2949775338
Comparison of Liąuid Propellcmt Rocket Engine Feed Systems - 1 - 5
where, my. Pressurizing gas mass (kg).
pp: Propellant tank instantaneous pressure (Pa). py Gas tank instantaneous pressure (Pa). py Pressurizing gas initial pressure (Pa).
T„: Pressurizing gas initial temperaturę (K).
Vy Propellant volume (m3).
Ry Pressurizing gas constant (J/kgK). yy Pressurizing gas specific heat ratio.
To foresee the scope of this analysis, the assumptions that allow deriving the previous eąuation will be enunciated:
• Adiabatic process.
• Ideał gas.
• Negligible initial mass inside the pipes and propellant tanks.
It will be assumed that the instantaneous pressure in the gas and propellants tanks is the same, that is, there are no losses in the pipes connecting them. Also, it is interesting to refer all feed system pressures to the combustion chamber pressure, which is a project parameter [3], Based on this criterion, the following constant is defined:
k
p
where pc is the combustion chamber pressure (Pa).
Not all the volume of a propellant tank is occupied by this one. A smali part is occupied by gas and that portion of the total volume of the tank is denominated ułlage. This is the necessary space to allow the propellant thermal expansion, the accumulation of gases that were originally dissolved in the propellants and to contain the reaction products of the slow reactions, which occurs during storage [1], To assess this ąuantity in the analysis, an additional constant relating both volumes will be introduced, also assuming that it is the same for both tanks:
k„
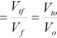
(2.1.3)
where, V,/ Fuel tank volume (m3).
V,y Oxidizer tank volume (m3).
V/ Fuel volume (m3).
V„: Oxidizer volume (m3).
Besides, the gas constant should be expressed in terms of their molar mass, thus:
Rg =
(2.1.4)
where, Ry Universal gas constant (8314.41 J/kmolK). My Pressurizing gas molar mass (kg/kmol).
Furthermore, volume and mass will be related through the following expressions:
Wyszukiwarka
Podobne podstrony:
Comparison of Liąuid Propellant Rocket Engine Feed Systems -1-9 At this point, is convenient to refe
Comparison of Liąuid Propellant Rocket Engine Feed Systems - 1 - 10 meepopllc 1 SLV (2.4.3) Replacin
Comparison of Liąuid Propellant Rocket Engine Feed Systems - 1 - 11 According to the previously expo
Comparison of Liąuid Propellant Rocket Engine Feed Systems - 1 - 12 Finally, combining the above exp
Comparison of Liąuid Propellant Rocket Engine Feed Systems - 1 - 15 The turbinę inlet gases are to a
Comparison of Liąuid Propellant Rocket Engine Feed Systems - 1 - 16 3.7. Materials It is necessary t
Comparison of Liąuid Propellant Rocket Engine Feed Systems - 1 - 1 REPORT N21COMPARISON OF LIOUID PR
Comparison of Liąuid Propellant Rocket Engine Feed Systems -1-2 CONTENTS I.
Comparison of Liąuid Propellant Rocket Engine Feed Systems - 1 - 3 I. INTRODUCTION Since several dec
Comparison of Liąuid Propellant Rocket Engine Feed Systems - 1 - 4 current from the battery to altem
Comparison of Liąuid Propellant Rocket Engine Feed Systems - 1 - 6 0/F mf pfvf - -mp = mppa \+OIF) 1
Comparison of Liąnid Propellant Rocket Engine Feed Systems - 1 - 8 2.2.2. Propellant tank mass In a
Comparison ofLiąuid Propellant Rocket Engine Feed Systems -1-7 (N II £ (2.2.2) where, Ap: Sphere
Comparison ofLiąuid Pr opel fant Rocket Engine Feed Systems - 1 - 13 . gg-RTin (2.9.4) where, Rgg: T
Comparison ofLiguid Pr opel font Rocket Engine Feed Systems - 1 - 14 For each calculation routine, i
Comparison ofLiąuid PropeUant Rocket Engine Feed Systems - 1 - 17 Figurę 2: Synchronous motor specif
Comparison ofLiąuid Pr opel lani Rocket Engine Feed Systems - 1 - 18 Table 6: Proposed batteries t
Research Report.ELECTRIC FEED SYSTEMS FOR LIQUID PROPELLANT ROCKET ENGINES.Pablo Rachov, Hernan
RESEARCH REPORTSELECTRIC FEED SYSTEMS FOR LIOUID PROPELLANT ROCKET ENGINESPablo RACHOV LABORATO
więcej podobnych podstron