
. 179 .
Chinese Journal of Traumatology 2008; 11(3):179-185
Implanting hydroxyapatite-coated porous titanium with
bone morphogenetic protein-2 and hyaluronic acid into
distal femoral metaphysis of rabbits
PENG Lei彭磊, BIAN Wei-guo边卫国 *, LIANG Fang-hui梁芳慧 and XU Hua-zi徐华梓
Department of Orthopedic Surgery, Second Affiliated
Hospital & Yuying Children
’s Hospital, Wenzhou Medical
College, W enzhou 325000, China (Peng L, Bian W G and
Xu HZ)
Northwest Institute for Non-ferrous Metal Research,
Xi
’an 710016, China (Liang FH)
*Corresponding author: Tel: 86-577-88879008, E-mail:
Objective: To assess the osseointegration capability
of hydroxyapatite-coated porous titanium with bone mor-
phogenetic protein-2 (BMP-2) and hyaluronic acid to repair
defects in the distal femur metaphysis in rabbits.
Methods: Porous titanium implants were made by sin-
tering titanium powder at high temperature, which were
coated with hydroxyapatite by alkali and heat treatment and
with BMP-2 combined with bone regeneration materials. And
hyaluronic acid was further used as delivery system to pro-
long the effect of BMP-2. The implants were inserted into
the metaphysis of the distal femur of rabbits. The animals
were killed at 6, 12 and 24 weeks to accomplish histological
and biomechanical analyses.
Results: According to the result of histological analysis,
the osseointegration in BMP-2 group was better than that
of the HA-coated porous titanium group. In push-out test,
all the samples had bigger shear stress as time passed by.
There was statistical difference between the two groups in
6 and 12 weeks but not in 24 weeks.
Conclusion: Hydroxyapatite-coated porous titanium
with BMP-2 and hyaluronic acid has a good effect in repair-
ing defects of distal fumur in rabbits, which is a fine bio-
technology for future clinical application.
Key words: Porous titanium; Bone morphogenetic
protein-2; Hydroxyapatite; Osteointegration; Hyaluronic
acid
Chin J Traumatol 2008; 11(3):179-185
S
ince Urist
1
discovered bone morphogenetic
protein (BMP), studies have kept focusing on
many kinds of bone growth factors and the
delivery systems. A multitude of studies have proved
that transforming growth factor-
β
(TGF-
β
), insulin-like
growth factor-I (IGF-I), BMP-3, and BMP-7 could en-
hance bone formation.
2-5
But as we all know, BMP-2 is
the most powerful bone growth factor. It can induce
undifferentiated mesenchymal cells to propagate and
differentiate, so much more attention is paid to it.
6,7
Meanwhile, some biomaterials, such as hydroxyapatite,
coral, cancellated bone and collagen, can combine with
bone growth factors to enhance bone formation because
of dual effects of bone conduction and bone induction.
8-11
At present, titanium alloy implants with porous coat-
ing can be produced by many technologies. This makes
it possible that new bone can grow into the pores to
improve the strength of synosteosis by mechanical lock
between the interface of bones and implants
12
and to
prolong the action time of bone growth factors. Some
researchers have discovered that hyaluronic acid has
good biocompatibility, can fill in porous structure, en-
hance cell adhesion and won
’t disturb the regional
osteanagenesis. So it is a good retarder to guarantee
the long-time effective concentration of BMP-2.
13
In this
research, we made some composite implants, which
had three-dimensional structure, bone conduction and
bone induction abilities by using hydroxyapatite-coated
porous titanium combined with BMP-2, with hyaluronic
acid as the retarder. The implants were inserted into
the distal femoral metaphysis of rabbits to accomplish
histological and biomechanical analyses.
METHODS
Implants
The size of the porous titanium implants (Ti-6Al-
4V, Northwest Institute for Non-ferrous Metal Research,
Xi
’an, China) was 4 mm×10 mm. The samples were
cleaned ultrasonically in acetone, ethanol and distilled
water for 20 minutes, respectively, and then immersed
in 5.0 mol/L NaOH aqueous solution at 60
°C for 24
. 180 .
Chinese Journal of Traumatology 2008; 11(3):179-185
hours. After washed by distilled water and dried in air
for 24 hours, the samples were heated in an electrical
furnace at heating rate of 5
°C/min and kept at 600°C for
1 hour and cooled with the furnace. Subjected to alkali-
heat, the samples were put in 0.5 mol/L Na
2
HPO
4
for
24 hours and then in saturated Ca(OH)
2
for 5 hours.
Then the samples were washed by distilled water and
soaked in 30 ml simulated body fluid (SBF) at 36.5
°C
to get hydroxyapatite coat. SBF was refreshed every
48 hours and prepared with ion concentrations nearly
equal to those of the human blood plasma, and buffered at
pH=7.40 with Tris-HCl at room temperature. The sam-
ples were taken out at 28 days and washed with dis-
tilled water and dried in air. Titanium samples were
cleaned ultrasonically in acetone, ethanol and distilled
water for 20 minutes, respectively. Then the implants
were put in the tootings, respectively. BMP-2 (provided
by East China Gene Research Institute, Hangzhou,
China) and hyaluronic acid were stirred evenly at the
ratio of 8 mg/g under aseptic conditions and dissolved
in buffered saline solution. Soak the samples in and
pump to vacuum under negative pressure. Take them
out after shaping. Finally hydroxyapatite-coated porous
titanium carrying about 12
µg BMP-2 was obtained.
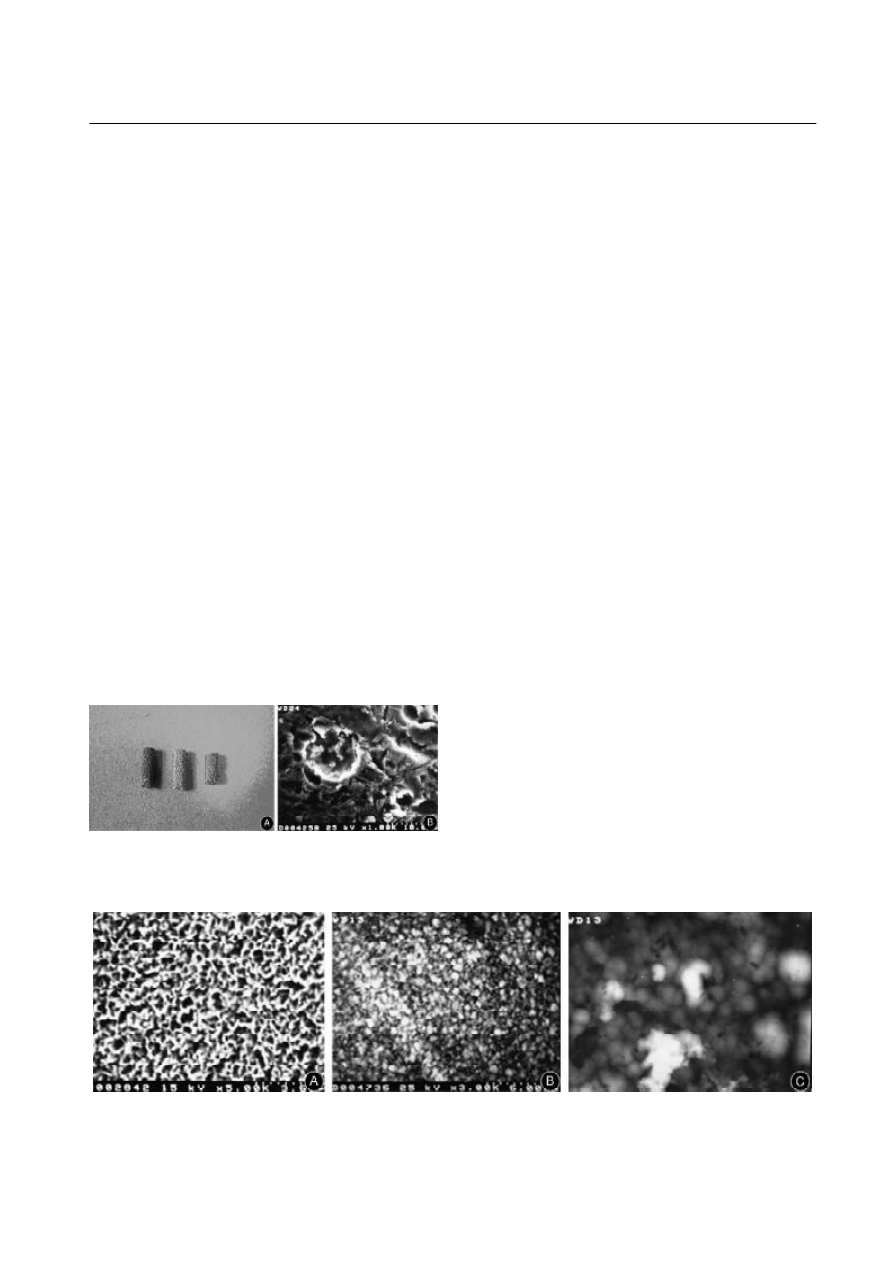
The photos and the size of the titanium implants are
shown in Fig.1.
Surface analysis of implants
Naturally-disrupted samples were made with the im-
plants obtained before. The disrupted surface was
etched with 20 ml/L methanoic acid mixed with acid
hydroc and 100 ml/L chlorinated soda, respectively. It
was dehydrated and dried and vacuum coating was
obtained by spraying. Then it was observed under a
scanning electron microscope (SEM, JSM
35C, Hitachi,
Japan) under different enlargements (Fig.2).
Animals and surgery
All the experiments on animals were performed un-
der the approval of the institutional review board. The
implants were inserted into the bilateral distal femora of
36 adult New Zealand rabbits ( body weight > 3.5 kg).
Under anesthesia with Ketanest-Rompun, an incision
was made proximally in the lateral femur condyle and
the area of 1 cm in diameter at the lateral femur condyle
was exposed. Just above the knee joint, an implant lo-
cation of 4 mm
×10 mm for porous titianium sample was
made by a bone drill. The longitudinal axes of the chan-
nels were perpendicular to the femoropatellar joint from
the medial to the lateral femur. The porous hydroxyapa-
tite-coated implant was inserted press-fit into the meta-
physis of the distal femora. Each animal received one
implant carrying about 12
µg BMP-2 (Group I). A hy-
droxyapatite-coated porous implant was placed as a
control in the contralateral femur in the same manner
(Group II). Wounds were closed in layers. The animals
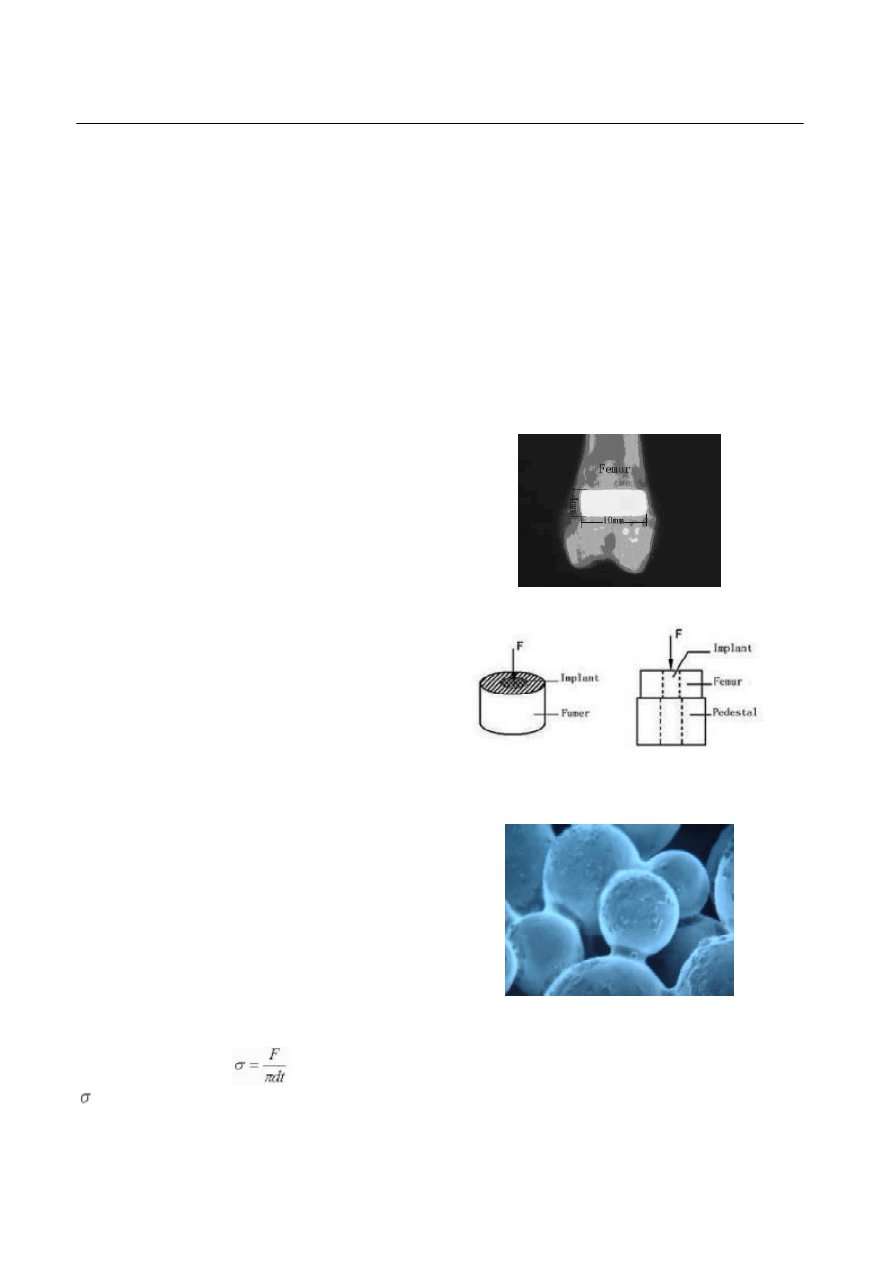
were bred in the same condition as before operation. X-
ray image of the sample is shown in Fig.3. Before
sacrificed, three animals were injected subcutaneously
with a fluorescent tetracyclin (25 mg/kg body weight,
Merck, Darmstadt, Germany) 5 days before in order to
label the process of bone formation.
Fig.2.A: Image of porous titanium subjected to alkali-heat: there is some tiny grid shaped formation on the surface under a scanning
electric microscope (
×5). B: Image of the sample subjected to calcification: the grid is covered by massive substance under a scanning
electric microscope (
×1000). C: Image of the sample in SBF for 4 days: the surface is coated with some compact substance under a
scanning electron microscope (
×5000).
Fig.1. A: Photos of titanium implants. B: The porosity of porous
layer of the implant is approximately 40% and the maximal pore
size is about 250
µm.
. 181 .
Chinese Journal of Traumatology 2008; 11(3):179-185
Implant collection and evaluation
Euthanasia was performed on the animals by using
carbon dioxide after 6, 12, and 24 weeks of operation,
respectively. Following euthanasia, the implants with
their surrounding tissues were collected and prepared
for histological (n = 6 for each material and each time
period) and mechanical (n = 6 for each material and
each time period) analyses.
Histological observation
The femur samples were fixed in 4% phosphate
buffered formaldehyde solution (pH = 7.4), dehydrated
in a graded series of ethanol, and embedded in
methylmethacrylate. The specimens were etched by
hydrochloric ethanol for 15 seconds, stained with me-
thylene blue for 1 minute, and stained with basic fuch-
sin for 30 seconds. Following polymerization, 10-
µm
thick sections were prepared from each implant using
a modified sawing microtome technique. The prepared
sections were examined under a light microscope. In
addition to the thin sections described above, two addi-
tional 30-
µm thick sections were prepared from each
sample of the three animals who received fluorochromes.
These sections were not stained but evaluated with a
fluorescence microscope equipped with an excitation
filter of 470-490 nm. The light and fluorescent micro-
scopic assessment consisted of a complete morpho-
logical description of the tissue response to the differ-
ent implants. In addition, quantitative information was
obtained on the amount of bone growing into the vari-
ous mesh implants.
Mechanical testing
Soft tissues covering the bones were removed from
freshly-excised specimens by using a scalpel blade.
The specimens containing implants were scoured to a
flat surface, perpendicular to the long axis of the implant.
The specimens were stored in physiological saline so-
lution until mechanical evaluation, which was performed
within 2 hours after retrieval. The pull-out tests were
conducted on a material testing machine (Instron 1185,
Siemens, China) linked to a computer at the speed of 1
mm/min to test the shear stress (Fig.4). The interface
shear strength was calculated by the following formula:
RESULTS
Scanning electron micrograph
The particles of hyaluronic acid with BMP-2 were
uniformly mixed and distributed over hydroxyapatite-
coated porous titanium irregularly. Part of the particles
uniformly distributed in the porous titanium as globule-
shaped or needle-shaped could combine with the newly-
formed mixture in a multi-punciform or multi-extent
manner. The surface of the particle was exposed ex-
cept for the contacted area and there were many ir-
regular fissures for 100-200
µm interconnected to each
other among them (Fig.5).
Histological observation
After 6 weeks
’ implantation, there were no signs of
inflammation and few newly-formed bones filled in the
interface gap of purely hydroxyapatite-coated implants.
The interface of the soft tissues was obviously observed.
But for BMP-2-coated implants, more newly-formed
Fig.3 X-ray image of the sample.
Fig.5. BMP-2 and hyaluronic acid on hydroxyapatite-coated po-
rous titanium (
×50).
Fig.4. Illustration of mechanical testing. The specimens are put on
a cylinder pedestal with pore diameter of 42 mm and a metal perch
is used to push the specimens gradually.
= shear strength; F = max load when collapse; d=
diameter of the specimen; t =height of the specimen.
. 182 .
Chinese Journal of Traumatology 2008; 11(3):179-185
bones could be observed in the interface gap than the
control group. Bones grew into the cylinder pore and
contacted with titanium sphere in some extent. Bones
had also formed along the outer surface of the cylinder.
After 12 weeks
’ implantation, more newly-formed bones
and obvious reconstruction could be observed in purely
hydroxyapatite-coated implants. And more newly-formed
and completely-calcified bones could be obtained in
BMP-2-coat group. Bones filled in almost all the gaps
and even the junction of the titanium sphere. After 24
weeks
’ implantation, newly-formed bones filled in al-
most all the gaps in both groups and there was no dif-
ference between the two groups.
The growth of new bones could be clearly differenti-
ated and recognized by observation of fluorescence
microscope. The newly-formed bones were deposited
initially on the surface and then grew into the pores.
Organized bone formation with clear ossification was
not observed in any of the implants. In all specimens,
diffusely-stained deposits of tetracycline (and some-
times calcein) were mainly localized in contact with
the Ti fibers (Fig.6).
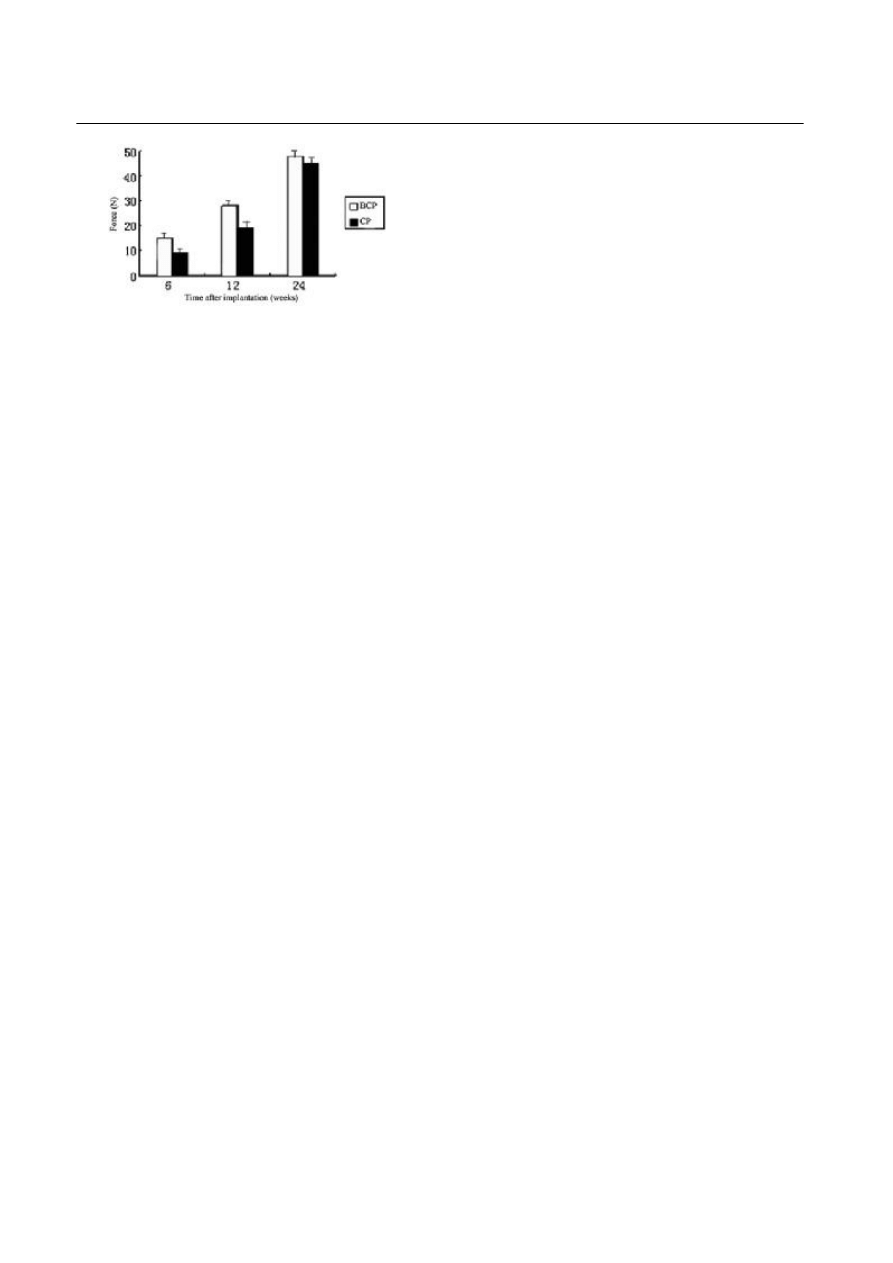
Mechanical testing
The results of pull-out test of purely hydroxyapatite-
coated implants and BMP-2 groups are listed in Table 1
and Fig. 7. There were 36 implants being prepared for
mechanical tests, but 5 were excluded because of un-
expected death or technical error during preparation or
pullout (n=5). There was a significant increase in shear
strength over time for all coatings. Shear strength val-
ues for the BMP-2-coated implants were higher than
the hydroxyapatite-coated implants in 6 and 12 weeks
(P<0.05). But in 24 weeks there was no significant sta-
tistical difference between the two groups.
Name of samples 6 weeks (MPa) 12 weeks (MPa) 24 weeks (MPa)
Table 1. Results of pull-out test
BCP
C P
15.1248
±0.89451 (
n=6)
10.2415
±0.4548 (
n=6)
27.2562
±2.025421 (
n=5)
21.3481
±2.25455 (
n=5)
48.1587
±3.15425 (
n=5)
43.2698
±3.24782 (
n=4)
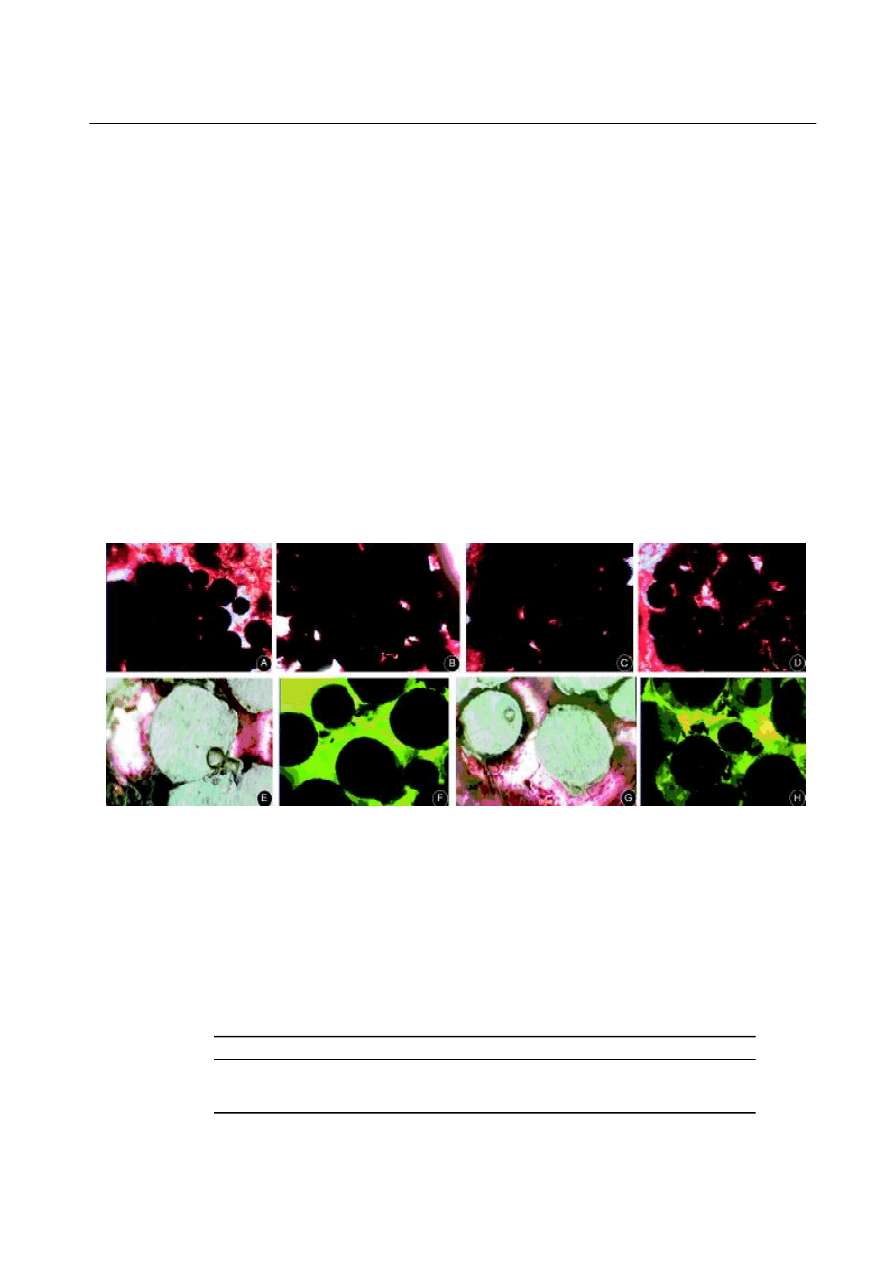
Fig. 6. A: After 6 weeks,
few newly-formed bones could be observed on the edge of purely
hydroxyapatite
-coated implants and there
was still some space around and in the implants.
B: After 6 weeks, the red substance filled most of the space around titanium sphere in
BMP-2 group. On the interface of the implants, tight binding could be observed. C: After 12 weeks,
more newly-formed bones could be
observed on the edge of purely
hydroxyapatite
-coated implants and there was still some space in the implants.
D: After 12 weeks, the red
substance filled most of the space in titanium sphere in BMP-2 group. More newly-formed bones could be observed in the implants. E:
After 24 weeks, well-calcificated red substance and compact filled in all the space around the titanium sphere even the corner area
of
purely
hydroxyapatite
-coated implants
. F: Fluorescence almost filled all the pores of the implants in
purely
hydroxyapatite
-coated
implants
. Only few blank area had been left. G: After 24 weeks, considerable setuliform or lamellar bone trabeculae could be observed
in BMP-2 group. H: Fluorescence almost filled all the pores of the implants in BMP-2
implants
group, few stained-deposits of tetracycline
could be observed.
BCP: BMP
2
hydroxyapatite
-coated porous titanium; CP:
hydroxyapatite
-coated porous titanium.
. 183 .
Chinese Journal of Traumatology 2008; 11(3):179-185
DISCUSSION
At present, many new researches about bony tis-
sues and engineering focus on precursor cells of bone
formation, implants and bone growth factors. Studies
show that marrow stroma cells (MSCs) have the ability
of multi-directional differentiation and bone formation,
but the results of implantation are not the only things
as expected. Some studies show that combined MSCs
with hydroxyapatite or BMP-2 can improve the ability
of bone formation greatly. It proved that good implanta-
tion with growth factors and MSCs could get the best
effect of bone formation
2-11
because it could gain better
interface of impaction and sooner healing. The pattern
of binding between bones and implants is the major
criterion to judge the biocompatibility and bioactivity.
There might be some fibrae encysts of different thick-
ness forming around the implants with negative biocom-
patibility and being thickened as time passes by.
Eventually, fluidify, inflammation or necrosis occurs
between the implants and the encysts can cause loos-
ening or displacement leading to abortive implantation.
Lamellate fibrous tissues soon form on the interface
between implants and bones after insertion of titanium.
It becomes thinning by time-lapse and does not lead to
some adverse effects such as inflammation. Ideal synos-
teosis is direct osseointegration on the interface of im-
plants and bones. After implantation, environmental
newly-formed osteoblasts lead to obv ious bone
formation, so compact and direct osseointegration is
getting sooner and better immediate union.
14,15
All these
require implants to have better bone tissue biocompat-
ibility and bioactivity including bone conductivity and
osteoinduction.
16
After inserting the cylinders of porous titanium treat-
ed by alkali-heat for 6 weeks, newly-formed bones were
observed on the interface and direct osseointegration
was obtained to speed up healing of porous titanium,
which means that hydroxyapatite coating can lead to
bone formation satisfactorily because of its good
biocompatibility.
The bone growth after implantation of porous tita-
nium is due to its surface structure, porous framework,
survival ability of bony bed of the host, coating with
bioactivity and reconstruction of environmental bones.
The rough surface of porous titanium can provide good
interface for bone growth.
17,18
Studies showed that the
tiniest diameter of pore which required bone growth and
good blood supply was 100
µm. Experimental result of
cell culture in vitro showed that hydroxyapatite coating
could help osteoblasts to adhere on the surface of
implants, to proliferate and diffuse. Meanwhile, Liang
et al proved that bone formation of implants with hy-
droxyapatite coating was faster than those without hy-
droxyapatite coating whether on the surface or in the
pores in vivo. Copious athletic osteoblasts around the
coating form new woven bones, which makes it easier
to grow into the pores, to maturate and reconstruct.
Hydroxyapatite coating can also eliminate the shield of
stress caused by porous structure, which allows new
bones to form along the surface of porous structure
into inner parts even the impaction of titanium globule,
so more direct conjunctive interfaces are obtained. All
these can speed up the healing of porous titanium and
bone tissues and profit stabilization in the early period.
It does benefit a lot to the recovery of bone defect, the
fusion of vertebra and arthro-replacement.
There are three essential pacing factors for mesen-
chymal cells to induce bone formation: inducing factor,
target cells and eligible environment. BMP-2 as induc-
ing factor, which causes the differentiation of mesen-
chymal cells, can stimulate bone formation. The target
cells of BMP-2 include undifferentiated mesenchymal
cells existing in muscles and connective tissues around
blood vessels, MSCs including determined osteogenic
precursor cells (DOPCs) and inducible osteogenic pre-
cursor cells (IOPCs) and connective tissue cells in
periosteum. MSCs are more sensitive to BMP-2 than
others.
17
Studies showed that imbed MSCs from au-
tologous bone marrow in bone defect could enhance
the inducing ability of BMP-2 greatly.
19,20
It is not the
truth that induction happens everywhere BMP-2 imbeds,
but the induction of BMP-2 requires fav orable
environment, the strongest in bone marrow, muscles
Fig.7. Results of pull-out test. BCP: BMP
2
hydroxyapatite-coated
porous titanium; CP: hydroxyapatite-coated porous titanium.

. 184 .
Chinese Journal of Traumatology 2008; 11(3):179-185
and brain tissues, and the weakest in spleen, liver and
kidney, etc. This research showed that hydroxyapatite
coating could enhance the combination of bones and
implants, but the effect was not as satisfactory as that
in 6 weeks after BMP-2 imbedding. More bone forma-
tion and osseointegration were observed than purely
hydroxyapatite-coated implants especially the inner part
of porous titanium to urge speeding up of healing. By
all these means BMP-2 has a good effect of induction
and can further enhance the effect of hydroxyapatite
coating.
There are four kinds of breaking patterns between
bones and implants: implants and tissues, woven bones
and mature bones, fracture faces in the mature bones
and in the coating, which passing through the coating
but not through the bone surface. The rough surface of
porous titanium can provide more interfaces for apposi-
tive growth. Porous structure can complicate the inter-
face of bones and titanium and diffuse the strength on
the surface including shear strength, pressure and ten-
sile force, etc. The internal inlaying locking can obtain
more conjunctive strength than some coatings on the
smooth surface.
21
And if hydroxyapatite coating was
added to the porous titanium, bigger conjunctive strength
can be obtained.
22
In this research, direct osseointe-
gration formed between bones and implants. The me-
chanical inlaying impaction was caused by newly-
formed bones in the pores and the gradient structure
between coating and implants enhanced conjunctive
strength. According to the results of pull-out tests, po-
rous structure and hydroxyapatite coating could pro-
mote conjunctive strength in the intermediate and long-
term stage, but the induction of BMP-2 in the earlier
period after implantation could not be ignored. Newly-
formed bones could grow into the pores and formed
mechanical inlaying impaction faster. The results might
be influenced by the position of pedestal (the distance
of inner edge and interface of bone and implants) in this
research. Besides, the size of titanium powders, sur-
face roughness, the way making coating and its thick-
ness might influence the results, too.
REFERENCES
1. Urist MR. Bone: formation by autoinduction. Science
1965;150 (698):893-899.
2. Kandziora F, Scholz M, Pflugmacher R, et al. Experimen-
tal fusion of the sheep cervical spine. Part II: Effect of growth
factors and carrier systems on interbody fusion. Chirurg 2002;73
(10):1025-1038.
3. Hiller T, Pflugmacher R, Mittlmeier T, et al. Bone mor-
phogenetic protein-2 application by a poly (D,L-lactide)-coated
interbody cage: in vivo results of a new carrier for growth factors.
J Neurosurg 2002;97(1 Suppl):40-48.
4. Lind M, Overgaard S, Jensen TB, et al. Effect of osteo-
genic protein 1/collagen composite combined with impacted al-
lograft around hydroxyapatite-coated titanium alloy implants is
moderate. J Biomed Mater Res 2001;55(1):89 -95.
5. Esenwein SA, Esenwein S, Herr G, et al. Osteogenetic
activity of BMP-3-coated titanium specimens of different surface
texture at the orthotopic implant bed of giant rabbits. Chirurg
2001;72(11):1360-1368.
6. Yan MN, Tang TT, Zhu ZA, et al. Effects of bone mor-
phogenetic protein-2 gene therapy on the bone-implant interface:
an experimental study with dogs. Chin J Med 2005;85:1521-1525.
7. Sachse A, Wagner A, Keller M, et al. Osteointegration of
hydroxyapatite-titanium implants coated with nonglycosylated
recombinant human bone morphogenetic protein-2 (BMP-2) in
aged sheep. J Bone 2005;37(5):699-710.
8. Kusakabe H, Sakamaki T, Nihei K,et al. Osseointegration
of a hydroxyapatite-coated multilayered mesh stem. Biomaterials
2004;25(15):2957-2969.
9. Kim HW, Lee EJ, Jun IK, et al. On the feasibility of phos-
phate glass and hydroxyapatite engineered coating on titanium. J
Biomed Mater Res A 2005;75(3):656-667.
10. Li LH, Kim HW, Lee SH, et al. Biocompatibility of tita-
nium implants modified by microarc oxidation and hydroxyapa-
tite coating. J Biomed Mater Res A 2005;73(1):48-54.
11. Coathup MJ, Blackburn J, Goodship AE, et al. Role of
hydroxyapatite coating in resisting wear particle migration and
osteolysis around acetabular components. Biomaterials 2005;26
(19):4161-4169.
12. Takemoto M, Fujibayashi S, Neo M, et al. Mechanical
properties and osteoconductivity of porous bioactive titanium.
Biomaterials 2005;26(30):6014-6023.
13. Aebli N, Stich H, Schawalder P, et al. Effects of bone
morphogenetic protein-2 and hyaluronic acid on the osseointegra-
tion of hydroxyapatite-coated implants: an experimental study in
sheep. J Biomed Mater Res A 2005;73(3):295-302.
14. Dorr LD, Wan Z, Song M, et al. Bilateral total hip arthro-
plasty comparing hydroxyapatite-coating to porous-coated
fixation. J Arthroplasty1998;13(7):729-736.
15. Tanzer M, Kantor S, Rosenthall L, et al. Femoral remodel-
ing after porous-coated total hip arthroplasty with and without
hydroxyapatite-tricalcium phosphate coating: a prospective ran-
domized trial. J Arthroplasty 2001;16(5):552-558.
16. Hench LL. Bioactive ceramics: theory and clin ical

. 185 .
Chinese Journal of Traumatology 2008; 11(3):179-185
applications. In: Andersson ÖH, Happonen RP, and Yli-Urpo A,
eds. Oxford: Pergamon.1994:3-14.
17. Popa C, Simon V, Vida-Simiti I, et al. Titanium--hydroxya-
patite porous structures for endosseous applications. J Mater Sci
Mater Med 2005;16(12):1165-1171.
18. Simon M, Lagneau C, Moreno J, et al. Corrosion resis-
tance and biocompatibility of a new porous surface for titanium
implants. Eur J Oral Sci 2005;113(6):537-545.
19. Knabe C, Howlett CR, Klar F, et al. The effect of different
titanium and hydroxyapatite-coated dental implant surfaces on
phenotypic expression of human bone-derived cells. J Biomed
Mater Res A 2004;71(1):98-107.
20. Minamide A, Yoshida M, Kawakami M, et al. The use of
cultured bone marrow cells in type I collagen gel and porous hy-
droxyapatite for posterolateral
lumbar spine fusion. Spine 2005;
30(10):1134-1138.
21. Fujisawa A. Investigation for bone fixation effect of thin
HA coated layer on Ti implants. Kokubyo Gakkai Zasshi 2005;72
(4):247-253.
22. Kold S, Rahbek O, Zippor B, et al. No adverse effects of
bone compaction on implant fixation after resorption of com-
pacted bone in dogs. Acta Orthop 2005; 76(6):912-919.
(Received November 26, 2007)
Edited by LIU Yang-e
Wyszukiwarka
Podobne podstrony:
9 bmp vga id 613453 Nieznany (2)
bmp 47860af740d9a[1] id 90729 Nieznany (2)
bmp 493f6ace6f6db id 90730 Nieznany
Abolicja podatkowa id 50334 Nieznany (2)
4 LIDER MENEDZER id 37733 Nieznany (2)
katechezy MB id 233498 Nieznany
metro sciaga id 296943 Nieznany
perf id 354744 Nieznany
interbase id 92028 Nieznany
Mbaku id 289860 Nieznany
Probiotyki antybiotyki id 66316 Nieznany
miedziowanie cz 2 id 113259 Nieznany
LTC1729 id 273494 Nieznany
D11B7AOver0400 id 130434 Nieznany
analiza ryzyka bio id 61320 Nieznany
pedagogika ogolna id 353595 Nieznany
Misc3 id 302777 Nieznany
więcej podobnych podstron