
1483
Combination Therapy with Famciclovir and Interferon-a for the Treatment of
Chronic Hepatitis B
Adriana R. Marques, Daryl T. Y. Lau, Robin McKenzie,
1
Stephen E. Straus, and Jay H. Hoofnagle
Laboratory of Clinical Investigation, National Institute of Allergy and
Infectious Diseases, and Liver Disease Section, National Institute of
Diabetes and Digestive and Kidney Diseases, National Institutes of
Health, Bethesda, Maryland
Interferon-a (IFN-a) treatment results in long-term remissions in only 25%–40% of
patients with chronic hepatitis B virus (HBV) infection. Famciclovir, the oral prodrug of
penciclovir, inhibits HBV DNA replication. Five adults with chronic HBV infection in
whom previous IFN-a therapy had failed were treated in a pilot study of overlapping IFN-
a and famciclovir therapy totaling 20 weeks. HBV DNA levels decreased by 0.9 log units
during the initial 4-week period of famciclovir alone, followed by a further decrease of 1.8
logs during the middle 12-week period of combination therapy. HBV DNA rose by 0.9 log
during the final 4-week period of IFN-a alone. Two patients cleared HBV DNA, and their
liver disease improved by clinical and histologic criteria. The combination of famciclovir and
IFN-a appeared to be at least additive in suppressing HBV DNA. Efficacy trials of combi-
nation therapy with famciclovir and IFN-a are warranted.
Hepatitis B virus (HBV) has infected 2 billion people living
today, of whom 350 million are chronically infected. Of the
estimated 52 million people who died in 1995, HBV is believed
to have contributed to the deaths of
∼1.1 million of them [1].
Interferon-a (IFN-a) is the only drug approved by the US
Food and Drug Administration for the treatment of chronic
HBV infection. IFN-a induces long-term remissions in only
25%–40% of treated patients. Therefore, the majority of pa-
tients with chronic HBV infection do not benefit from IFN-a
therapy.
Famciclovir is the oral prodrug of penciclovir, a nucleoside
analogue that is active against herpesviruses. Famciclovir has
been approved for treatment of acute herpes zoster and herpes
simplex virus infections. Penciclovir is also a potent inhibitor
of the HBV DNA polymerase [2] and has been shown to inhibit
HBV replication in vitro and in vivo [3].
The aim of this pilot study was to evaluate the safety and
antiviral and clinical effects of famciclovir alone and when used
Received 15 December 1997; revised 5 June 1998.
Presented in part: 37th Interscience Conference on Antimicrobial Agents
and Chemotherapy, Toronto, 28 September to 1 October 1997 (paper H-
035).
This study was approved by the Institutional Review Board of the Na-
tional Institute of Allergy and Infectious Diseases, and written informed
consent was obtained from each patient. This study was conducted under
an independent IND held by the investigators and followed the guide-
lines for the conduct of research involving human subjects at the National
Institutes of Health.
1
Present affiliation: Johns Hopkins Bay View Medical Center, Baltimore.
Reprints or correspondence: Dr. Adriana R Marques, National Insti-
tutes of Health, Bldg. 10, Room 11N228, 10 Center Dr., Bethesda, MD
(amarques@atlas.niaid.nih.gov).
The Journal of Infectious Diseases
1998; 178:1483–7
This article is in the public domain.
0022-1899
in combination with IFN-a in patients with chronic HBV in-
fection who did not respond to previous therapy with IFN
alone.
Subjects and Methods
Five patients with chronic HBV infection entered and completed
the study. All patients had received previous therapy with IFN-a
without achieving prolonged HBV clearance. These patients were
1
18 years of age; had documented presence of hepatitis B surface
antigen (HBsAg) in serum for at least 6 months; had positive tests
for both hepatitis B e antigen (HBeAg) and HBV DNA in serum
and elevated alanine aminotransferase levels on two or more oc-
casions, at least 1 month apart, during the 6 months before entry;
had compensated liver disease (prothrombin time
!
2 s longer than
normal, serum albumin level
≥3 g/dL, serum bilirubin level ≤4.0
mg/dL, and no history of ascites, wasting, hepatic encephalopathy,
or bleeding esophageal varices); had received no antiviral or im-
munomodulatory therapy for chronic HBV infection within 6
months before study entry; had no significant systemic illnesses
other than liver disease; and had no preexisting bone marrow
compromise, history of pancreatitis, renal insufficiency (creatinine
clearance of
!
60 mL/min or serum creatinine level
1
2.0 mg/100
mL), or coinfection with human immunodeficiency virus, hepatitis
C virus, or delta hepatitis virus.
Patients received famciclovir, 500 mg orally three times a day,
for a total of 16 weeks. After the first 4 weeks of therapy, IFN-a,
5 million units (MU) subcutaneously each day, was added and was
continued for 16 weeks. Thus, therapy was for 20 weeks: an initial
period of 4 weeks with famciclovir alone, a middle 12-week period
with both drugs, and a final period of 4 weeks with IFN-a alone.
Before treatment, all patients initially underwent a thorough
medical evaluation, a battery of blood tests, abdominal ultra-
sound, and percutaneous liver biopsy. During therapy, patients
http://jid.oxfordjournals.org/
Downloaded from
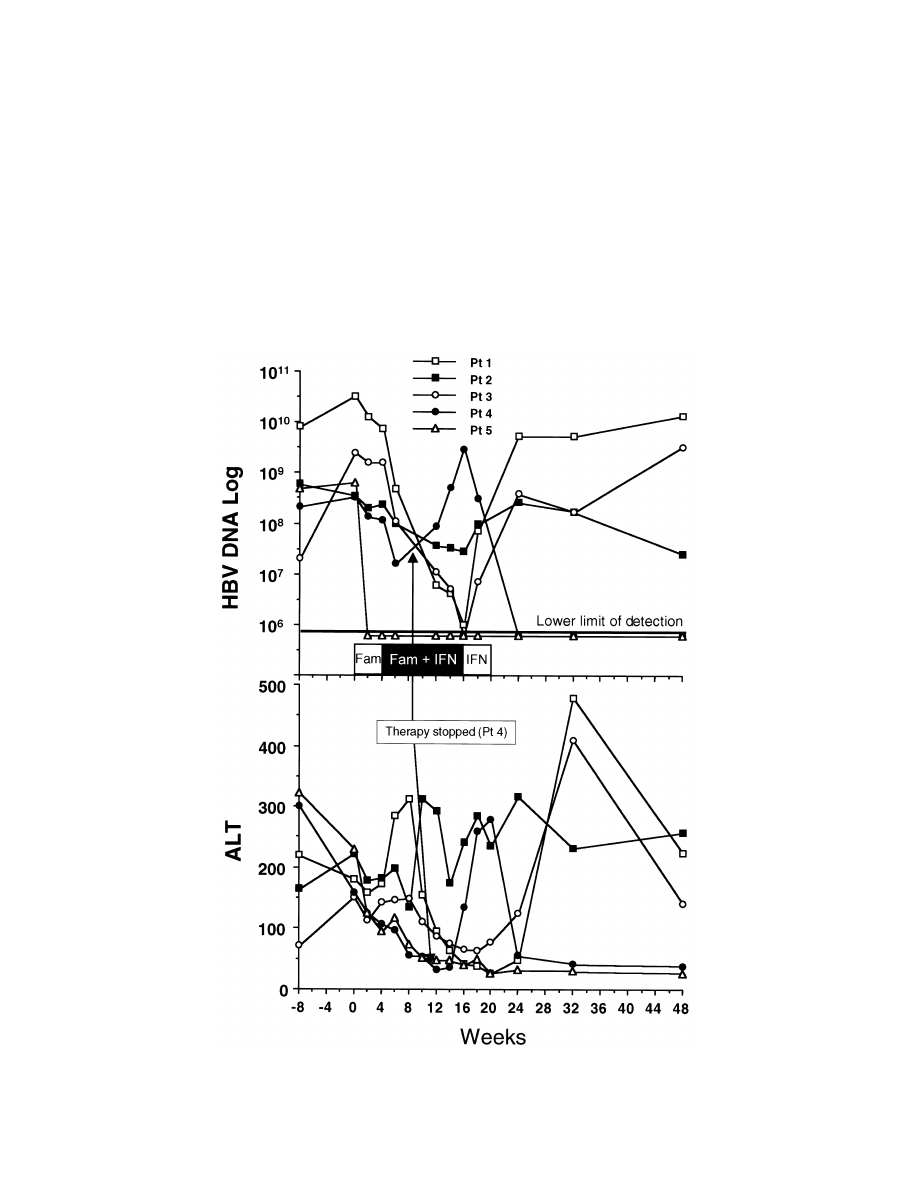
1484
Concise Communications
JID 1998;178 (November)
Figure 1.
Serum alanine aminotransferase (ALT) values and log changes in serum HBV DNA levels (copies/mL) over time in 5 patients treated
sequentially with famciclovir (Fam), combination therapy (
), and IFN.
Fam
1 IFN
were seen and evaluated every 2 weeks for biochemical and sero-
logic markers of hepatitis and for monitoring of side effects. Pa-
tients were then reevaluated at 1- to 2-month intervals and under-
went a repeat liver biopsy 12 months after starting treatment.
Serum aminotransferases were determined by a sequential
multiple autoanalyzer. HBsAg and HBeAg testing was done by
commercial EIAs (Abbott Laboratories, North Chicago). HBV
DNA was quantitated in serum by branched DNA assay (Chiron,
Emeryville, CA), the lower limit of detection being
6
∼ 0.7 3 10
genome equivalents/mL.
The virologic and clinical criteria for responses to treatment
were as follows: A complete virologic response was defined as clear-
ance of both HBV DNA and HBeAg from the serum, whereas a
biochemical response was defined as the fall of serum alanine
aminotransferase levels to within the normal range. Liver biopsy
specimens were graded with respect to the degree of necrosis, in-
flammation, and fibrosis according to the histology activity index
scoring system [4]. A histologic response was defined as a decrease
of at least 3 points in the inflammation score without an increase
in the fibrosis score.
http://jid.oxfordjournals.org/
Downloaded from

JID 1998;178 (November)
Concise Communications
1485
Table 1.
Serum alanine aminotransferase (ALT) levels, hepatitis B e antigen (HBeAg), histology activity index score, and liver biopsy pathology
in patients before treatment (week 0) and 7 months after stopping therapy (week 48).
Patient
ALT
a
HBV DNA
b
HBeAg
Histology activity
index score
c
Liver pathology
Week 0
Week 48
Week 0
Week 48
Week 0
Week 48
Week 0
Week 48
Week 0
Week 48
1
180
223
32,160
13,140
1
1
15
17
Chronic hepatitis with
marked activity and
bridging fibrosis
Chronic hepatitis with
marked activity and
bridging fibrosis
2
222
256
344
25
1
1
12
12
Chronic hepatitis with
moderate activity and
bridging fibrosis
Chronic hepatitis with
moderate activity and
bridging fibrosis
3
147
140
2490
3176
1
1
11
14
Chronic hepatitis with
mild activity and ex-
tensive nodular frag-
ments, consistent with
cirrhosis
Chronic hepatitis with
marked activity and
bridging fibrosis (in-
complete cirrhosis)
4
158
38
331
!
0.7
1
1
12
8
Chronic hepatitis with
moderate activity and
portal fibrotic
expansion
Chronic hepatitis with
mild activity and early
bridging fibrosis
5
230
25
630
!
0.7
1
2
16
4
Chronic hepatitis with
marked activity and
bridging fibrosis
Chronic hepatitis with
minimal activity and
portal fibrotic
expansion
a
Normal,
!
41 U/L.
b
HBV DNA was quantitated by branched DNA assay. Lower limit of detection:
genome equivalents/mL.
6
0.7
3 10
c
Range of scores: 0–22.
Results
Five men, 25–44 years old, were studied. Three were Cau-
casian and had acquired HBV infection during adulthood; the
other 2 patients were Asian and were believed to have acquired
the infection during infancy or childhood. Symptoms of liver
disease were either absent or mild, fatigue being the only com-
mon symptom. Alanine aminotransferase levels at entry ranged
from 147 to 230 U/L (normal,
!
41). HBV DNA levels ranged
from
to
genome equivalents/mL. The his-
8
10
3.31
3 10
3.2
3 10
tology activity index ranged from 11 to 16 (potential range of
scores, 0–22).
Famciclovir was generally well tolerated; 1 patient required
a dose reduction because of headaches and vertigo. Symptoms
resolved promptly without recurrence despite continuation of
therapy. IFN-a was associated with its expected side effects [5].
Both famciclovir and IFN were stopped after 7 weeks of the
combination in 1 patient (patient 4) because of thrombocyto-
penia (platelet count, 27,000/mm
3
). Platelet counts gradually
returned to baseline levels 4 weeks after stopping therapy.
The changes in the HBV DNA and serum alanine amino-
transferase levels over time for each patient are shown in figure
1. During the initial 4 weeks of famciclovir therapy, HBV DNA
levels decreased in all patients (average decrease, 0.9 log units).
During the combination phase of therapy, HBV DNA levels
decreased on average by a further 1.8 logs. In the final 4 weeks
of IFN-a alone, HBV DNA levels rose from the nadir by an
average of 0.9 log. In general, serum aminotransferase levels
decreased with the decrease of HBV DNA levels during ther-
apy. After stopping therapy, serum aminotransferase levels
increased concurrently with HBV DNA levels. HBV DNA be-
came undetectable during combination therapy in 1 patient who
became HBeAg-negative on follow-up, with normalization of
his serum alanine aminotransferase levels. A second patient,
whose therapy was stopped early because of thrombocytopenia,
had spontaneous resolution of his chronic hepatitis during fol-
low-up. After discontinuation of therapy, his HBV DNA levels
increased, followed by an increase in his serum alanine ami-
notransferase levels. The serum alanine aminotransferase levels
peaked
∼4 weeks after the peak HBV DNA values, associated
with decreasing HBV DNA values. He subsequently had nor-
malization of serum alanine aminotransferase levels and be-
came HBV DNA–negative and intermittently HBeAg-negative.
Both patients had improvement in the inflammation score of
the histology activity index. Despite transient reduction in virus
load during treatment, the other 3 patients continued to be
HBeAg- and HBV DNA–positive. They presented with a mild
flare of serum aminotransferases after stopping therapy, asso-
ciated with an increase in HBV DNA levels. Two patients also
had mild elevations in serum alanine aminotransferase levels
during therapy. Repeat liver biopsies 6–8 months after stopping
therapy showed no improvement in disease activity in these
patients (table 1).
Discussion
Chronic HBV infection can lead to cirrhosis, hepatocellular
carcinoma, and death. The natural history of liver disease
caused by persistent infection with HBV, however, can be quite
http://jid.oxfordjournals.org/
Downloaded from

1486
Concise Communications
JID 1998;178 (November)
variable. Nonetheless, the frequent finding of chronic active
hepatitis or cirrhosis on liver biopsy and the strong link between
HBV infection and hepatocellular carcinoma underscore the
need for effective antiviral therapy [6].
Currently, IFN-a is the only approved treatment for chronic
HBV infection. A 4- to 6-month course of IFN-a in doses of
5 MU daily or 10 MU thrice weekly results in suppression of
HBV replication and improvement in liver disease in 25%–40%
of patients [5]. Studies have shown that individuals maintain
their response during long-term follow-up, and sustained re-
sponders are more likely to have loss of serum HBV DNA
detection by polymerase chain reaction and HBsAg, suggesting
virus clearance [7, 8].
Unfortunately, the majority of patients with chronic HBV
infection do not respond to IFN therapy. Several patient var-
iables have been reported to influence the response to IFN-a.
The features that best predict a beneficial response to treat-
ment are high initial serum aminotransferase levels, low copy
number of circulating viral DNA, active histologic changes
(inflammation and necrosis) and fibrosis in the liver biopsy, a
short duration of the disease, and an absence of complicating
illnesses [9].
Management of the many patients in whom IFN-a therapy
fails is controversial. Retreatment seems to be rarely beneficial.
In a study of retreatment of 10 children with chronic HBV
infection, the response to a second course of IFN therapy
was poor, with only 1 patient becoming HBeAg- and HBV
DNA–negative [10]. In another study of IFN-a retreatment
of adult patients with chronic HBV infection, 2 (11%) of 18
responded to therapy, and 2 additional patients became HBV
DNA–negative with sustained HBeAg positivity [11].
Other approaches to the management of patients in whom
IFN-a therapy initially fails include retreatment with longer
courses of IFN-a, retreatment with IFN-a preceded by a short
course of corticosteroids, or retreatment when the patient
appears to be a better candidate for therapy, that is, when
alanine aminotransferase levels are higher, HBV DNA levels
are lower, or both.
Famciclovir is the oral form of the antiviral compound
penciclovir (9[4-hydroxy-3-hydroxymethylbut-1-yl] guanine).
Penciclovir inhibits duck HBV replication in vitro and in
animal experiments [2, 3]. Recent clinical trials showed fam-
ciclovir to be well-tolerated and to suppress HBV replication
in patients with chronic HBV infection [12, 13]. Famciclovir
has also been shown to decrease HBV replication and improve
liver function in patients with decompensated cirrhosis or re-
current hepatitis B after transplantation [14].
Because the baseline HBV DNA level is an independent
predictor of a response to IFN-a, we tested the hypothesis of
using a nucleoside analogue inhibitor of HBV DNA replication,
famciclovir, to augment the potential for a response to IFN-a.
This strategy underlies phase III studies in which IFN is used
in combination with lamivudine, another nucleoside analogue
with potent activity against HBV, to treat IFN-naive patients
and patients in whom IFN has failed. In a study of 226
IFN-naive patients, who were randomized to lamivudine and
IFN-a or the respective monotherapies, there was no significant
increase in the rate of HBeAg seroconversion [15]. The results
from the combination therapy trial in patients who did not
respond to IFN are not yet available.
In this pilot study, the combination of famciclovir and
IFN-a appeared to be safe and possibly additive in suppress-
ing HBV DNA and resulted in clearance of HBV DNA by
branched DNA assay in 2 of 5 patients, with accompanying
improvement of their liver disease as demonstrated by nor-
malization of serum aminotransferase levels and improvement
of the histology activity index on liver biopsy. One patient also
had clearance of serum HBeAg. It is of interest that both pa-
tients who responded to therapy were Asian men, who probably
acquired the infection in infancy or childhood and are thus less
likely to respond to IFN-a alone. This is an uncontrolled study,
and further follow-up and larger studies are needed to better
discern the rate and durability of serologic and biochemical
responses to this combination treatment regimen.
Acknowledgments
We thank Yoon Park for her help in the care of study participants.
We also thank the members of the Data Safety Monitoring Board,
Judith Falloon, Leslye D. Johnson, and Emil Miskovsky.
References
1. The world health report 1996—fighting disease, fostering development. World
Health Forum 1997; 18:1–8.
2. Korba BE, Boyd MR. Penciclovir is a selective inhibitor of hepatitis B virus
replication in cultured human hepatoblastoma cells. Antimicrob Agents
Chemother 1996; 40:1282–4.
3. Lin E, Luscombe C, Wang YY, Shaw T, Locarnini S. The guanine nucleo-
side analog penciclovir is active against chronic duck hepatitis B virus
infection in vivo. Antimicrob Agents Chemother 1996; 40:413–8.
4. Knodell RG, Ishak KG, Black WC, et al. Formulation and application of
a numerical scoring system for assessing histological activity in asymp-
tomatic chronic active hepatitis. Hepatology 1981; 1:431–5.
5. Hoofnagle JH, di Bisceglie AM. The treatment of chronic viral hepatitis. N
Engl J Med 1997; 336:347–56.
6. Perrillo RP. Interferon in the management of chronic hepatitis B. Dig Dis
Sci 1993; 38:577–93.
7. Niederau C, Heintges T, Lange S, et al. Long-term follow-up of HBeAg-
positive patients treated with interferon alfa for chronic hepatitis B. N
Engl J Med 1996; 334:1422–7.
8. Lau D, Everhart J, Kleiner D, et al. Long term follow-up of patients with
chronic hepatitis B treated with interferon alfa. Gastroenterology 1997;
113:1660–7.
9. Perrillo RP, Schiff ER, Davis GL, et al. A randomized, controlled trial of
interferon alfa-2b alone and after prednisone withdrawal for the treatment
of chronic hepatitis B. N Engl J Med 1990; 323:295–301.
10. Giacchino R, Timitilli A, Cristina E, et al. Repeated course of interferon
treatment in chronic hepatitis B in childhood. Arch Virol Suppl 1992; 4:
273–6.
11. Janssen HL, Schalm SW, Berk L, de Man RA, Heijtink RA. Repeated courses
of alpha-interferon for treatment of chronic hepatitis type B. J Hepatol
1993; 17:S47–51.
http://jid.oxfordjournals.org/
Downloaded from

JID 1998;178 (November)
Concise Communications
1487
12. Trepo C, Atkinson GF, Boon RJ. Efficacy of famciclovir in chronic hepatitis
B: results of a dose finding study [abstract 247]. Hepatology 1996; 24:
188A.
13. Main J, Brown JL, Howells C, et al. A double blind, placebo-controlled study
to assess the effect of famciclovir on virus replication in patients with
chronic hepatitis B virus infection. J Viral Hepat 1996; 3:211–5.
14. Singh N, Gayowski T, Wannstedt CF, Wagener MM, Marino IR. Pretrans-
plant famciclovir as prophylaxis for hepatitis B virus recurrence after liver
transplantation. Transplantation 1997; 63:1415–9.
15. Heathcote J, Schalm S, Cianciara J, et al. Lamivudine and intron A com-
bination treatment in patients with chronic hepatitis B infection. [abstract
GS2/O7]. J Hepatol 1998; suppl 1:43.
http://jid.oxfordjournals.org/
Downloaded from
Wyszukiwarka
Podobne podstrony:
Chronic Pain Syndromes After Ischemic Stroke PRoFESS Trial
Chronic Pain for Dummies
Chronic Pain Syndromes After Ischemic Stroke PRoFESS Trial
Ebsco Farezadi Chronic pain and psychological well being
Evidence for Therapeutic Interventions for Hemiplegic Shoulder Pain During the Chronic Stage of Stro
Chronic Prostatitis A Myofascial Pain Syndrome
Lumbar lordosis and pelvic inclinations in adults with chronic lumbar pain
Serum cytokine levels in patients with chronic low back pain due to herniated disc
Treating Non Specific Chronic Low Back Pain Through the Pilates Method
Chronic Hepatitis
1-Kefir chroni przed mutacjami w DNA, ZDROWIE-Medycyna naturalna, Poczta Zdrowie
Dozwolony użytek chronionych utworów, Kulturoznawstwo UAM, Ochrona właśności intelektualnej
Nauczyciele będą chronieni jak funkcjonariusze publiczni, 03. DLA NAUCZYCIELI
Akumulator do?UTZ?HR COMBINE HARVESTERS M3370 M3380
Jak chronić własne dziecko przed narkotykami
DIY Combination Solar Water and Nieznany
więcej podobnych podstron