92665
2261
J. Salrh et ai./FuetS9 (2010) 2260 2266
CH, — O
CH — O
CH:— O
O
L
0
1
R
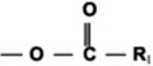
CH: — OH
— R:
— R>
(Triglyccridc)
Catalyst -f 3ROH ◄-►
(Mixturc of fatty cstcrs)
(Alcohol)
(Glyccrol)
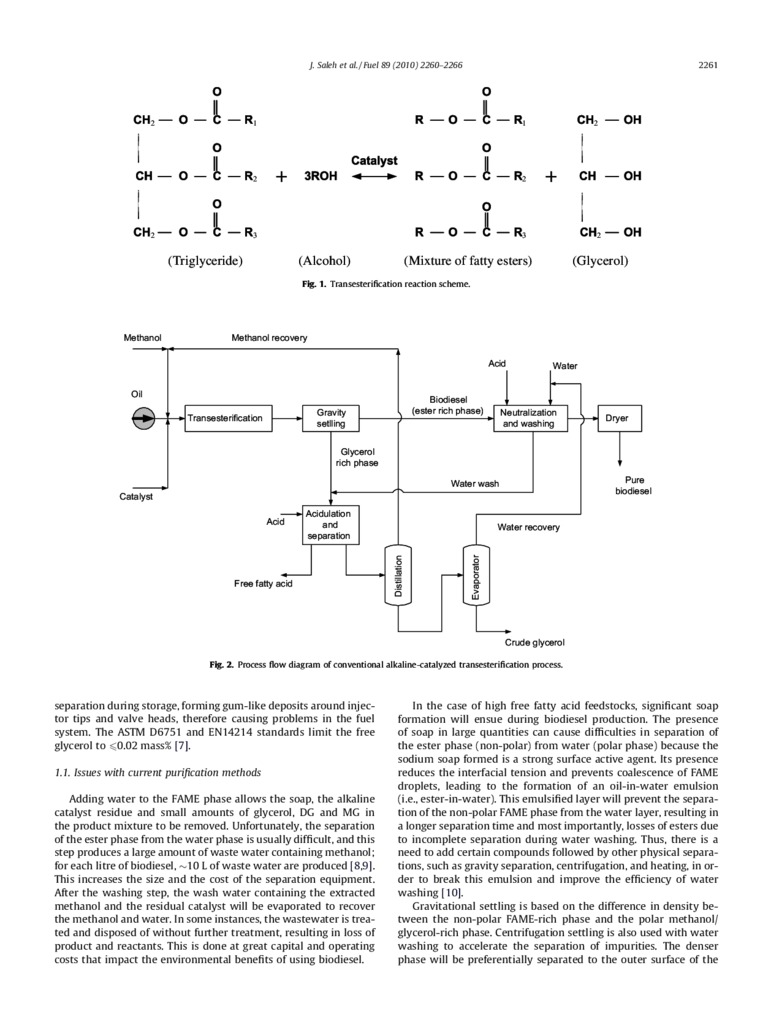
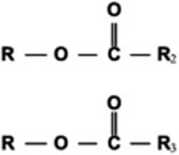
FI*. i. Transesterification rcaction schcmo.
Methanoi Methanol recovery
Crude gtycerol
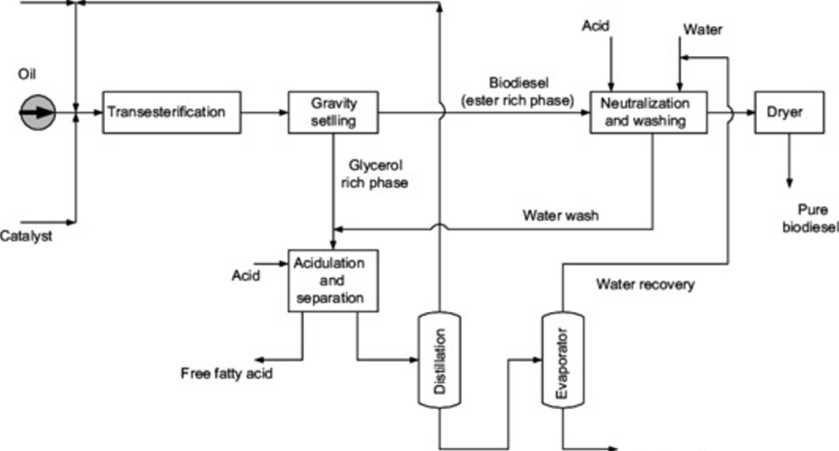
Fijc. 2. Proces* rtow diagram of convrntional alkalinc<alaly2cd Iranscstcriftcation proces*.
separation cluring storage.forminggum-like deposits around injec-tor tips and valve heads. (herefore causing problem* in the fuel system. The ASTM D6751 and EN 14214 standards limit the free glycerol to <0.02 mass% |7|.
1.1. Issues with currenl purification methods
Adding water to the FAMĘ phase allows the soap. the alkaline catalyst residue and smali amounts of glycerol. DG and MG in the product mixture to be removed. Unfortunately. the separation of the ester phase from the water phase is usually difficult. and this step produces a large amount of waste water containing methanol; foreach litre of biodiesel, ~-10 Lof waste water are produced |8.9|. This increases the size and the cost of the separation equipment. After the washing step. the wash water containing the extracted methanol and the residual catalyst will be evaporated to recover the methanol and water. In some instances. the wastewater is trea-ted and disposed of without further treatment. resulting in loss of product and reactants. This is done at great Capital and operating costs that impact the environmental benefits of using biodiesel.
In the case of high free fatty acid feedstocks. significant soap formation will ensue during biodiesel production. The presence of soap in large quantities can cause difhculties in separation of the ester phase (non-polar) from water (polar phase) because the sodium soap formed is a strong surface active agent. Its presence reduces the interfacial tension and prevents coalescence of FAMĘ droplets. leading to the formation of an oil-in-water emulsion (i.e.. ester-in-water). This emulsified layer will prevent the separation of the non-polar FAMĘ phase from the water layer. resulting in a longer separation time and most importantly. losses of esters due to incomplete separation during water washing. Thus. there is a need to add certain compounds followed by other physical separa-tions. such as gravity separation. centrifugation. and heating. in order to break this emulsion and improve the efficiency of water washing (10|.
Gravitational settling is based on the difference in density be-tween the non-polar FAME-rich phase and the polar methanol/ glycerol-rich phase. Centrifugation settling is also used with water washing to accelerate the separation of impurities. The denser phase will be preferentially separated to the outer surface of the
Wyszukiwarka
Podobne podstrony:
186 S. Takahashi et ai (D For the beam chamber side Q,/2 = h x A x AT Q i
Architektura snu u pacjenta z depresją Bu/sse OJ et ai In Siei n DJ et ał. eds Textbook of Mooó Diso
908 B. Rihn et ai carcinogenesis (Keith et al., 1995). Both males and females spontaneously develop
528 M. Daszykowski et ai To interpret differences among the groups of sagę samples, score and loadin
149 Levitsky, Steven et Lucan Way. 2010, Competitive Authoritarianism. Hybrid Regimes After the Cold
Kopańska et ai Journal of Orthopaedic Surgery and Research (2017) 12:51 DOI 10.1186/S13018 017 0542
Zdjęcie039(3) V/
Zdjęcie059 Złoża Fe-TA/ w Kompleksie Bustweld (ryc. Bolewski et ai. 1992 na podstawie
ETUDE SI R LE DIALKCTE BERBERE DES ZA1AN ET Ai r SOOUGOU 1° Toujours, apres ma : Suppression de ał:
152 Mei, H., Chen, W., Dellinger, A., He, J., Wang, M., Yau, C., Srinivasan, S. et Be-renson, G. (20
KAMSIAH IBRAHIM, M.A. AUGUSTIN ANI) A.S.H. ONG terioration of oil during frying (Gwo et ai 1985). Ho
img057 Chengjang (ci A.śj- GcuScicrsccRC,Cathaymyrus diadexus Shu et ai., 1999 -12 cm -
więcej podobnych podstron