628996302
P.-J. Ro\vr et al.
402 Cul turę Medium and DC Maturation
Countciflowcenlrilugation-purifled monocytcs
1D» I0« 1C» 10*
CO 14

'3r-
A!MV k. AIMV
mDC
(poły I:C) to inducc maturation. Yicld, ccii viability, maturation and thc functional capacitics of rhcsc mDC to acthratc I -ccII populatioas wcrc comparcd. Our icsults indicatc that only dcpcnding on thc culturc medium or thc protein adjuvant, DC havc distinct viabilitics, phcnotypcs and immunc capacitics. In our hands, optimal DC procluction in terms of maturation (phcnorypic and functional), and quality control rcquircmcnrs wcrc obtaincd with a protein-frcc culturc medium (i.c. RPMI-1640) supplcmcntcd with HSA that miLst be tested bcforc usc. Thus, thc present study highlights, for thc first timc, that thc composition of thc admrc medium and particularly their protein supplc-mentations arc as important as other thcrapcutic pnxlucts, such as cytokincs and maturation agents and thcrcforc must be taken into account, when establishing standard operating proccdurcs for thc generation of DC.
Materials and methods
Culturc media. The foliowing culmrc media wcrc tested: AIMV (Gibco BRL, Paislcy, UK), CTM-DC (Mabio, Toureoing, France), Ccłl-GRO DC' medium (CcllGcnix, Ficiburg, Germany), X-VIVO 15 (Biowhitaker, Walkcrsvillc, MD, USA) and RPMI (Life Technologies, Cergy Pontoisc, France). AU media wcrc supplcmcntcd with 1% pcnicillin/strcptomycin and 2 mM L-glutaminc (all purchascd from Life Technologies). FCS was purchascd from Eurobio (Les Ulis, France) and HSA from Laboratoirc Franęais dc Fractionncmcnt et de Biotcchnologics (Les Ulis, France). Autologous pląs ma (AP) and human serum (HS) wcrc produccd locally in our laboratory. FCS, HS and AP wcrc hcat inactivatcd for 30 min at 56 C.
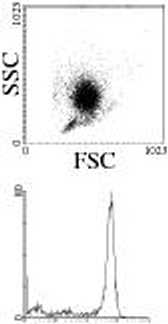
DC preparat ion. Immaturc Mo-DC wcrc generated from leukapheresis products of HIA-AX0201 hcalthy donors. Ihcsc products wcrc collectcd from donors at Etablissement Franęais du Sang. After obtaining infbrmcd conscnt, pcripheral blood mononuclcar cells wcrc isolated by Ficoll-Paquc density gradient centrifugation (Amersham, Uppsala, Sweden). Monocytcs wcrc then enriched by clu tria -tion (countcrflow ccntriftigation) asing a Bcckman Avanti J20 ccntriftigc cquippcd with a JE5.0 rotor and a 5-ml cłutriation chambcr. Cells wcrc ccntriftigcd at 602 g with an incieasing flow ratę (20, 25 and 35 ml/min, respcctively) to separate thc difterent celi populations. Threc 200-ml ffactions wcrc thus collectcd, thc last of which was enriched in monocytcs. Routincly, purity ofclutriatcd monocytcs was > 80%, as asscsscd by flow cytomctry bascd on thc dctcction of thc CDI4 marker. Monocytcs wcrc washed in culmrc medium (Fig. 1) bcforc they wcrc sccdcd in 6-we 11 platcs at 2x 106 cdLs/ml with 500 IU/ml GM-CSF and 200IU/ml IL-4 (AbCys SA, Paris, France). Cells wcrc then allowcd to differentiate for 6 days.
Phagocytosis of apoptońe tumour cells. Human mclanoma BEU cells (kindly providcd by Professor J.F. Dore, Lyon, France) wcrc labcllcd with PKH-67 membranę dyc
RPMI 1
X-VlVO-
► ilX'-
Cdj-GR(
crM-oci
DilTeicntiation. Maturation, GM-CSF IL-4 TNFct+poly I:C
Figurę 1 Deiulritic cells (1X3) generation. Monocytcs purification was analyscd by flow cyromctry, bascd on morpholcęical properties and CD 14 cxprcssion. Then. monocytcs wcrc culturcd with GM-CSF and II.-4 in different media for 1X3 generation. After 6 days. immaturc DC3 wcrc activated with TNF3 and poły I:C for 4S h.
(Sigma, St Qucntin Fallavicr, France) according to thc manufacturcr protocol. Apoptosis of tumour cells wras induccd by 1-min cxposurc to UV'B irradiatioas. After 48 h, celi dcath was asscsscd using FITC-labellcd annexin V and staining with propidium iodidc (BD Bioscicnccs, Le Pont dc C!aix, France). Apoptotic cells wrerc harvcsted and culturcd with iDC at 2:1 ratio. After 24 h, DC wrcrc lalx1llcd with PE-conjugatcd anti-CD86 monoclonal anti-body (Immunotcch, Y^illcpinte, France), and phagtKytosis wras asscsscd by flow cytomctry as thc pcrccntagc of dou-blc-positivc cells.
DC maturation. After 6 days, iDC wcrc harvcstcd and platcd in 24-wrdl platcs in fresh medium supplcnK1nted with GM-CSF (500 IU/ml) and IL-4 (200 IU/ml) at I06cdls/ml. Platcs wcrc coated with poly(2- hydroxycthy lnx- t.K ryl ate) (Sigma) to prcvcnt celi adhesi(xi. Mamration wras induccd by an additional 48-h treatment with 20 ng/ml TTMFot (AbCys SA) and 50pg/ml poły I:C (Sigma) as prcviously descrilxxl [13|.
Vlow cytometry. FFFC or PE-conjugatcd monoclonal antibtxlics directcd agaiast thc following antigens wrcrc uscd for phcnotypic analysis: CDI4, CD80, C’D83, CD86, HLA-ABC (Immunotcch) and HLA-DR (Caltag, Burlingamc, CA, USA). Isotypc-matchcd antilxxlics wrcrc uscd as Controls (Immunotcch). Lalx1lling wras carricd out at 4 C for 30min in RPMI with 10% FCS. Cells wrcrc wrashed twicc in PBS and analvscd on a FACScalibur cytomctrc asing thc Ceil Quest Pro software applicition. Fhc DC population was gated bascd on its forward-scatter and sidc-scattcr profile. Dcad ccILs wcrc staincd with 1 pM FO-PRO-3 iodidc (Molccular Probes, Montluęon, France) and cxcludcd from thc analysis. Rcsults arc cxprcsscd as median fluorcsccncc intensity (MI I).
Cytokine deteetion. Supernatants from 48-h DC culturcs wrcrc collectcd and stored at — 20 °C until they wrcrc ascd for cytokine quantiftcation. IL-12p70 and IL-10 produc-tions wcrc asscsscd by FLISA (BD Bioscicnccs) according
2006 Blackwell Puhlishing Ltd. Scandinaman Journal of Immunolog 63. 401-409
Wyszukiwarka
Podobne podstrony:
P.-J. Royer et al. 408 Cul turę Medium and DC Maturation uncicr GMP conditions, thc culnirc protocol
NF-kB and radioscnsi(ivity • C. DlDELOT et al. 1355 mediated transcriptional activation and provides
196 T. Nishidono et al. 196 T. Nishidono et al. nanow fingcrs base tn A O RO. 5 contact projection (
X Foreword surface runoff from smali to medium watersheds in Niger (Desconnets et al.) and in Senega
Image29 (X ro) + t/^- E[E(iro)+T] Et E(iro) + l]ft(x™D = O) Eó + (ro)Ed^ = (m)v
img024 Motor Control Rałionale Biblioaraphy Berg, K., et al., 1989 Measuring balance in the elderly:
img086 Chengjang Shimeid and Holland 2000Myllokunmingia fengjiaoa Shu et al., 1999 - &nb
Slajd19 (27) Klasyfikacja klastrów - wg Sterling T. et al. How to Build Beowulf T2-1 - Modele oblicz
1255Vol. 322 No. IK TKKATMKNT OK MAI.AOAPTATION TO NICII I* WOKK — O/.KISI.KR ET Al during ihis limc
» Vo1. 322 No. 18 TKEATMRNT OF MAI.ADAPTATION TO NICIIT WORK — CZF.1SLF.R ET Al.. 1257 Comroi r«ne a
288 Z. KORUBA ET AL. Fig. 4. (a) Time-dependent profile of the terrain unevenness (bump); (b) time-d
więcej podobnych podstron