dried in a fluid dryer. After sieving the
granules, over- and undersized materials are milled
and recycled to the premix.
42.3.2 G
RANULATION BY THE
P
RILLING
P
ROCESS
The basic princi ple of this pro cess (
) is that
the total mixture of ingredients is transferred into a
TABLE 42.8
Effect of Salts Addition on Spray Drying and Storage
Losses of Fungal Phytase
Salt Type
Spray Drying
Losses (%)
Storage
Losses (%) after
8 Weeks at 358C
None
6
52
Magnesium sulfate
7
15
Magnesium chloride
26
43
Magnesium nitrate
32
27
Zinc sulfate
5
9
Zinc chloride
48
5
Calcium chloride
40
18
Calcium nitrate
44
13
Sodium sulfate
11
51
Potassium sulfate
17
36
Ammonium sulfate
6
46
Patent reference: US 5 972 669.
Enzyme: Fungal phytase concentrate (Gist-brocades) 17 w/w %
þ
800 mM salt.
Drying technology: Lab-scale Buchi 190 mini spray dryer T
in
:
1408C; T
out
: 808C.
ß
2006 by Taylor & Francis Group, LLC.
molten mass of low viscosity in which insoluble ingre-
dients have to be homogeneously distributed. The melt
is pumped through insulated tubes to the top of a
tower where it is sprayed by nozzles or a rotating disk.
The size of the droplets that fall down the tower is
determined by the diameter of the nozzle, the rotation
frequency of the disk, the surface tension, and the
viscosity of the melt. The heat capacity and the melt-
ing heat of the droplets dictate the distance needed to
solidify the droplets to nearly ideal balls and, there-
fore, also the height of the prill tower. The final steps
are cooling in continuous fluid beds and sieving of the
solidified prills. Over- and undersized materials are
separated and recycled.
With this process, it is of some disadvantage that
all the ingredients, including the enzyme, have to be
anhydrous. Therefore, the enzyme has to be brought
into a dry state, which is often costly because of
energy demand, enzymes losses, and inactivation
because of the (high) drying temperature. The pre-
paration of the melt at elevated temperatures also
inactivates a certain amount of the enzyme, which
additionally increases the cost of the process.
As prilling agents, meltable ingredients, such as
polyethylene glycols, are used as binders. Salts are
used to make the particles brittle and tough, and
they combine the advantage of being cheap.
42.3.3 G
RANULATION BY
E
XTRUSION
P
ROCESS
The extrusion process is very well established in the
plastic and food industry. This technology for the
manufacturing of the enzymes-containing granulate
combines the advantage of a homogeneous particle
size distribution with low-cost ingredients.
After mixing the dry ingredients, the dry premix-
ture and the liquid enzyme concentrate are fed batch-
wise in a mixing system to obtain a moist doughlike
mixture. This mass is fed into a twin-screw screen-
type extruder and presses through thin perforated
metal sheets with holes that are the diameter of the
desired particle size. The extruded noodles fall by
batchwise feeding into a spheronizer. This machine,
equipped with a fast-rotating disk, breaks the noodles
down to cylindrical particles, which are then trans-
ported on the disk to the walls of the apparatus.
After drying the particles in a fluid-bed dryer and
removal of over- and undersized materials by sieving,
the beads are coated in a fluid-bed coater with one or
two coating layers to obtain white or colored beads
and to have a tough protecting layer that completely
avoids the development of enzymatically active dust.
42.3.4 G
RANULATION BY
S
PRAY
C
OATING
OF
C
ORE
P
ARTICLES
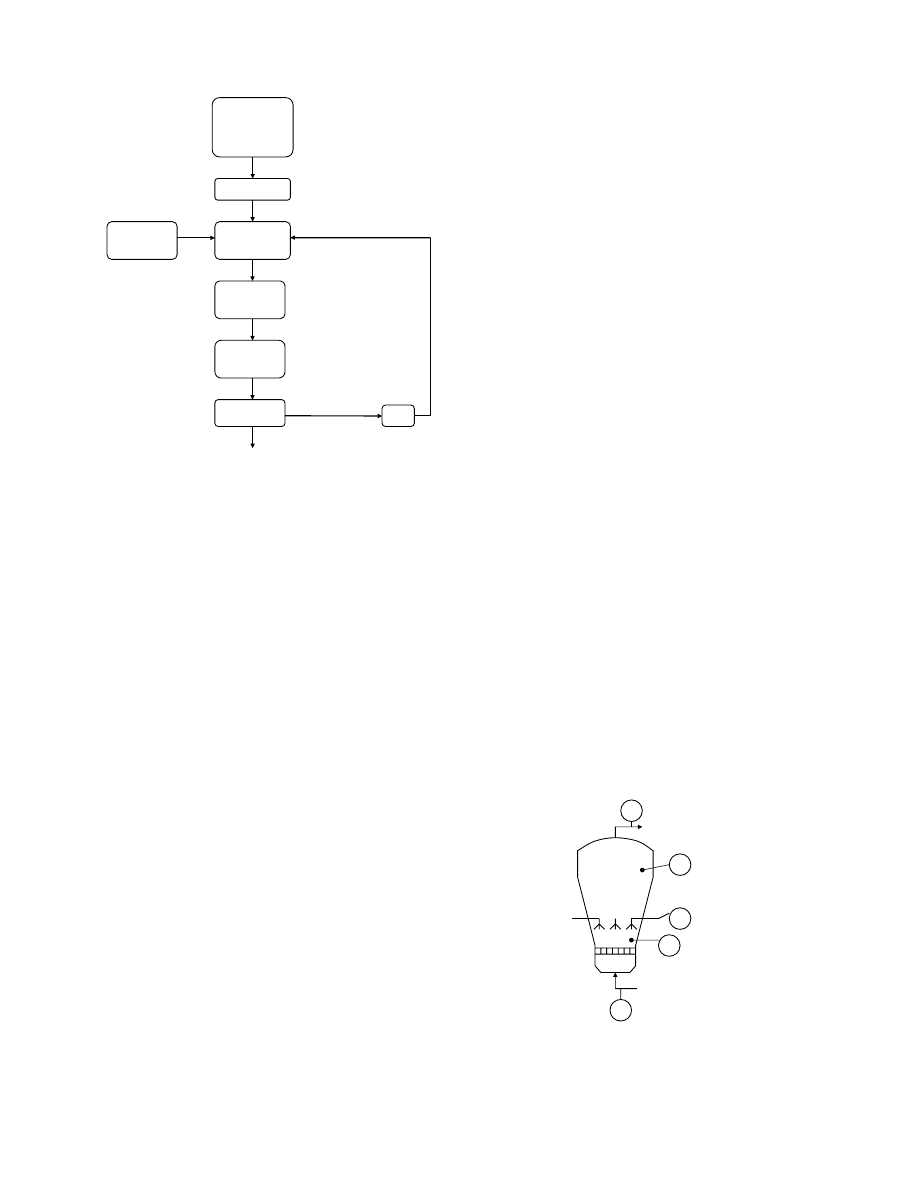
Granulation by spray coating generally refers to pro-
ducing a particle having an average size between 20
and 400 mm by fluidizing a core material in a heated
airstream to pass through an area of atomized liquid
(Figure 42.3). The atomized liquid droplets, which
contain dissolved or suspended solids, form a film
on the surface of the core material. The coated core
material is then transferred from the spray zone into a
drying zone. The solvent in the liquid—generally
water—is dried, leaving the dissolved or suspended
solids as a film on the core material. This process is
continued until the desired amount of film is formed.
Nonstandard size
Melt
preparation
Prilling
tower
Fluid-bed
coater
Sieve
mill
Meltable
component
Spray drying
Liquid
enzyme
concentrate
Enzyme granule
FIGURE 42.2 Prilling process.
Air outlet
4
5
2
3
Air inlet
1
FIGURE 42.3 Principle of the core-coating fluid-bed appar-
atus: (1) air inlet; (2) product bowl; (3) spray nozzles; (4) ex-
pansion chamber; (5) air outlet.
ß
2006 by Taylor & Francis Group, LLC.

Finally, the coated particles, which have an onion-like
structure, may be screened to obtain the desired range
of particles.
Methods for making low-dust granules include:
1. Loading a suitable carrier as a core material
into a fluid-bed granulator
2. Blending an aqueous enzyme source and one or
more suitable binders
3. Spraying the blend of enzyme and binder of
step 2 on the carrier
4. Spraying the product of step 3 with a water-
soluble, food-grade polymer at a rate to form a
coating and to reach the desired particle size
Typical carriers used as core materials and binders
are listed in Table 42.9. As used herein, ‘‘binder’’
indicates one or more materials, which act either
alone or in combination with sugars (such as sorbitol)
to bind the enzymes to the carrier material.
Two objectives are attained during the spraying
process: (1) the enzyme is attached to the carrier
and (2) the particle is built up to a granular form
(within the desired size range). A suitable food-grade
polymer is then sprayed onto the granulated particles
to envelope the enzyme and to hold the agglomerate
or granule together.
This process is economically attractive because the
moisture sprayed onto the carrier is ‘‘flashed off’’ as the
liquid is sprayed on the carrier, and thus a large amount
of aqueous enzyme can be loaded on the carrier.
Atomizing spraying can be done countercurrently
(down spray) to the fluidizing air or cocurrently (up
spray) with the fluidizing air. Down spray usually
results in more agglomeration and is useful when
fine powders are coated to increase the particle size,
resulting in lower dust granules. Cocurrent spraying
(up spray) results in less agglomeration and is used
when the core particle size already approximates the
final product size [20].
Many variables affect the efficiency of the coating
process. The three most important parameters for
manufacturing the microgranules are bed tempera-
ture, fluidization air rate, and spray rate. Careful
adjustment of all the engineering parameters is re-
quired to set the optimal conditions for a dust-free
granulate of desired specifications.
ACKNOWLEDGMENTS
Authors acknowledge the financial support from Uni-
ersidad de Buenos Aires, Consejo Nacional de Investi-
gaciones Cientı´ficas y Te´cnicas, and Agencia Nacional
de Promocio´n Cientı´fica y Tecnolo´gica de la Repu´blica
Argentina.
REFERENCES
1. S. Nath and G.R. Satpathy, A systematic approach for
investigation of spray drying process, Drying Technol.,
16:1173 (1998).
2. E. Dumoulin and J.J. Bimbenet, The properties of
water in foods, ISOPOW 6 (D.S. Reid, ed.), Blackie
Academic and Professional, New York, 1998.
3. Z. Pakowski and A.S. Mujumdar, Drying of pharmaceut-
ical products, Handbook of Industrial Drying (A.S.
Mujumdar, ed.), Marcel Dekker, New York, 1987, p. 605.
4. W. Bewert and G. Schwarz, Production of enzyme
preparations comprising an enzyme and finely divided
hydrophobic silica, US Patent 5 318 903 (1994).
5. Flair-Flow Europe, Are all dried foods the same? Flair-
Flow Reports; F-FE 110/93:1 (1993).
6. S. Yamamoto and Y. Sano, Drying of enzymes: En-
zyme retention during drying of a single droplet, Chem.
Engi. Sci., 47:177 (1992).
7. M. Adler and G. Lee, Stability and surface activity of
lactate
dehydrogenase
in
spray-dried
trehalose,
J. Pharm. Sci., 88:199 (1999).
8. H. Helsing, Latest developments in spray drying of
chemical and pharmaceutical products, Danish Tech-
nical Days, A/S Niro, Warsaw and Krakow, Poland
(1990).
9. A.M.R. Pilosof and M.R. Terebiznik, Spray and freeze-
drying of enzymes, Drying Technology in Agriculture
and Food Science (A.S. Mujumdar, ed.), Science Pub-
lishers, Enfield, NH, 2000, p. 167.
10. F. Franks, R.H. Hatley, and S.F. Mathias, Process for
storage of materials, US Patent 5 928 469 (1999).
11. J.F. Carpenter and J.H. Crowe, An infrared spectro-
scopic study of the interactions of carbohydrates with
dried proteins, Biochemistry, 28:3916 (1989).
TABLE 42.9
Typical Carriers and Binders Used in Granulation
of Enzymes by Spray Coating
Binders
Carriers Used as Core Materials
Alginate
Sodium chloride
Carrageenan
Sodium carbonate
Cellulose fibers
Urea
Gelatins
Calcium alginate
Xanthan gum
Saccharose beads
Locust bean gum
Polyvinyl alcohol beads
Gellan gum
Starch
Soluble or hydrolyzed starch
Soy flour, guts
Polyethylene glycols
Corn flour
Ethoxylated fatty alcohols
Cellulose-type materials
Polyvinyl alcohols
Polyvinyl pyrrolidones
Ethoxylated phenols
ß
2006 by Taylor & Francis Group, LLC.

12. J.H. Crowe, J.F. Carpenter, and L.M. Crowe, The role
of vitrificaton in anhydrobiosis, Annu. Rev. Physiol.,
60:73 (1998).
13. J.F. Carpenter and J.H. Crowe, Modes of stabilization
of a protein by organic solutes during desiccation,
Cryobiology, 25:459 (1988).
14. J.F. Carpenter, J.H. Crowe, and T. Arakawa, Compari-
son of solute-induced protein stabilization on aqueous
solution and in the frozen and dried states, J. Dairy
Sci., 73:3627 (1990).
15. B.C. Hancock and G. Zografi, Characteristics and signifi-
cance of the amorphous state in pharmaceutical systems,
Am. Chem. Soc. and Am. Pharm. Assoc., 86:1 (1997).
16. N. Yamada and Y. Shoga, Solid enzyme preparation
and process for producing the same, EP Patent 0 501
375 A1 (1992).
17. H.-P. Harz and J.B. Roland, Salt-stabilized enzyme
preparations, US Patent 5 972 669 (1999).
18. Chem. Mark Reporter, January 16, p. SR 18 (1995).
19. M.S. Showell, Powdered detergents, Powdered Detergents
(M.S. Showell, ed.) Marcel Dekker, New York, 1997, p. 1.
20. H.A. Herrmann, I. Good, and A. La¨ufer, Manufactur-
ing and downstream processing of detergent enzymes,
Powdered Detergents (M.S. Showell, ed.) Marcel
Dekker, New York, 1997, p. 251.
ß
2006 by Taylor & Francis Group, LLC.

Document Outline
- Table of Contents
- Chapter 042: Drying of Enzymes
Wyszukiwarka
Podobne podstrony:
Modeling with shrinkage during the vacuum drying of carrot (daucus carota) (Arévalo Pinedo, Xidieh M
Influence of drying methods on drying of bell pepper (Tunde Akintunde, Afolabi, Akintunde)
Far infrared and microwave drying of peach (Jun Wang, Kuichuan Sheng)
Microwave Application in Vacuum Drying of Fruits (Drouzaf, H SchuberP)
Microwave vacuum drying of model fruit gels (Drouzas, Tsami, Saravacos)
041 Drying of Polymers
039 Drying of Biotechnological Products
Modeling and minimizing process time of combined convective and vacuum drying of mushrooms and parsl
022 Drying of Fish and Seafood
026 Drying of Herbal Medicines and Tea
Characterization of microwave vacuum drying and hot air drying of mint leaves (Mentha cordifolia Opi
Microwave Application in Vacuum Drying of Fruits (Drouzaf, H SchuberP)
040 Drying of Coated Webs
Microwave Drying of Parsley Modelling, Kinetics, and Energy Aspects
James Axler Deathlands 042 Way of the Wolf
Microwave Finish Drying of Diced Apples in a Spouted Bed
043 Drying of Coal
więcej podobnych podstron