lewulino
Glicyna i kwas glutaminowy jako prekursor porfiryn
|
(a) |
[( |
1 O O r | ||||
|
( |
:oo- |
( |
:h2 | |||
|
( |
h2 |
CoA-SH |
( |
'H, |
co2 | |
|
< |
'H2 + < l—S-CoA |
pHa—NHa zoo |
._9 , |
>—o |
9 | |
|
1 |
f> -aminolevulinate •yntha*c |
V \ |
:h—ńh;i |
ć-aminol«vuhnute synthasc | ||
|
( |
) |
( |
:oo | |||
|
Succinyl-CoA |
| Glycine | |
a-Amino-0- ketoadipate_ | ||||
ciułania U*- 1 -ocmiaJdcKydc aminomutase
(b)
coo
CH*
COO
I
ch2
CHo
I ♦
hc-nh3
c=o
tRNAc,u
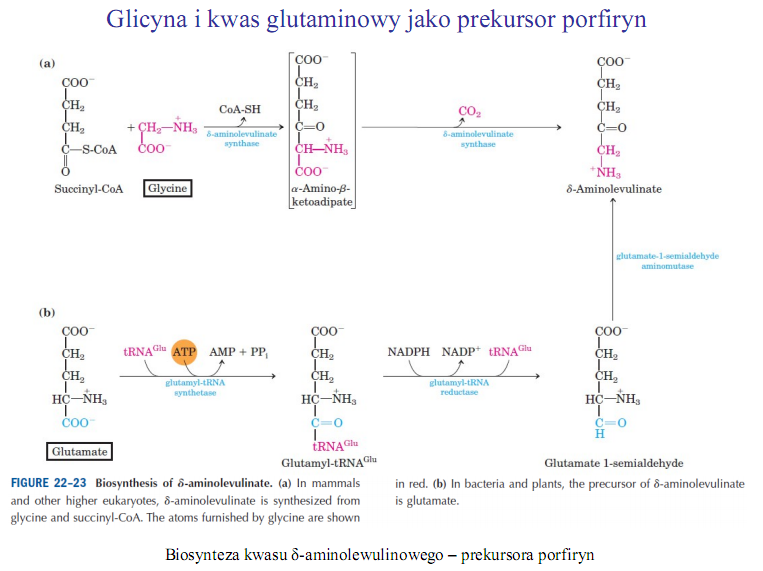
Glutamyl-tRNA01" FIGURĘ 22-23 Biosynthosis ot «S-aminolcvulinate. (a) In mammals and other higher eukaryotes, 5-aminolevulinate is synthesized from glycine and succinyl-CoA. The atoms furnished by glycine are shown
HC-NH,
COO
Glutamate I
tRNAGłu
glutnmyl-tRNA syn tlw lane
NADPH
glutiimyl-lRNA
rcduclasc
COO
CHo
CHo
I ♦
HC—NH3
C=0
H
Glutamate 1-semialdehyde
in red. (b) In bacteria and plants, the precursor of 8-aminolevulinate is glutamate.
Wyszukiwarka
Podobne podstrony:
DSC03252 AMINOKWASY W OUN * Kwa* y-aminomaSłowy (GABA) Glicyna Kwas glutaminowy Kwas asparaginowy Kw
DSC 20 Glutation Tripeptyd - cysteina, kwas glutaminowy, glicyna Zredukowany glutation = GSH Jak zap
37123 IMGP4647 (1024x667) .............. , ir»Glutatión Tripeptyd - cysteina, kwas glutaminowy, glic
Image005 kwas kwas glutaminowy. Smak fen nazwano urnami. Niedługo po odbyciu rozpoczęto pr^ dukcję p
skanuj0045 (7) Kwas octowy (jako słaby kwas) powoduje wiązanie jonów CNO,,2 z wytworzeniem HC204 : C
skanuj0045 4 Kwas octowy (jako słaby kwas) powoduje wiązanie jonów C2O42 z wytworzeniem HC2O4*:CH3CO
DSC00023 (15) HfH—CH ęHt + MAD kwas glutaminom] dehydrogenaza głutaminianowa y **i f“° n -f HjO mmmm
Maszczak T.: Jędrzej Śniadecki jako prekursor wychowania fizycznego specjalnego. Wychowanie Fizyczne
35 NARODZINY GENETYKI MOLEKULARNEJ nowicie, w tym miejscu, gdzie u zdrowych ludzi występuje kwas glu
DSC00010 Smaki - prelercnfjc ■ I numi — (kwas glutaminowy) ■ Sło
więcej podobnych podstron