Oblicz pH roztworu zawierającego w 1 dm3 0.020 M Na2SO4 i 0.020 M HCl.
cNa+ = 2 *cNa2SO4 = 2*0.020 M = 0.040 M
cSO42- = 0.020 M
cCl- = 0.020 M
I = ½ (0.040*12 + 0.020*22 + 0.020*12 +0.020*12) =0.080
Dla jonu H+: a*B = 3.0, A = 0.51
![]()
![]()
![]()
![]()
![]()
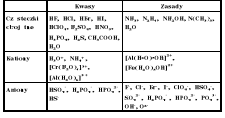
ogniwa: K(+)-red | A(-)-utl elektro: K(-)-red | A(+)-utl |
![]()
→(+)e.joniz.,powin.e-(-)char.metal. ↓(+)char.metal.(-)e.joniz.,powin.e-,elektrouj. |
![]()
[H3O+]=10-pH |
[HCOOH] |
[H3O+] |
[HCOO−] |
pocz. |
0.10 |
0 |
0 |
zmiana |
−4.2 × 10-3 |
+4.2 × 10-3 |
+4.2 × 10−3 |
równow. |
0.10 − 4.2 × 10−3 = 0.0958 = 0.10 |
4.2 × 10−3 |
4.2 × 10−3 |
![]()
![]()
![]()

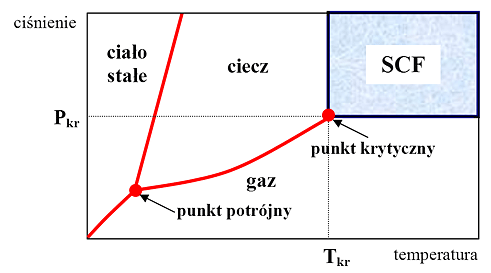
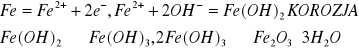
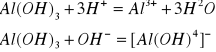
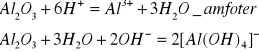
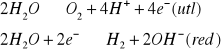
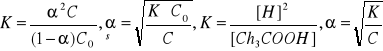
![]()
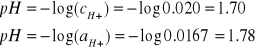
![]()
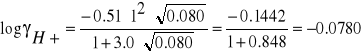
![]()
Wyszukiwarka
Podobne podstrony:
sciaga egz nieorg2, podstawy chemii nieorganicznej
Podstawy chemii nieorganicznej egzamin 13
,podstawy chemii nieorganicznej L, Pojemność buforu
,podstawy chemii nieorganicznej L,Równowagi w roztworach elektrolitów
,podstawy chemii nieorganicznej L, Pojemność buforu
,podstawy chemii nieorganicznej L,stała dysocjacji słabego elektrolitu
program, podstawy chemii nieorganicznej, Chemia nieorganiczna laboratorium, MOJE
Podstawy chemii nieorganicznej egzamin id 366852
,podstawy chemii nieorganicznej L,Równowagi w roztworach elektrolitów
Bufory sprawko, podstawy chemii nieorganicznej, Chemia nieorganiczna laboratorium, MOJE
jastrzab lomozik bregier jarzebowska podstawy chemii nieorg
Bufory sprawko, Biotechnologia PWR, Semestr 2, Podstawy chemii nieorganicznej Laboratorium, Instrukc
inz chem sciaga egz, podstawy inżynierii chemicznej
więcej podobnych podstron