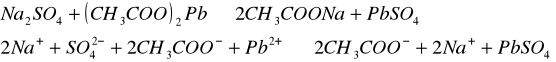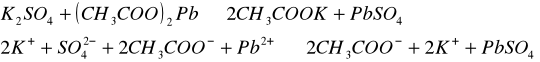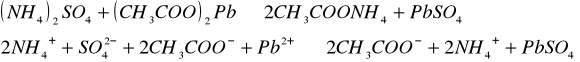[Author ID1: at Tue Mar 31 22:49:00 1998
]Korzeniewicz [Author ID1: at Sat Apr 4 00:59:00 1998
]Łukasz[Author ID1: at Sat Apr 4 01:09:00 1998
] [Author ID1: at Sat Apr 4 00:59:00 1998
]Korzeniewicz Łukasz [Author ID1: at Sat Apr 4 00:59:00 1998
] [Author ID1: at Sat Apr 4 01:09:00 1998
] [Author ID1: at Tue Mar 31 22:49:00 1998
] [Author ID1: at Tue Mar 31 22:50:00 1998
] [Author ID1: at Tue Mar 31 22:49:00 1998
]1998-03-27
ĆWICZENIA LABOR[Author ID1: at Tue Mar 31 20:53:00 1998
]L[Author ID1: at Tue Mar 31 20:53:00 1998
]ATORYJNE Z CHEMI NIEORGANICZNEJ.
[Author ID1: at Tue Mar 31 22:49:00 1998 ]Temat: DYSOCJACJA ELEKTROLITYCZNA.
W drugiej połowie XIX [Author ID1: at Fri Mar 27 22:05:00 1998
] w[Author ID1: at Fri Mar 27 22:05:00 1998
]w[Author ID1: at Fri Mar 27 22:05:00 1998
]. szwed[Author ID1: at Fri Mar 27 22:03:00 1998
]zki chemik S. A. Arrhenius doświadczalnie udowodnił, [Author ID1: at Fri Mar 27 22:06:00 1998
]że substancje chemiczne można podzielić na dwie grupy[Author ID1: at Fri Mar 27 22:07:00 1998
]. Do grupy [Author ID1: at Fri Mar 27 22:08:00 1998
]-->I [Author ID1: at Fri Mar 27 22:08:00 1998
][Author ID1: at Tue Mar 31 22:53:00 1998
]mo[Author ID1: at Fri Mar 27 22:08:00 1998
]ż[Author ID1: at Fri Mar 27 22:08:00 1998
]na zaliczyć takie, których roztwory wodne przewodzą prąd elektryczny[Author ID1: at Fri Mar 27 22:08:00 1998
], a do grupy [Author ID1: at Fri Mar 27 22:10:00 1998
]-->II [Author ID1: at Fri Mar 27 22:10:00 1998
][Author ID1: at Tue Mar 31 22:53:00 1998
]te, które w tych samych warunkach praktycznie nie przewodzą prądu[Author ID1: at Fri Mar 27 22:10:00 1998
]. Substancje gr[Author ID1: at Fri Mar 27 22:12:00 1998
]u[Author ID1: at Fri Mar 27 22:12:00 1998
]py [Author ID1: at Fri Mar 27 22:12:00 1998
]-->I[Author ID1: at Fri Mar 27 22:12:00 1998
][Author ID1: at Tue Mar 31 22:53:00 1998
] nazwał [Author ID1: at Fri Mar 27 22:12:00 1998
]elektrolitami[Author ID1: at Fri Mar 27 22:13:00 1998
], grupy [Author ID1: at Fri Mar 27 22:13:00 1998
]-->II[Author ID1: at Fri Mar 27 22:13:00 1998
][Author ID1: at Tue Mar 31 22:53:00 1998
]--> [Author ID1: at Fri Mar 27 22:18:00 1998
][Author ID1: at Tue Mar 31 22:53:00 1998
]-[Author ID1: at Fri Mar 27 22:14:00 1998
] [Author ID1: at Fri Mar 27 22:18:00 1998
]nieelektrolit[Author ID1: at Fri Mar 27 22:14:00 1998
]a[Author ID1: at Fri Mar 27 22:14:00 1998
]mi.[Author ID1: at Fri Mar 27 22:14:00 1998
][Author ID0: at Thu Nov 30 00:00:00 1899
]
Teorię tą można ująć ogólnie w postaci czter[Author ID1: at Fri Mar 27 22:19:00 1998 ]ech podstawowych, opartych na doświadczeniu założeń.[Author ID1: at Fri Mar 27 22:20:00 1998 ]
Elektrolity, a więc kwasy, zasady i sole podczas rozpuszczania w wodzie rozpadają się na elementy naładowane elektrycznie, czyli ulegają tzw. [Author ID1: at Fri Mar 27 22:22:00 1998 ]dys[Author ID1: at Fri Mar 27 22:25:00 1998 ]o[Author ID1: at Fri Mar 27 22:25:00 1998 ]cjacji elektrolitycznej.[Author ID1: at Fri Mar 27 22:25:00 1998 ] Elementy te nazwano [Author ID1: at Fri Mar 27 22:26:00 1998 ]jonami.[Author ID1: at Fri Mar 27 22:26:00 1998 ] Jony naładowane d[Author ID1: at Fri Mar 27 22:26:00 1998 ]o[Author ID1: at Fri Mar 27 22:26:00 1998 ]datnio nazywają się [Author ID1: at Fri Mar 27 22:26:00 1998 ]kationami, [Author ID1: at Fri Mar 27 22:27:00 1998 ]a ujemne[Author ID1: at Fri Mar 27 22:27:00 1998 ] [Author ID1: at Fri Mar 27 22:28:00 1998 ]anionami.[Author ID1: at Fri Mar 27 22:28:00 1998 ]-->[Author ID1: at Fri Mar 27 22:28:00 1998 ][Author ID1: at Fri Mar 27 22:19:00 1998 ]
Suma ładunków elektrycznych kationów i anionów, powstałych na skutek dysocjacji elektrolitycznej [Author ID1: at Fri Mar 27 22:28:00 1998 ]elektrolitów, jest [Author ID1: at Fri Mar 27 22:31:00 1998 ]zawsze [Author ID1: at Fri Mar 27 22:32:00 1998 ]równa [Author ID1: at Fri Mar 27 22:31:00 1998 ]zeru.[Author ID1: at Fri Mar 27 22:32:00 1998 ]-->[Author ID1: at Fri Mar 27 22:32:00 1998 ][Author ID1: at Fri Mar 27 22:19:00 1998 ]
Nieelektrolity[Author ID1: at Fri Mar 27 22:32:00 1998 ], tzn. substancje, które w roztworach i w stanie stopionym nie p[Author ID1: at Fri Mar 27 22:33:00 1998 ]rzewodzą prądu elektrycznego, nie ulegają dysocjacji elektro[Author ID1: at Fri Mar 27 22:36:00 1998 ]litycznej.[Author ID1: at Fri Mar 27 22:36:00 1998 ]-->[Author ID1: at Fri Mar 27 22:36:00 1998 ][Author ID1: at Fri Mar 27 22:19:00 1998 ]
Własności [Author ID1: at Fri Mar 27 22:37:00 1998 ]chemiczne jonów różnią się zupełnie od własności obojętnych atomów lub cząsteczek. Z [Author ID1: at Fri Mar 27 22:38:00 1998 ]tego względu obecność jonów w roztworach n[Author ID1: at Fri Mar 27 22:39:00 1998 ]a[Author ID1: at Fri Mar 27 22:39:00 1998 ]daje im ch[Author ID1: at Fri Mar 27 22:39:00 1998 ]a[Author ID1: at Fri Mar 27 22:39:00 1998 ]rakterystyczne [Author ID1: at Fri Mar 27 22:39:00 1998 ]cechy chemiczne i fizyczne.[Author ID1: at Fri Mar 27 22:41:00 1998 ]-->[Author ID1: at Fri Mar 27 22:42:00 1998 ][Author ID1: at Fri Mar 27 22:19:00 1998 ]
Kwasami [Author ID1: at Fri Mar 27 22:42:00 1998 ] [Author ID1: at Fri Mar 27 22:42:00 1998 ]s[Author ID1: at Fri Mar 27 22:59:00 1998 ]ą [Author ID1: at Fri Mar 27 22:42:00 1998 ]to [Author ID1: at Fri Mar 27 22:59:00 1998 ]związki chemiczne[Author ID1: at Fri Mar 27 22:42:00 1998 ], które podczas rozpu[Author ID1: at Fri Mar 27 22:43:00 1998 ]szczania w wodzie d[Author ID1: at Fri Mar 27 22:43:00 1998 ]y[Author ID1: at Fri Mar 27 22:43:00 1998 ]socjują ca[Author ID1: at Fri Mar 27 22:43:00 1998 ]ł[Author ID1: at Fri Mar 27 22:43:00 1998 ]kowicie lub częściowo na kationy wodorowe i aniony reszt kwasowych.[Author ID1: at Fri Mar 27 22:43:00 1998 ][Author ID1: at Fri Mar 27 23:00:00 1998 ]
Zasady [Author ID1: at Fri Mar 27 23:01:00 1998 ]są to związki chemiczne, które [Author ID1: at Fri Mar 27 23:02:00 1998 ]podczas rozpuszczania w wodzie dys[Author ID1: at Fri Mar 27 23:03:00 1998 ]o[Author ID1: at Fri Mar 27 23:03:00 1998 ]cjują całkow[Author ID1: at Fri Mar 27 23:03:00 1998 ]i[Author ID1: at Fri Mar 27 23:03:00 1998 ]cie lub częściowo [Author ID1: at Fri Mar 27 23:03:00 1998 ]na aniony wodorotlenowe OH[Author ID1: at Fri Mar 27 23:05:00 1998 ]-[Author ID1: at Fri Mar 27 23:06:00 1998 ] i kationy metali.[Author ID1: at Fri Mar 27 23:06:00 1998 ]
Sole [Author ID1: at Fri Mar 27 23:07:00 1998 ]są[Author ID1: at Fri Mar 27 23:07:00 1998 ] produktami reakcji kwasów z zasadami. [Author ID1: at Fri Mar 27 23:07:00 1998 ]Związki [Author ID1: at Fri Mar 27 23:08:00 1998 ]te w temperaturze p[Author ID1: at Fri Mar 27 23:09:00 1998 ]o[Author ID1: at Fri Mar 27 23:09:00 1998 ]kojowej występują na ogół w stanie stałym, krystalicznym i mają budowę jonową, czyli składają się z kationów [Author ID1: at Fri Mar 27 23:09:00 1998 ]metali lub kationu [Author ID1: at Fri Mar 27 23:12:00 1998 ]amonowego [Author ID1: at Fri Mar 27 23:13:00 1998 ]i anionów[Author ID1: at Fri Mar 27 23:09:00 1998 ] reszt kwas[Author ID1: at Fri Mar 27 23:13:00 1998 ]o[Author ID1: at Fri Mar 27 23:13:00 1998 ]wych[Author ID1: at Fri Mar 27 23:13:00 1998 ].[Author ID1: at Fri Mar 27 23:09:00 1998 ][Author ID1: at Fri Mar 27 23:13:00 1998 ]
Do porównywania mocy elektrolitów wprowadzono pojęcie stopnia i stałej d[Author ID1: at Fri Mar 27 23:14:00 1998 ]y[Author ID1: at Fri Mar 27 23:14:00 1998 ]socjacji ele[Author ID1: at Fri Mar 27 23:14:00 1998 ]k[Author ID1: at Fri Mar 27 23:14:00 1998 ]trolitycznej.[Author ID1: at Fri Mar 27 23:14:00 1998 ]
Stopień dysocjacji [Author ID1: at Fri Mar 27 23:17:00 1998 ]określa [Author ID1: at Fri Mar 27 23:17:00 1998 ]się[Author ID1: at Fri Mar 27 23:18:00 1998 ] stosunkiem liczby moli cząsteczek zdysocjow[Author ID1: at Fri Mar 27 23:20:00 1998 ]a[Author ID1: at Fri Mar 27 23:20:00 1998 ]nych[Author ID1: at Fri Mar 27 23:20:00 1998 ] na jony do liczby moli cząsteczek substancji rozpuszczonej:[Author ID1: at Fri Mar 27 23:21:00 1998 ]
[Author ID1: at Fri Mar 27 23:23:00 1998
][Author ID1: at Fri Mar 27 23:23:00 1998
]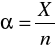
[Author ID1: at Fri Mar 27 23:23:00 1998
][Author ID1: at Fri Mar 27 23:23:00 1998
], [Author ID1: at Fri Mar 27 23:25:00 1998
][Author ID1: at Fri Mar 27 23:28:00 1998
]
gdzie[Author ID1: at Fri Mar 27 23:25:00 1998 ]-->:[Author ID1: at Fri Mar 27 23:25:00 1998 ][Author ID1: at Fri Mar 27 23:30:00 1998 ]--> [Author ID1: at Fri Mar 27 23:25:00 1998 ][Author ID1: at Fri Mar 27 23:29:00 1998 ]-->α[Author ID1: at Fri Mar 27 23:26:00 1998 ][Author ID1: at Fri Mar 27 23:29:00 1998 ] [Author ID1: at Tue Mar 31 22:54:00 1998 ]-->-[Author ID1: at Fri Mar 27 23:26:00 1998 ][Author ID1: at Fri Mar 27 23:29:00 1998 ] stopień dysocjacji[Author ID1: at Fri Mar 27 23:26:00 1998 ]-->, X[Author ID1: at Fri Mar 27 23:26:00 1998 ][Author ID1: at Fri Mar 27 23:29:00 1998 ] [Author ID1: at Tue Mar 31 22:54:00 1998 ]- liczba moli czą[Author ID1: at Fri Mar 27 23:26:00 1998 ]steczek zdysocjowanych na jony, [Author ID1: at Fri Mar 27 23:28:00 1998 ]-->n[Author ID1: at Fri Mar 27 23:28:00 1998 ][Author ID1: at Fri Mar 27 23:29:00 1998 ] [Author ID1: at Tue Mar 31 22:54:00 1998 ]- [Author ID1: at Fri Mar 27 23:28:00 1998 ]liczba moli cząsteczek substancji rozpuszczonej.[Author ID1: at Fri Mar 27 23:30:00 1998 ] Stopień dysocjacji jest odwrotnie [Author ID1: at Fri Mar 27 23:33:00 1998 ][Author ID1: at Fri Mar 27 23:36:00 1998 ]
proporcjonalny do[Author ID1: at Fri Mar 27 23:33:00 1998 ] [Author ID1: at Fri Mar 27 23:34:00 1998 ]stę[Author ID1: at Fri Mar 27 23:33:00 1998 ]ż[Author ID1: at Fri Mar 27 23:34:00 1998 ]enia i [Author ID1: at Fri Mar 27 23:33:00 1998 ]w rozcieńczeniu nieskończenie wielkim zbliża się do 100%[Author ID1: at Fri Mar 27 23:35:00 1998 ]. Jest [Author ID1: at Fri Mar 27 23:36:00 1998 ]on również zależny od temperatury i rośnie wraz z jej wzrostem. [Author ID1: at Fri Mar 27 23:37:00 1998 ]Dysocj[Author ID1: at Fri Mar 27 23:42:00 1998 ]a[Author ID1: at Fri Mar 27 23:42:00 1998 ]cja elektrolityczna jest procesem odwracalnym, więc w roztworze elektrolitów istnieje równowaga[Author ID1: at Fri Mar 27 23:42:00 1998 ],[Author ID1: at Fri Mar 27 23:45:00 1998 ] dla kt[Author ID1: at Fri Mar 27 23:42:00 1998 ]órej można napisać wyrażenie na stałą równowagi [K[Author ID1: at Fri Mar 27 23:45:00 1998 ]c[Author ID1: at Fri Mar 27 23:46:00 1998 ]]:[Author ID1: at Fri Mar 27 23:46:00 1998 ]
[Author ID1: at Fri Mar 27 23:47:00 1998
][Author ID1: at Fri Mar 27 23:47:00 1998
]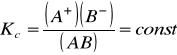
[Author ID1: at Fri Mar 27 23:47:00 1998
], jeżeli [Author ID1: at Fri Mar 27 23:51:00 1998
]t=co[Author ID1: at Fri Mar 27 23:52:00 1998
]nst[Author ID1: at Fri Mar 27 23:52:00 1998
], [Author ID1: at Fri Mar 27 23:52:00 1998
][Author ID1: at Fri Mar 27 23:53:00 1998
]
gdzie [Author ID1: at Fri Mar 27 23:52:00 1998 ](A[Author ID1: at Fri Mar 27 23:52:00 1998 ]+[Author ID1: at Fri Mar 27 23:52:00 1998 ]),(B[Author ID1: at Fri Mar 27 23:53:00 1998 ]-[Author ID1: at Fri Mar 27 23:53:00 1998 ])[Author ID1: at Fri Mar 27 23:53:00 1998 ] [Author ID1: at Tue Mar 31 22:53:00 1998 ]- rzeczywiste[Author ID1: at Fri Mar 27 23:53:00 1998 ] stężenie jonów w stanie równowagi, [Author ID1: at Fri Mar 27 23:54:00 1998 ](AB)[Author ID1: at Fri Mar 27 23:54:00 1998 ] - rzeczywiste stężenie cząsteczek niezdysocjowanych[Author ID1: at Fri Mar 27 23:54:00 1998 ] [Author ID1: at Fri Mar 27 23:56:00 1998 ]w [Author ID1: at Fri Mar 27 23:54:00 1998 ]stanie równowagi.[Author ID1: at Fri Mar 27 23:56:00 1998 ][Author ID0: at Thu Nov 30 00:00:00 1899 ]
Teoria kwasów i zasad według Br[Author ID1: at Tue Mar 31 20:55:00 1998 ]önsteda mówi, że cząsteczka kwasu po odd[Author ID1: at Tue Mar 31 22:42:00 1998 ]a[Author ID1: at Tue Mar 31 22:42:00 1998 ]niu protonu staje się cząsteczką lub jonem zasady i odwrotnie tzn. że cząsteczka zas[Author ID1: at Tue Mar 31 22:42:00 1998 ]a[Author ID1: at Tue Mar 31 22:42:00 1998 ]dy po przejęciu protonu staje się cząstką kwasu. Według [Author ID1: at Tue Mar 31 22:42:00 1998 ]tej teori[Author ID1: at Tue Mar 31 22:45:00 1998 ]i, kwas może wyk[Author ID1: at Tue Mar 31 22:46:00 1998 ]a[Author ID1: at Tue Mar 31 22:46:00 1998 ]zywać swoje właściwości kwasowe tylko wobec zasad[Author ID1: at Tue Mar 31 22:46:00 1998 ]y, która przyjmuje proton i o[Author ID1: at Tue Mar 31 22:47:00 1998 ]d[Author ID1: at Tue Mar 31 22:47:00 1998 ]wrotnie zasada może nią być tylko w obecności [Author ID1: at Tue Mar 31 22:47:00 1998 ]kwasu, który oddaje proton.[Author ID1: at Tue Mar 31 22:47:00 1998 ]
[Author ID1: at Tue Mar 31 22:49:00 1998 ]Doświadczenie 1.[Author ID0: at Thu Nov 30 00:00:00 1899 ]
![]()
[Author ID1: at Tue Mar 31 23:28:00 1998
]Dysocjacja chlorku miedziowego w obecności [Author ID1: at Tue Mar 31 22:51:00 1998
]H[Author ID1: at Tue Mar 31 22:52:00 1998
]2[Author ID1: at Tue Mar 31 22:52:00 1998
]O.[Author ID1: at Tue Mar 31 22:52:00 1998
][Author ID1: at Tue Mar 31 22:54:00 1998
]
Przebieg reakcji zależy od stałej dielektrycznej rozpuszczalnika [Author ID1: at Tue Mar 31 23:37:00 1998 ]-->ε[Author ID1: at Tue Mar 31 23:38:00 1998 ][Author ID1: at Tue Mar 31 23:40:00 1998 ]-->.[Author ID1: at Tue Mar 31 23:38:00 1998 ][Author ID1: at Tue Mar 31 23:40:00 1998 ] Gdy [Author ID1: at Tue Mar 31 23:38:00 1998 ]-->ε[Author ID1: at Tue Mar 31 23:38:00 1998 ][Author ID1: at Tue Mar 31 23:40:00 1998 ]--><10[Author ID1: at Tue Mar 31 23:39:00 1998 ][Author ID1: at Tue Mar 31 23:40:00 1998 ] dysocjacja nie zachodzi. Gdy [Author ID1: at Tue Mar 31 23:40:00 1998 ]10<[Author ID1: at Tue Mar 31 23:40:00 1998 ]ε[Author ID1: at Tue Mar 31 23:41:00 1998 ]<[Author ID1: at Tue Mar 31 23:40:00 1998 ]40[Author ID1: at Tue Mar 31 23:41:00 1998 ] dysocjacja zachodzi częściowo, gdy[Author ID1: at Tue Mar 31 23:41:00 1998 ] [Author ID1: at Tue Mar 31 23:43:00 1998 ]ε[Author ID1: at Tue Mar 31 23:43:00 1998 ]<40[Author ID1: at Tue Mar 31 23:43:00 1998 ] d[Author ID1: at Tue Mar 31 23:43:00 1998 ]y[Author ID1: at Tue Mar 31 23:43:00 1998 ]socjacja zachodzi całkowicie. Następuje tu zmiana barwy z żółtej na niebieską. Nat[Author ID1: at Tue Mar 31 23:43:00 1998 ]o[Author ID1: at Tue Mar 31 23:43:00 1998 ]miast [Author ID1: at Tue Mar 31 23:43:00 1998 ]dysocjacja [Author ID1: at Tue Mar 31 23:44:00 1998 ]-->CuCl[Author ID1: at Tue Mar 31 23:45:00 1998 ][Author ID1: at Tue Mar 31 23:45:00 1998 ]-->2[Author ID1: at Tue Mar 31 23:45:00 1998 ][Author ID1: at Tue Mar 31 23:45:00 1998 ]--> [Author ID1: at Tue Mar 31 23:41:00 1998 ][Author ID1: at Tue Mar 31 23:45:00 1998 ]w obecności acetonu, [Author ID1: at Tue Mar 31 23:46:00 1998 ]zachodzi częściowo. Na [Author ID1: at Tue Mar 31 23:47:00 1998 ]dnie znajdują się niebieskie jony [Author ID1: at Tue Mar 31 23:48:00 1998 ]Cu[Author ID1: at Tue Mar 31 23:48:00 1998 ]2+[Author ID1: at Tue Mar 31 23:48:00 1998 ], a u góry żółte niezdysocjowane [Author ID1: at Tue Mar 31 23:49:00 1998 ]CuCl[Author ID1: at Tue Mar 31 23:49:00 1998 ]2[Author ID1: at Tue Mar 31 23:49:00 1998 ]. Oznacza to, że w obecności acetonu dysocjacja zachodzi częściowo.[Author ID1: at Tue Mar 31 23:50:00 1998 ]
Doświadczenie 2.[Author ID1: at Tue Mar 31 23:15:00 1998 ]
Dysocjacja chlorku kobaltowego w obecności [Author ID1: at Tue Mar 31 23:16:00 1998 ]H[Author ID1: at Tue Mar 31 23:17:00 1998 ]2[Author ID1: at Tue Mar 31 23:17:00 1998 ]O.[Author ID1: at Tue Mar 31 23:17:00 1998 ][Author ID1: at Tue Mar 31 23:28:00 1998 ]
[Author ID1: at Tue Mar 31 23:28:00 1998
][Author ID1: at Tue Mar 31 23:28:00 1998
]![]()
[Author ID1: at Tue Mar 31 23:28:00 1998
][Author ID0: at Thu Nov 30 00:00:00 1899
]
Stała równowagi dla tej reakcji:[Author ID1: at Tue Mar 31 23:28:00 1998 ]
[Author ID1: at Tue Mar 31 23:28:00 1998
][Author ID1: at Tue Mar 31 23:28:00 1998
]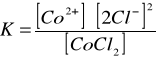
[Author ID1: at Tue Mar 31 23:28:00 1998
].[Author ID1: at Tue Mar 31 23:28:00 1998
][Author ID1: at Tue Mar 31 23:35:00 1998
]
Dodatek wspólnego jonu chlorkowego [Author ID1: at Tue Mar 31 23:36:00 1998 ]Cl[Author ID1: at Tue Mar 31 23:36:00 1998 ]-[Author ID1: at Tue Mar 31 23:36:00 1998 ] cofnął dysocjacje. Roztwór zmienił barwę z różowej na niebieską. Nadmiar jonu chlorkowego przesuwa równowagę w stronę zdysocjowanego [Author ID1: at Tue Mar 31 23:36:00 1998 ]CoCl[Author ID1: at Tue Mar 31 23:36:00 1998 ]2[Author ID1: at Tue Mar 31 23:36:00 1998 ].[Author ID1: at Tue Mar 31 23:36:00 1998 ][Author ID0: at Thu Nov 30 00:00:00 1899 ]
[Author ID1: at Fri Apr 3 21:07:00 1998 ]Doświadczenie 3.[Author ID1: at Fri Apr 3 21:06:00 1998 ][Author ID0: at Thu Nov 30 00:00:00 1899 ]
Do probówki zawierającej trzy krople (siarczanu żelaza III) [Author ID1: at Fri Apr 3 22:45:00 1998 ]Fe[Author ID1: at Fri Apr 3 22:46:00 1998 ]2[Author ID1: at Fri Apr 3 22:46:00 1998 ](SO[Author ID1: at Fri Apr 3 22:46:00 1998 ]4[Author ID1: at Fri Apr 3 22:46:00 1998 ])[Author ID1: at Fri Apr 3 22:46:00 1998 ]3[Author ID1: at Fri Apr 3 22:46:00 1998 ] dol[Author ID1: at Fri Apr 3 22:47:00 1998 ]al[Author ID1: at Fri Apr 3 22:50:00 1998 ]i[Author ID1: at Fri Apr 3 22:50:00 1998 ]śmy[Author ID1: at Fri Apr 3 22:50:00 1998 ] [Author ID1: at Fri Apr 3 22:47:00 1998 ]([Author ID1: at Fri Apr 3 22:49:00 1998 ]ti[Author ID1: at Fri Apr 3 22:47:00 1998 ]o[Author ID1: at Fri Apr 3 22:48:00 1998 ]cyjani[Author ID1: at Fri Apr 3 22:47:00 1998 ]a[Author ID1: at Fri Apr 3 22:48:00 1998 ]nu[Author ID1: at Fri Apr 3 22:47:00 1998 ] potasowego[Author ID1: at Fri Apr 3 22:48:00 1998 ]) [Author ID1: at Fri Apr 3 22:49:00 1998 ]KSCN[Author ID1: at Fri Apr 3 22:49:00 1998 ], otrzymany roztwór zabarwiony był na czerw[Author ID1: at Fri Apr 3 22:49:00 1998 ]o[Author ID1: at Fri Apr 3 22:49:00 1998 ]no. Po [Author ID1: at Fri Apr 3 22:49:00 1998 ]dodaniu wody destylowanej nastąpiła dysocjacja elektrolityczna, w wyniku kt[Author ID1: at Fri Apr 3 22:51:00 1998 ]ó[Author ID1: at Fri Apr 3 22:51:00 1998 ]rej [Author ID1: at Fri Apr 3 22:51:00 1998 ]roztwór odbarwił się na pomarańczowo.[Author ID1: at Fri Apr 3 22:52:00 1998 ]
Dysocjacja roztworu [Author ID1: at Fri Apr 3 22:58:00 1998 ]Fe(SCN)[Author ID1: at Fri Apr 3 22:59:00 1998 ]3[Author ID1: at Fri Apr 3 23:00:00 1998 ]:[Author ID1: at Fri Apr 3 23:00:00 1998 ]-->[Author ID1: at Fri Apr 3 22:58:00 1998 ][Author ID1: at Fri Apr 3 23:00:00 1998 ]
-->[Author ID1: at Fri Apr 3 22:54:00 1998
][Author ID1: at Fri Apr 3 23:52:00 1998
]-->[Author ID1: at Fri Apr 3 22:54:00 1998
][Author ID1: at Fri Apr 3 23:52:00 1998
]-->[Author ID1: at Fri Apr 3 23:52:00 1998
]-->![]()
[Author ID1: at Fri Apr 3 23:52:00 1998
]-->[Author ID1: at Fri Apr 3 22:54:00 1998
][Author ID1: at Fri Apr 3 23:52:00 1998
]-->.[Author ID1: at Fri Apr 3 23:00:00 1998
][Author ID1: at Fri Apr 3 23:52:00 1998
]
Do zdysocjowanowego rodanku w pierwszym przypadku dodaliśmy [Author ID1: at Fri Apr 3 23:00:00 1998 ]Fe[Author ID1: at Fri Apr 3 23:02:00 1998 ]2[Author ID1: at Fri Apr 3 23:02:00 1998 ](SO[Author ID1: at Fri Apr 3 23:02:00 1998 ]4[Author ID1: at Fri Apr 3 23:02:00 1998 ])[Author ID1: at Fri Apr 3 23:02:00 1998 ]3[Author ID1: at Fri Apr 3 23:02:00 1998 ] co spowodowało [Author ID1: at Fri Apr 3 23:03:00 1998 ]to zabarwienie roztworu na kolor krwist[Author ID1: at Fri Apr 3 23:05:00 1998 ]o[Author ID1: at Fri Apr 3 23:06:00 1998 ] czerwoną[Author ID1: at Fri Apr 3 23:05:00 1998 ]. Obecność wspólnego jonu [Author ID1: at Fri Apr 3 23:08:00 1998 ]Fe[Author ID1: at Fri Apr 3 23:09:00 1998 ]3+[Author ID1: at Fri Apr 3 23:10:00 1998 ] cofnęła dysocjację na stronę [Author ID1: at Fri Apr 3 23:10:00 1998 ]Fe(SCN)[Author ID1: at Fri Apr 3 23:11:00 1998 ]3[Author ID1: at Fri Apr 3 23:11:00 1998 ]. W drugim przypadku [Author ID1: at Fri Apr 3 23:11:00 1998 ]d[Author ID1: at Fri Apr 3 23:12:00 1998 ]o[Author ID1: at Fri Apr 3 23:12:00 1998 ]daliśmy [Author ID1: at Fri Apr 3 23:12:00 1998 ]KSCN[Author ID1: at Fri Apr 3 23:13:00 1998 ], miało to taki sam [Author ID1: at Fri Apr 3 23:13:00 1998 ]wynik jak w pierwszym przypadku. [Author ID1: at Fri Apr 3 23:14:00 1998 ]Wspólnym j[Author ID1: at Fri Apr 3 23:11:00 1998 ]o[Author ID1: at Fri Apr 3 23:11:00 1998 ]nem [Author ID1: at Fri Apr 3 23:11:00 1998 ]w tym przypadku był [Author ID1: at Fri Apr 3 23:15:00 1998 ]SCN[Author ID1: at Fri Apr 3 23:16:00 1998 ]-[Author ID1: at Fri Apr 3 23:16:00 1998 ]. Również tutaj obecność cofnęła[Author ID1: at Fri Apr 3 23:16:00 1998 ] dysocjację[Author ID1: at Fri Apr 3 23:17:00 1998 ] [Author ID1: at Fri Apr 3 23:18:00 1998 ]w stronę [Author ID1: at Fri Apr 3 23:17:00 1998 ]Fe(SCN)[Author ID1: at Fri Apr 3 23:17:00 1998 ]3[Author ID1: at Fri Apr 3 23:17:00 1998 ].[Author ID1: at Fri Apr 3 23:17:00 1998 ][Author ID0: at Thu Nov 30 00:00:00 1899 ]
Do trzech probówek wprowadziliśmy następujące związki: [Author ID1: at Fri Apr 3 23:19:00 1998 ]Pb(NO[Author ID1: at Fri Apr 3 23:20:00 1998 ]3[Author ID1: at Fri Apr 3 23:21:00 1998 ])[Author ID1: at Fri Apr 3 23:21:00 1998 ]2[Author ID1: at Fri Apr 3 23:21:00 1998 ],[Author ID1: at Fri Apr 3 23:21:00 1998 ] (CH[Author ID1: at Fri Apr 3 23:21:00 1998 ]3[Author ID1: at Fri Apr 3 23:22:00 1998 ]COO)[Author ID1: at Fri Apr 3 23:22:00 1998 ]2[Author ID1: at Fri Apr 3 23:22:00 1998 ]Pb[Author ID1: at Fri Apr 3 23:22:00 1998 ] oraz [Author ID1: at Fri Apr 3 23:22:00 1998 ]PbCl[Author ID1: at Fri Apr 3 23:23:00 1998 ]2[Author ID1: at Fri Apr 3 23:23:00 1998 ]. Następnie kolejno do każdej z nich nala[Author ID1: at Fri Apr 3 23:23:00 1998 ]liśmy kwasu sia[Author ID1: at Fri Apr 3 23:24:00 1998 ]r[Author ID1: at Fri Apr 3 23:24:00 1998 ]kowego[Author ID1: at Fri Apr 3 23:24:00 1998 ] (po dwie krople). W [Author ID1: at Fri Apr 3 23:24:00 1998 ]pierwszej probówce [Author ID1: at Fri Apr 3 23:25:00 1998 ]roztwór [Author ID1: at Fri Apr 3 23:27:00 1998 ]zmienił konsystencję i w[Author ID1: at Fri Apr 3 23:25:00 1998 ]y[Author ID1: at Fri Apr 3 23:25:00 1998 ]dzielił się z nieg[Author ID1: at Fri Apr 3 23:25:00 1998 ]o osad. W [Author ID1: at Fri Apr 3 23:27:00 1998 ]drugiej także wydzielił się osad[Author ID1: at Fri Apr 3 23:28:00 1998 ], to samo, ale z mniejszą intensywnością, zaszło w trzeciej probówce.[Author ID1: at Fri Apr 3 23:29:00 1998 ]
-->[Author ID1: at Fri Apr 3 23:31:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:31:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->
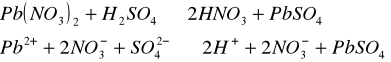
[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:31:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:37:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:37:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:37:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->
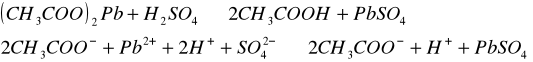
[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:37:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:45:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:45:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:45:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:45:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->
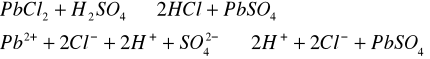
[Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:45:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]-->[Author ID1: at Fri Apr 3 23:52:00 1998 ][Author ID1: at Fri Apr 3 23:52:00 1998 ]
W drugiej [Author ID1: at Fri Apr 3 23:52:00 1998 ]części doświadczenia do kolejnych trzech probówek wl[Author ID1: at Fri Apr 3 23:53:00 1998 ]aliśmy[Author ID1: at Fri Apr 3 23:56:00 1998 ] [Author ID1: at Fri Apr 3 23:57:00 1998 ]k[Author ID1: at Fri Apr 3 23:53:00 1998 ]o[Author ID1: at Fri Apr 3 23:53:00 1998 ]lejno [Author ID1: at Fri Apr 3 23:53:00 1998 ]Na[Author ID1: at Fri Apr 3 23:54:00 1998 ]2[Author ID1: at Fri Apr 3 23:54:00 1998 ]SO[Author ID1: at Fri Apr 3 23:54:00 1998 ]4[Author ID1: at Fri Apr 3 23:54:00 1998 ], [Author ID1: at Fri Apr 3 23:54:00 1998 ]K[Author ID1: at Fri Apr 3 23:54:00 1998 ]2[Author ID1: at Fri Apr 3 23:54:00 1998 ]SO[Author ID1: at Fri Apr 3 23:54:00 1998 ]4[Author ID1: at Fri Apr 3 23:54:00 1998 ], [Author ID1: at Fri Apr 3 23:54:00 1998 ](NH[Author ID1: at Fri Apr 3 23:55:00 1998 ]4[Author ID1: at Fri Apr 3 23:55:00 1998 ])[Author ID1: at Fri Apr 3 23:55:00 1998 ]2[Author ID1: at Fri Apr 3 23:55:00 1998 ]SO[Author ID1: at Fri Apr 3 23:55:00 1998 ]4[Author ID1: at Fri Apr 3 23:56:00 1998 ]. Do każdej z nich doda[Author ID1: at Fri Apr 3 23:56:00 1998 ]liśmy[Author ID1: at Fri Apr 3 23:57:00 1998 ] po[Author ID1: at Fri Apr 3 23:56:00 1998 ] dwie krople [Author ID1: at Fri Apr 3 23:57:00 1998 ]Pb(NO[Author ID1: at Fri Apr 3 23:57:00 1998 ]3[Author ID1: at Fri Apr 3 23:57:00 1998 ])[Author ID1: at Fri Apr 3 23:57:00 1998 ]2[Author ID1: at Fri Apr 3 23:57:00 1998 ],[Author ID1: at Fri Apr 3 23:57:00 1998 ] [Author ID1: at Fri Apr 3 23:57:00 1998 ]co spowodowało wytrącenie się osadu w każdej probówce.[Author ID1: at Fri Apr 3 23:58:00 1998 ][Author ID1: at Sat Apr 4 00:00:00 1998 ]
Doświadczenie 5.[Author ID1: at Sat Apr 4 00:15:00 1998 ]
Do dwóch[Author ID1: at Sat Apr 4 00:17:00 1998 ] probówek zawierających (kwas octowy) [Author ID1: at Sat Apr 4 00:18:00 1998 ]CH[Author ID1: at Sat Apr 4 00:19:00 1998 ]3[Author ID1: at Sat Apr 4 00:19:00 1998 ]COOH[Author ID1: at Sat Apr 4 00:19:00 1998 ] dodaliśmy oranż metylowy[Author ID1: at Sat Apr 4 00:19:00 1998 ]. Otrzymany [Author ID1: at Sat Apr 4 00:22:00 1998 ]roztwór miał barwę pomarańczową. Do jednej probówki dodaliśmy [Author ID1: at Sat Apr 4 00:23:00 1998 ]CH[Author ID1: at Sat Apr 4 00:24:00 1998 ]3[Author ID1: at Sat Apr 4 00:24:00 1998 ]COONa[Author ID1: at Sat Apr 4 00:24:00 1998 ] przez co roztwór zmienił barwę na lekko żółtą. Obecność [Author ID1: at Sat Apr 4 00:24:00 1998 ]wspólnego jonu[Author ID1: at Sat Apr 4 00:25:00 1998 ] [Author ID1: at Sat Apr 4 00:27:00 1998 ] CH[Author ID1: at Sat Apr 4 00:26:00 1998 ]3[Author ID1: at Sat Apr 4 00:26:00 1998 ]COO[Author ID1: at Sat Apr 4 00:26:00 1998 ]-[Author ID1: at Sat Apr 4 00:27:00 1998 ] cofnęła dysocjację[Author ID1: at Sat Apr 4 00:25:00 1998 ] w stronę [Author ID1: at Sat Apr 4 00:27:00 1998 ] CH[Author ID1: at Sat Apr 4 00:26:00 1998 ]3[Author ID1: at Sat Apr 4 00:26:00 1998 ]COOH[Author ID1: at Sat Apr 4 00:26:00 1998 ].[Author ID1: at Sat Apr 4 00:27:00 1998 ]
Dysocjacja kwasu octowego[Author ID1: at Sat Apr 4 00:45:00 1998 ] (CH[Author ID1: at Sat Apr 4 00:48:00 1998 ]3[Author ID1: at Sat Apr 4 00:48:00 1998 ]COOH)[Author ID1: at Sat Apr 4 00:48:00 1998 ]:[Author ID1: at Sat Apr 4 00:48:00 1998 ][Author ID1: at Sat Apr 4 00:30:00 1998 ]
[Author ID1: at Sat Apr 4 00:30:00 1998
][Author ID1: at Sat Apr 4 00:30:00 1998
]![]()
[Author ID1: at Sat Apr 4 00:30:00 1998
].[Author ID0: at Thu Nov 30 00:00:00 1899
]
Stała dysocjacji[Author ID1: at Sat Apr 4 00:32:00 1998 ] kwasu octowego [Author ID1: at Sat Apr 4 00:33:00 1998 ]([Author ID1: at Sat Apr 4 00:49:00 1998 ]CH[Author ID1: at Sat Apr 4 00:33:00 1998 ]3[Author ID1: at Sat Apr 4 00:33:00 1998 ]COOH[Author ID1: at Sat Apr 4 00:33:00 1998 ])[Author ID1: at Sat Apr 4 00:49:00 1998 ]:[Author ID1: at Sat Apr 4 00:33:00 1998 ]
[Author ID1: at Sat Apr 4 00:34:00 1998
][Author ID1: at Sat Apr 4 00:34:00 1998
]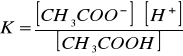
[Author ID1: at Sat Apr 4 00:34:00 1998
].[Author ID0: at Thu Nov 30 00:00:00 1899
]
W drugiej części [Author ID1: at Sat Apr 4 00:37:00 1998 ]doświadczenia do dwóch probówek wprowadziliśmy [Author ID1: at Sat Apr 4 00:38:00 1998 ]NH[Author ID1: at Sat Apr 4 00:39:00 1998 ]4[Author ID1: at Sat Apr 4 00:39:00 1998 ]OH[Author ID1: at Sat Apr 4 00:39:00 1998 ], który zabarwiliśmy fenoloftaleiną (kolor malinowy). Dodanie [Author ID1: at Sat Apr 4 00:39:00 1998 ]do jednej z dwóch pr[Author ID1: at Sat Apr 4 00:41:00 1998 ]o[Author ID1: at Sat Apr 4 00:41:00 1998 ]bówek [Author ID1: at Sat Apr 4 00:41:00 1998 ]NH[Author ID1: at Sat Apr 4 00:42:00 1998 ]4[Author ID1: at Sat Apr 4 00:42:00 1998 ]Cl[Author ID1: at Sat Apr 4 00:42:00 1998 ]2[Author ID1: at Sat Apr 4 00:42:00 1998 ] spowodowało całkowite odbarwienie roztworu.[Author ID1: at Sat Apr 4 00:42:00 1998 ] Również [Author ID1: at Sat Apr 4 00:43:00 1998 ]tu obecność wspólnego jonu cofnęła dysocjację.[Author ID0: at Thu Nov 30 00:00:00 1899 ]
[Author ID1: at Sat Apr 4 00:44:00 1998 ]Dysocjacja wodorotlenku amonowe[Author ID1: at Sat Apr 4 00:45:00 1998 ]go[Author ID1: at Sat Apr 4 00:45:00 1998 ] (NH[Author ID1: at Sat Apr 4 00:49:00 1998 ]4[Author ID1: at Sat Apr 4 00:49:00 1998 ]OH)[Author ID1: at Sat Apr 4 00:49:00 1998 ]:[Author ID1: at Sat Apr 4 00:45:00 1998 ]-->[Author ID1: at Sat Apr 4 00:33:00 1998 ][Author ID1: at Sat Apr 4 00:39:00 1998 ]
[Author ID1: at Sat Apr 4 00:28:00 1998
][Author ID1: at Sat Apr 4 00:28:00 1998
]![]()
[Author ID1: at Sat Apr 4 00:28:00 1998
].[Author ID1: at Sat Apr 4 00:47:00 1998
]
Stała dysocjacji tej zasady wynosi:[Author ID1: at Sat Apr 4 00:48:00 1998 ]
[Author ID1: at Sat Apr 4 00:51:00 1998
][Author ID1: at Sat Apr 4 00:51:00 1998
]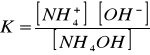
[Author ID1: at Sat Apr 4 00:51:00 1998
].[Author ID1: at Sat Apr 4 00:51:00 1998
]
[Author ID1: at Fri Apr 3 23:56:00 1998 ]-->[Author ID1: at Fri Apr 3 23:51:00 1998 ][Author ID1: at Fri Apr 3 23:56:00 1998 ]
[Author ID1: at Tue Mar 31 23:18:00 1998
][Author ID1: at Tue Mar 31 23:18:00 1998
]![]()
[Author ID1: at Tue Mar 31 23:18:00 1998
]-->[Author ID1: at Tue Mar 31 23:01:00 1998
][Author ID1: at Tue Mar 31 23:14:00 1998
]
[Author ID1: at Tue Mar 31 22:45:00 1998 ]-->[Author ID1: at Fri Mar 27 22:52:00 1998 ][Author ID1: at Fri Mar 27 23:54:00 1998 ]
![]()
Wyszukiwarka
Podobne podstrony:
Ćw.ch.4, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Ćw.ch.5, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Ćw.ch.4(1), Szkoła, penek, Przedmioty, Chemia, Laboratoria
Poprawka Cw 3, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Chemia kataliza, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Roztwory, Szkoła, penek, Przedmioty, Chemia, Laboratoria
KOROZJA1, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Hydroliza, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Szybkość reakcji, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Pierwiastki 2, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Redox2, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Elektroliza, Szkoła, penek, Przedmioty, Chemia, Laboratoria
w03 Dysocjacja elektrolity, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Sprawozdanie 5 ćwiczenia 4 i 5, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Reakcje redoks, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Korozja, Szkoła, penek, Przedmioty, Chemia, Laboratoria
Korozja-now, Szkoła, penek, Przedmioty, Chemia, Laboratoria
więcej podobnych podstron