372596247
74
RIKEN Accel. Próg. Rep. 24 (1990)
111*3-10. A Multitracer Study of the Adsorption of Metal Elements
on Hydrated Ferric Oxide
S.Y. Chen, S. Ambe, Y. Ohkubo, M. Iwamoto,
Y. Kobayashi, N. Takematsu, and F. Ambe
Adsorption is one of the most important pro- ume of the suspension was then adjusted to 10 ml
cesses in geochemical behavior of metal elements. with artificial seawater, and its pH to 7.5. Shaken
A multitracer techniąue using radioactive nuclides in an 8-shape modę with a shaker at 25°C, the sus-
produced by heavy-ion irradiation is especially pension was found to attain the adsorption eąuilib-
effective in studying the characteristic behavior of rium within 60 min. After centrifugation, 5 ml of the
different elements simultaneously. This report solution was pipetted from the supematant solution.
describes a multitracer study on the adsorption of The y-ray spectrum of radioactive nuclides in the
various metal elements on hydrated ferric oxide. solution was measured with a Ge detector.
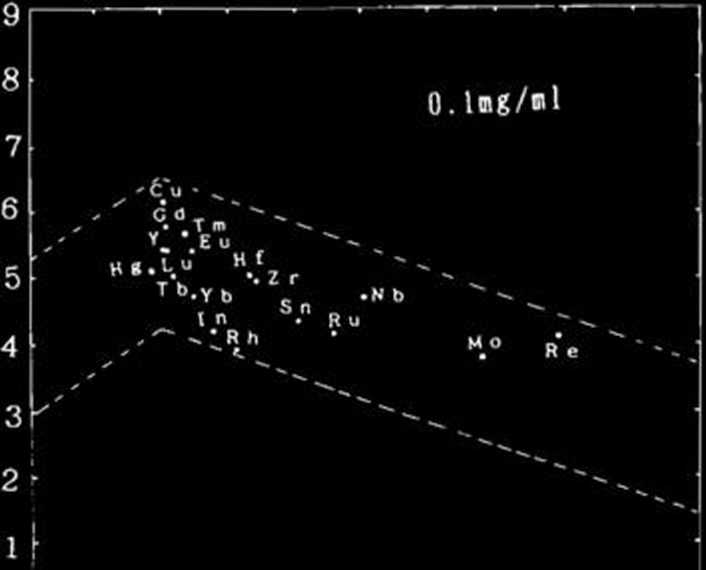
Carrier- and salt-free radioactive multitracer The distribution coefficients (Ad) of Y, Zr, Hf, Solutions were prepared from gold foil irradiated Mo, Re, Ru, Rh, Cu, Hg, In, Eu, Gd, Tb, Tm, with a 14N beam of 135 MeWnucleon as described Yb, Sn, and Nb were determined from the activities elsewhere.11 of their radioactive isotopes. The results are shown
Eight milliliters of artificial seawater and 10 /u.1 of in Table 1. The logarithmic plot of Ka against the
a multitracer solution was put into a polyethylene electron binding energy I2 of M4 2 is given in Fig. 1.
bottle. After pH was adjusted to 7.5 with a 1 mol These data provide a basis for understanding the
dm-3 Na2C0;j solution, 0.5 ml of adsorbent suspen- removal mechanism of metal elements from sea-sion (2 mg/ml) was added to the solution. The vol- water.2)
Tabie 1. Distribution coefficients of metal elements for hydrated ferric oxide (ml mg-1).*
Fe(0H):i
|
Y |
2.63 x 10s |
|
Zr |
9.12 x 104 |
|
Hf |
1.07 x 105 |
|
Mo |
6.46 x 103 |
|
Re |
1.35 x 104 |
|
Ru |
1.50 x 104 |
|
Rh |
8.32 x 103 |
|
Cu |
1.41 x 10B |
|
Hg |
1.26 x 105 |
|
In |
1.58 x 104 |
|
Eu |
2.51 x 105 |
|
Gd |
5.75 x 105 |
|
Tb |
1.05 x 105 |
|
Tm |
3.72 x 10r> |
|
Lu |
2.63 x 105 |
|
Yb |
5.27 x 104 |
|
Sn |
2.27 x 104 |
|
Nb |
5.39 x 104 |
* The concentration of a sorbent is 0.1 mg-ml \
OL--1- . 4 A- A , ■ I *■ —-*--1-->
o 20 40 60 80 100
Electron Binding Energy. I?. (eV>
Fig. 1. Logarithmic distribution coefficients Kd of metal elements are plotted against electron binding energies.
References
1) S. Ambe, S.Y. Chen, Y. Ohkubo, Y. Kobayashi, M. Iwamoto, and F. Ambe: This Report, p. 73.
2) S.Y. Chen, S. Ambe, Y. Ohkubo, M. Iwamoto, Y. Kobayashi, N. Takematsu, and F. Ambe: ibid., p.
p. 75.
Wyszukiwarka
Podobne podstrony:
105 RIKEN Accel. Próg. Rep. 24 (1990)111-5-10. Velocity Distribution of IGISOL lon Beams M. Koizumi,
108 RIKEN Accel. Próg. Rep. 24 (1990)111-5-12. Status Report of the RIKEN Swinger-Magnetic Analyzer
12 RIKEN Accel. Próg. Rep. 24 (1990)111-1-3. Coulomb Breakup Reaction of 90 MeV/u 140 T. Takei, T. M
12 RIKEN Accel. Próg. Rep. 24 (1990)111-1-3. Coulomb Breakup Reaction of 90 MeV/u 140 T. Takei, T. M
92 RIKEN Accel. Próg. Rep. 24 (1990)111-5. Instrumentation1. Design of a Microbeamline for a Compact
94 RIKEN Accel. Próg. Rep. 24 (1990)111-5-2. Design of a Decay Muon Channel Using an Axially Symmetr
102 RIKEN Accel Próg. Rep. 24 (1990)111-5-8. Performance of Isotopic Separation in RIPS T.Nakamura,
103 RIKEN Accel. Próg. Rep. 24 (1990)111-5-9. Test Experiment of the GARIS/IGISOL K. Morita, T. Nomu
110 RIKEN Accel. Próg. Rep. 24 (1990)111-5-14. Test for Dispersive-Mode Beam Transportto the SMART
116 RIKEN Accel. Próg. Rep. 24 (1990)111-5-19. Responses of Large Position-Sensitive Detectorsto Hea
RIKEN Accel. Próg. Rep. 24 (1990)111-5-25. High Speed Serial Data Link for PC-9801 J. Fujita > PC
11 RIKEN Accel. Próg. Rep. 24 (1990)111-1-2. Three a Disintegration of 12C in the Field of208Pb Nucl
29 RIKEN Accel. Próg. Rep. 24 (1990)111-1-19. Dissociation Cross Sections of nLiK. Soutome, S. Yamaj
RIKEN Accel. Próg. Rep. 24 (1990)111-1-20. Induced Fission Studied with a Multi-DimensionalLangevin
48 RIKEN Accel. Próg. Rep. 24 (1990)111-2-15. High Resolution L X-Ray Angular Distribution Measureme
56 RIKEN Accel. Próg. Rep. 24 (1990)111-2-22. Electron Spectra from Doubly Excited Boroń lonsProduce
więcej podobnych podstron