
Regulation of Milk Protein Gene Expression in Normal
Mammary Epithelial Cells by Tumor Necrosis Factor*
WENDY K. SHEA-EATON†, PING-PING HWANG LEE,
AND
MARGOT M. IP
Grace Cancer Drug Center, Roswell Park Cancer Institute, Buffalo, New York 14263
ABSTRACT
Tumor necrosis factor-
␣ (TNF) is a physiologically significant reg-
ulator of mammary gland development, stimulating growth and
branching morphogenesis of mammary epithelial cells (MEC) and
modulating functional differentiation. The present studies were per-
formed to determine the mechanism by which TNF modulated func-
tional differentiation. In rat MEC in primary culture, TNF inhibited
accumulation of whey acidic protein and
-casein messenger RNAs in
a time- and concentration-dependent manner. In contrast, levels of
transferrin messenger RNA, the product of another milk protein gene,
were not inhibited by TNF, suggesting selectivity. Using a nuclear
run-on assay in the immortalized HC11 mammary epithelial cell line
and the transcriptional inhibitor actinomycin D in MEC in primary
culture, the effects of TNF were shown to be mediated by both a
decrease in transcription and a decrease in the stability of the whey
acidic protein and
-casein transcripts. Additionally, TNF stimulated
the binding of nuclear factor-
B to a consensus B-oligonucleotide,
increased the stability of matrix metalloproteinase-9 (MMP-9) tran-
scripts, and increased MMP-9 activity. Together, these data suggest
that TNF may exert its effects on milk protein gene expression either
directly via nuclear factor-
B modulation of transcription, or indi-
rectly via MMP-9-induced remodeling of the architectural or hor-
monal environment surrounding the MEC. (Endocrinology 142:
2558 –2568, 2001)
A
LTHOUGH BEST KNOWN for its role in the immune
system, tumor necrosis factor-
␣ (TNF) also plays a
critical role in endocrine tissue, including the ovary, uterus,
and placenta (1, 2). In addition, our laboratory has demon-
strated that TNF plays a key role in the mammary gland
(3–7). Specifically, TNF was shown to stimulate growth as
well as induce extensive branching and alveolar morpho-
genesis of isolated rat mammary epithelial cells (MEC) in
primary culture and under optimal medium conditions to
inhibit the accumulation of casein proteins (3–5). Under sub-
optimal conditions, however, namely in the absence of epi-
dermal growth factor (EGF), the effects of TNF on casein
were biphasic, with low levels stimulating casein accumu-
lation in parallel with a stimulatory effect on morphogenesis,
and higher levels inhibiting casein protein levels. Further-
more, using agonistic antibodies specific to each of the re-
ceptors, we found that the p55 TNF receptor (TNFR) medi-
ates the stimulatory effect of TNF on proliferation as well as
the inhibitory effect on casein accumulation. In contrast, the
p75 TNFR mediates an increase in casein accumulation (4).
Recent studies suggest that these effects of TNF on MEC
are physiologically relevant. First, we found that secondary
and tertiary branching of the mammary epithelium was in-
hibited in TNF null mice during puberty (7). Second, MEC
were shown to express TNF as well as its two receptors, p55
and p75 TNFR, in a developmentally regulated manner (4).
TNF messenger RNA (mRNA) increased markedly during
pregnancy, then gradually decreased throughout lactation
and involution; concomitant with this, the 26-kDa membrane
form of TNF protein was first detected during pregnancy and
was significantly elevated during lactation, but was not de-
tected in pubescent rats or during involution. In contrast, p55
TNFR mRNA was elevated during pregnancy and early lac-
tation, declining thereafter, and p75 TNFR mRNA increased
during lactation and remained elevated through early invo-
lution. Taken together, these studies support the hypothesis
that TNF, acting through the p55 TNFR, may play an im-
portant role in stimulating growth and morphogenesis dur-
ing pregnancy; moreover, together with progesterone, TNF
may inhibit the expression and secretion of milk proteins at
this time. During lactation, however, the increased levels of
p75 TNFR together with a significantly increased expression
of the 26-kDa membrane form of TNF, which is thought to
act selectively through the p75 TNFR (8), may stimulate
functional differentiation, thus permitting the extensive syn-
thesis and secretion of milk proteins seen at this develop-
mental stage.
The regulation of milk proteins, the functional differenti-
ation products of the mammary gland, has been studied by
a number of investigators. In general, these studies have
shown that several hormones act in concert to exert regula-
tion at both transcriptional and posttranscriptional levels (9,
10). In vitro, PRL and a glucocorticoid, together with insulin
are required for optimal transcription of the
-casein and
whey acidic protein (WAP) genes (10, 11), whereas proges-
terone is inhibitory (12–15). No information is available on
the mechanism by which TNF inhibits casein expression.
Thus, the first objective of the work reported here was to
determine whether the effect of TNF on casein accumulation
was exerted at the RNA level. Once this was established, our
second objective was to determine the mechanism by which
steady state mRNA levels were inhibited by TNF, with focus
on both mRNA stability as well as changes in transcription.
Received October 30, 2000.
Address all correspondence and requests for reprints to: Dr. Margot Ip,
Grace Cancer Drug Center, Roswell Park Cancer Institute, Elm and Carlton
Streets, Buffalo, New York 14263. E-mail: margot.ip@roswellpark.org.
* This work was supported by NIH Grant CA-77656 and the shared
resources of NIH Core Grant CA-16056.
† Current address: Department of Obstetrics and Gynecology, Uni-
versity of South Florida, Tampa, Florida 33606.
0013-7227/01/$03.00/0
Vol. 142, No. 6
Endocrinology
Printed in U.S.A.
Copyright © 2001 by The Endocrine Society
2558

As part of these investigations, we also determined whether
casein was the only milk protein altered by TNF, as changes
in at least one other protein would provide further support
for a physiological role of TNF in the mammary gland. Fi-
nally, having determined that TNF inhibited the expression
of both
-casein and WAP by altering transcription as well
as mRNA stability, the final objective was to carry out pre-
liminary studies to provide leads as to how TNF might exert
these effects. These latter studies focused on matrix metal-
loproteinase-9 (MMP-9), which we have recently found to
play an important role in TNF-induced branching morpho-
genesis of MEC (6).
Materials and Methods
Materials
[
␣-
32
P]Deoxy-CTP, [
␥-
32
P]deoxy-ATP, and [
␣-
32
P]UTP were pur-
chased from NEN Life Science Products (Boston, MA). Insulin, proges-
terone, hydrocortisone, transferrin, ascorbic acid, fatty acid-free BSA,
phenylmethylsulfonylfluoride, actinomycin D, and phenol red-free
DMEM/Ham’s F-12 (1:1) tissue culture medium containing 15 mm
HEPES were products of Sigma (St. Louis, MO). RPMI 1640, gentamicin,
and TRIzol were purchased from Life Technologies, Inc. (Grand Island,
NY). Collagenase class III was obtained from Worthington Biochemical
Corp. (Freehold, NJ). Grade II dispase, leupeptin, and the RNA labeling
kit (SP6) were obtained from Roche Molecular Biochemicals (Indianap-
olis, IN). FBS and normal calf serum (NCS) were purchased from Hy-
Clone Laboratories, Inc. (Logan, UT). The Multiprime DNA Labeling Kit,
Hybond N nylon membrane, and the enhanced chemiluminescence
Western blotting detection reagents were products of Amersham Phar-
macia Biotech (Arlington Heights, IL). Mouse EGF and liquid dispase (50
caseinolytic units/ml) were products of Collaborative Research (Bed-
ford, MA). Ovine PRL (NIDDK oPRL-19) was a gift from Dr. A. Parlow
at the National Hormone and Pituitary Program, NIDDK. Donkey an-
tirabbit peroxidase-conjugated IgG was a product of Jackson Immu-
noResearch Laboratories, Inc. (West Grove, PA). The complementary
DNA (cDNA) probe for recombinant human glyceraldehyde-3-phos-
phate dehydrogenase (GAPDH) was purchased from CLONTECH Lab-
oratories, Inc. (Palo Alto, CA). The cDNA probes for rat WAP and rat
-casein were gifts from Dr. J. Rosen (Baylor University, Houston, TX),
the cDNA probe for rat 92-kDa gelatinase B (MMP-9) was a gift from Dr.
L. Matrisian (Vanderbilt University, Nashville, TN), and the riboprobe
for rat transferrin was a gift from Drs. M. Griswold and S. Sylvester
(Washington State University, Pullman, WA). The 220-bp mouse
-ca-
sein probe was generated by PCR using forward (positions 5921–5944)
and reverse (6996 –7017) primers from the mouse
-casein gene. Re-
combinant human TNF
␣ (2.5 ⫻ 10
6
U/mg), a gift from Asahi Chemical
Industry Co. (Fuji, Shizuoka, Japan), was used in all studies involving
primary MEC. Recombinant mouse TNF
␣ (1 ⫻ 10
7
U/mg), purchased
from Biosource International (Camarillo, CA), was used for experiments
with the mouse mammary HC11 cell line. The nuclear factor-
B (NFB)
antibodies used in the supershift study, p65 (sc-109X), p50 (sc-114X), p52
(sc-298X), and c-Rel (sc-070X), were obtained from Santa Cruz Biotech-
nology, Inc. (Santa Cruz, CA).
Animals
Virgin, 50- to 55-day-old female Sprague Dawley CD rats (Crl:CD BR),
purchased from Charles River Laboratories, Inc. (Wilmington, MA) were
used as the source of mammary glands in the time- and dose-curve
experiments, whereas Sprague Dawley [Tac:N(SD)fBR] rats from Tac-
onic Farms, Inc. (Germantown, NY), were used for all other experiments
reported herein. Female CD2F
1
mice purchased from NCI-Frederick
Cancer Research Facility, Biological Testing Branch (Frederick, MD)
were used to carry the Engelbreth-Holm-Swarm sarcoma. Animals were
fed chow diets (Teklad, Madison, WI) ad libitum and had free access to
water. All animal work was conducted using approved protocols from
the institute animal care and use committee, and met the highest stan-
dards of humane animal care.
Preparation of reconstituted basement membrane
The reconstituted basement membrane (RBM) matrix was extracted
from the Engelbreth-Holm-Swarm sarcoma as previously described (16).
Final dialysis was carried out using a dialysis membrane with a 14-kDa
cut-off.
Primary mammary epithelial organoid isolation, cell lines,
and culture conditions
Procedures for isolation of primary mammary epithelial organoids
have been described previously (16 –18). In brief, excised mammary
glands from 12–15 rats/experiment were minced finely, placed in di-
gestion solution (10 ml/g wet wt) consisting of 0.2% (wt/vol) colla-
genase type III and 0.2% (wt/vol) dispase grade II in phenol red-free
RPMI 1640 containing 5% (vol/vol) NCS and 50
g/ml gentamicin, and
incubated at 37 C for approximately 13 h. The digested tissue was then
pelleted, washed twice with RPMI 1640, resuspended in RPMI 1640, and
filtered initially through a 530-
m Nitex filter (Tetko, Depew, NY) and
then through a 60-
m Nitex filter to trap the epithelial organoids (which
were saved) but allow passage of small cell clusters and single cells
(which were discarded). The organoids were washed off the Nitex filter
with a 1:1 mixture of DMEM/Ham’s F-12 (phenol red-free), 5% NCS,
and 50
g/ml gentamicin; placed in a plastic tissue culture flask; and
incubated for 4 h at 37 C to facilitate the attachment and subsequent
removal of stromal contaminants. The cells within the nonadherent
mammary organoids were enumerated by isolation and counting of
nuclei after dilution into 0.1 m citric acid as described previously (16),
pelleted by centrifugation at 500
⫻ g for 10 min, and resuspended in
ice-cold RBM matrix at a concentration of 1.5
⫻ 10
6
cells/ml matrix. For
each experiment, 200
l of this cell-RBM suspension were plated on top
of 200
l solidified cell-free RBM in 24-well tissue culture plates and
incubated at 37 C for 3 h. After gelation of the cell-RBM suspension, 1 ml
serum-free medium was added to each well. Alternatively, for the RNA
studies, the mammary organoids were resuspended in ice-cold RBM
matrix at a concentration of 4
⫻ 10
6
cells/ml, and 2.5 ml of this sus-
pension were plated on top of 2.5 ml solidified RBM in petri dishes and
incubated at 37 C for 4 h. After gelation, 12 ml serum-free medium were
added to each dish.
The serum-free medium used in these studies consisted of phenol
red-free DMEM/F-12 (1:1) containing 10
g/ml insulin, 1 g/ml
progesterone, 1
g/ml hydrocortisone, 10 ng/ml EGF, 1 g/ml PRL,
5
g/ml transferrin, 5 m ascorbic acid, 1 mg/ml fatty acid-free BSA,
and 50
g/ml gentamicin. MEC were cultured for 7–10 days in
complete serum-free medium or in medium lacking hydrocortisone,
as noted. Vehicle (PBS) or TNF (0.4 – 40 ng/ml) was added either at
time zero (continuously present) or as noted in the text. Cells were
refed with fresh medium twice per week.
The mouse HC11 mammary epithelial cell line obtained from Dr. J.
Rosen (Baylor University, Houston, TX) with the permission of Dr. B.
Groner (Institute for Biomedical Research, Frankfurt/Main, Ger-
many) was grown to confluence and maintained for 4 days in RPMI
1640 medium supplemented with 10% FBS containing 10 ng/ml EGF,
2 mm glutamine, 5
g/ml insulin, and 50 g/ml gentamicin (growth
medium). The growth medium was then removed, and the cells were
switched to lactogenic medium (RPMI 1640 supplemented with 10%
FBS containing 5
g/ml insulin, 5 g/ml PRL, 1 g/ml hydro-
cortisone, 2 mm glutamine, and 50
g/ml gentamicin). For the nuclear
run-on studies, TNF (40 ng/ml) or vehicle (PBS) was added on day 3
of lactogenic medium (48 h point) or day 5 (2 and 4 h points), and all
cells were harvested on day 5 of culture in lactogenic medium.
Cell number
Viable cell number was determined for MEC using the 3-[4,5-
dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay as previ-
ously described (5). For HC11 cells, viable cell number was quantitated
by counting an aliquot of harvested cells using a hemocytometer and
trypan blue.
RNA isolation and Northern blot analysis
For MEC in primary culture, medium was removed from the culture
dishes, and 10 ml dispase (5 caseinolytic units/ml) in PBS were added.
TNF REGULATION OF GENE EXPRESSION IN MEC
2559
The RBM was dissociated with a cell scraper, followed by gentle up and
down pipetting, and was digested by incubation at 37 C for 30 min with
gentle stirring. The MEC were then collected by centrifugation for 10 min
at 500
⫻ g at 25 C. For studies using HC11 cells, cells were harvested from
T175 flasks by trypsinization. After harvesting MEC and HC11 cells, 1
ml TRIzol reagent was added per 10
7
cells, and total RNA was isolated
according to the manufacturer’s protocol. Denatured RNA (20 –30
g)
was separated by electrophoresis on a 1% (wt/vol) agarose gel contain-
ing 1
⫻ 3-[N-morpholino]propanesulfonic acid (MOPS) and 2.2 m form-
aldehyde and transferred by capillary action overnight to Hybond N
nylon membrane. The RNA was then cross-linked to the membranes by
UV irradiation (UV Stratalinker 1800, Stratagene, La Jolla, CA). Mem-
branes were prehybridized in a buffer containing 5
⫻ SSC (standard
saline citrate), 20 mm sodium phosphate (pH 6.5), 0.2% (wt/vol) SDS, 5
⫻
Denhardt’s solution, 250
g/ml salmon sperm DNA, and 50% form-
amide, using a modification of the standard method (19), for 4 h at 52
C. Hybridization with 2
⫻ 10
6
cpm/ml [
␣-
32
P]deoxy-CTP multiprimed
cDNA probes or 2
⫻ 10
6
cpm/ml [
␣-
32
P]UTP-labeled RNA probes was
performed in prehybridization buffer containing 10% dextran sulfate for
16 h at 52 C. The membranes were washed in 6
⫻ SSC and 0.1% SDS,
and autoradiography was performed at
⫺80 C with Kodak X-Omat AR
film using DuPont Cronex cassettes (Wilmington, DE) and intensifying
screens. The bands were scanned using a Molecular Dynamics, Inc.
(Sunnyvale, CA) laser densitometer, and quantitated using ImageQuant
software.
Nuclear run-on assay
Nuclei were prepared by a slight modification of the method of
Lamers et al. (20). Confluent HC11 cells were harvested by trypsinization
after 5 days in lactogenic medium (in the presence or absence of 40
ng/ml TNF for the indicated times) and gently resuspended in lysis
buffer (20 mm Tris HCl (pH 7.5), 2 mm MgCl
2
, and 10 mm NaCl). All
procedures were performed at 4 C unless otherwise noted. Nonidet P-40
(0.5% final concentration) was added to each sample, and the nuclei were
gently pipetted up and down on ice for 5–15 min with periodic exam-
ination under the microscope to check on the progress of cellular lysis.
When more than 60% of the cells were lysed, the samples were centri-
fuged at 500
⫻ g; nuclei were washed twice and resuspended in a buffer
containing 40% glycerol, 5 mm MgCl
2
, and 50 mm Tris-HCl (pH 7.5) at
4 C and 0.1 mm EDTA; and aliquots (5
⫻ 10
7
nuclei) were frozen in liquid
nitrogen and stored at
⫺80 C until use.
The transcriptional activity of the nuclei was measured by determin-
ing the incorporation of 250
Ci [␣-
32
P]UTP into RNA transcripts elon-
gated in vitro. For hybridization, a slot blot was prepared that contained
2.5
g linearized DNA (pGEM vector and transferrin), 50 ng purified
cDNA insert (GAPDH), 100 ng purified cDNA insert (WAP) or 100 ng
PCR-generated DNA (
-casein). Immobilization of DNA probes on ny-
lon membrane, hybridization, and washes were performed as previ-
ously described (21). Autoradiography was performed at
⫺80 C with
Kodak X-Omat AR film using DuPont Cronix cassettes and intensifying
screens. The bands were scanned using a Molecular Dynamics, Inc. laser
densitometer, and quantitated using ImageQuant software.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts from control and TNF-treated HC11 cells were pre-
pared according to the method of Olnes and Kurl (22). For EMSA, 20
g
nuclear protein and 1
g poly(dI-dC) were incubated for 20 min at 25
C in EMSA buffer [10 mm Tris HCl (pH 7.5), 50 mm NaCl, 1 mm
dithiothreitol, 1 mm EDTA, and 5% glycerol]. Seventy-five femtomoles
of the NF
B consensus oligonucleotide (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA), labeled with [
␥-
32
P]ATP using T4 polynucleotide ki-
nase (Promega Corp., Madison, WI), were added to the nuclear protein
samples, and the incubation was continued for an additional 13 min
before applying the samples to the gel. For the competition study,
nuclear extracts were incubated with a 50-fold excess of unlabeled NF
B
consensus oligonucleotide for 20 min at room temperature before ad-
dition of the
32
P-labeled NF
B oligonucleotide. To determine the protein
composition of the NF
B-DNA complexes, nuclear extracts were incu-
bated with
32
P-labeled NF
B oligonucleotide for 15 min at room tem-
perature, then 4
g antibody against p65, c-Rel, p52, or p50 were added.
The samples were incubated overnight at 4 C before loading on the gel.
F
IG
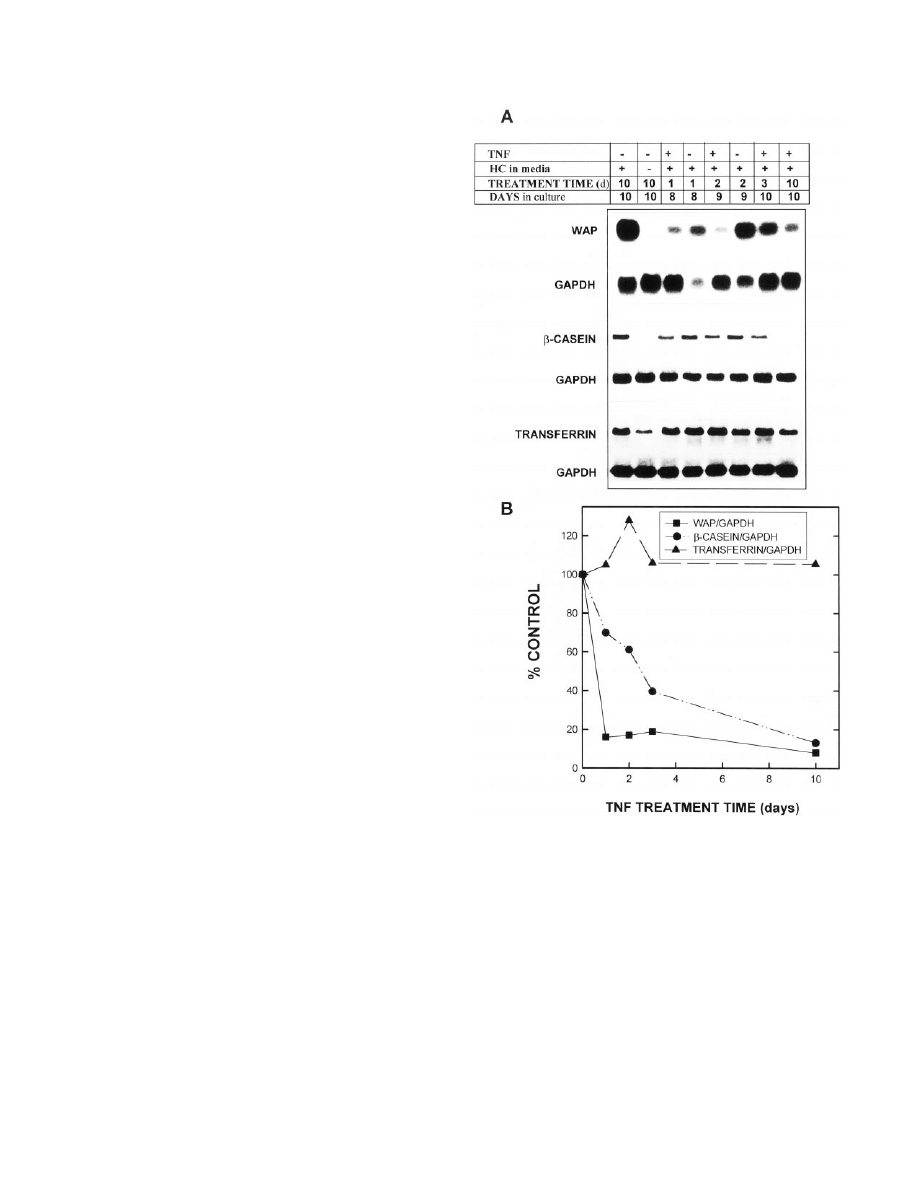
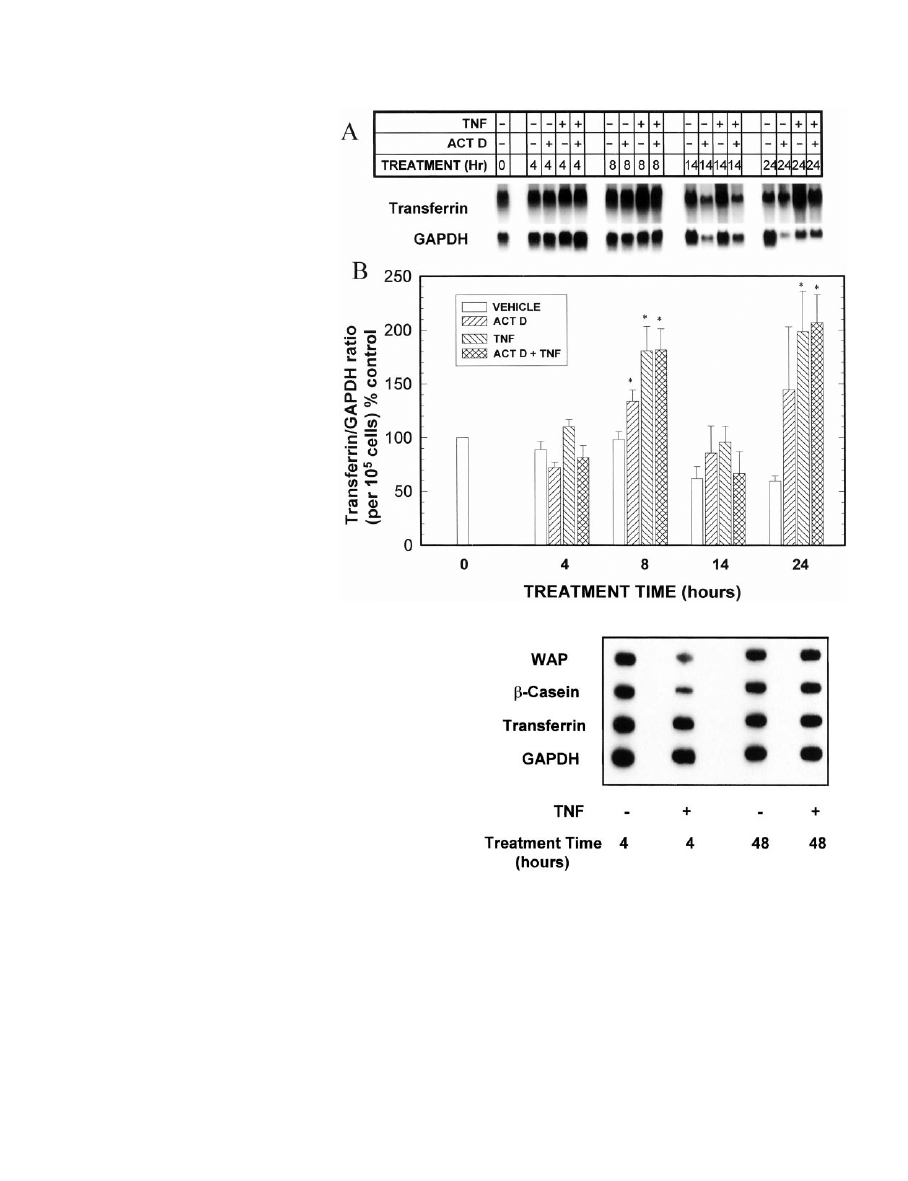
. 1. Effect of TNF on the time-dependent accumulation of WAP,
-casein, and transferrin mRNAs. MEC in primary culture were
grown in complete medium for 8 –10 days and digested out of the
RBM, and total RNA was isolated. TNF (40 ng/ml) or PBS vehicle was
added to complete medium on day 0 (with harvest on day 10; 10 days
of treatment) or on day 7 (with harvest on day 8, 9, or 10 of culture;
1, 2, or 3 days of treatment, respectively). Total RNA was isolated at
each of these harvest times. A, Northern blots of WAP,
-casein,
transferrin, and their respective GAPDH mRNAs. B, Quantitation by
scanning densitometry of autoradiograms from the experiment shown
in A, normalized to GAPDH and calculated as a percentage of the
appropriate vehicle control (i.e. controlled for time in culture). These
experiments represent two separate experiments for each probe from
MEC pooled from 12–15 rats in each experiment, each performed with
a duplicate set of dishes and RNA isolation. An additional group of
MEC was grown for 10 days in medium lacking hydrocortisone (HC)
as a negative control. Quantitation by scanning densitometry of MEC
grown in medium lacking HC revealed values of 3%, 11.7%, and 44.3%
of the vehicle control values for WAP,
-casein, and transferrin,
respectively.
2560
TNF REGULATION OF GENE EXPRESSION IN MEC
Endo
• 2001
Vol. 142
• No. 6
The samples were run on 4% or 5% native polyacrylamide gels in 0.5
⫻
TBE running buffer [50 mm Tris borate (pH 8.0) and 1 mm EDTA] for
75 min at 200 V. The gels were dried and exposed to x-ray film at
⫺80
C. Specific bands were scanned using a Molecular Dynamics, Inc. laser
densitometer and quantitated using ImageQuant software.
Zymography
Gelatinase activity in conditioned medium from MEC cultured for
7–10 days was analyzed by zymography on SDS-10% (wt/vol) poly-
acrylamide gels containing 1 mg/ml gelatin under nonreducing La-
emmli buffer conditions. Samples were loaded on an equal cell number
basis (conditioned medium from 20,000 cells for each sample). After
electrophoresis, the gel was washed in 2% (vol/vol) Triton X-100 and
incubated at 37 C overnight in substrate buffer [50 mm Tris-HCl, 5 mm
CaCl
2
, and 0.02% (wt/vol) sodium azide, pH 7.8, at 25 C]. After staining
with Coomassie blue, the gelatin-degrading enzymes appeared as clear
zones of lysis against a blue background.
Statistics
Data are presented as the mean
⫾ sem. When more than two groups
were compared, statistical significance was evaluated using one-way
ANOVA with the Tukey test for pairwise multiple comparisons. When
two groups were compared, statistical significance was evaluated using
Student’s t test. P
⬍ 0.05 was considered statistically significant.
Results
TNF inhibits accumulation of WAP and
-casein mRNAs in
a time- and concentration-dependent manner, but does not
alter transferrin mRNA levels
Our previous studies had demonstrated that TNF inhibits
the accumulation of casein proteins (3–5) in isolated MEC;
however, it was not known whether this effect was exerted
at the mRNA level, or if other milk proteins were similarly
inhibited. To assess this, MEC were cultured in optimal me-
dium or, as a negative control, in medium lacking hydro-
cortisone for up to 10 days, and the effects of TNF on the
expression of WAP and
-casein mRNAs were examined. In
the first experiment, 40 ng/ml TNF was added on day 7 of
culture, and WAP mRNA levels were examined 1, 2, and 3
days thereafter; alternatively, TNF was present continuously
from days 0 –10. As shown in Fig. 1, WAP mRNA was de-
creased within 1 day of addition of TNF to the culture me-
dium and remained below 20% of the control levels for up
to 10 days of treatment. Additionally, as expected from its
F
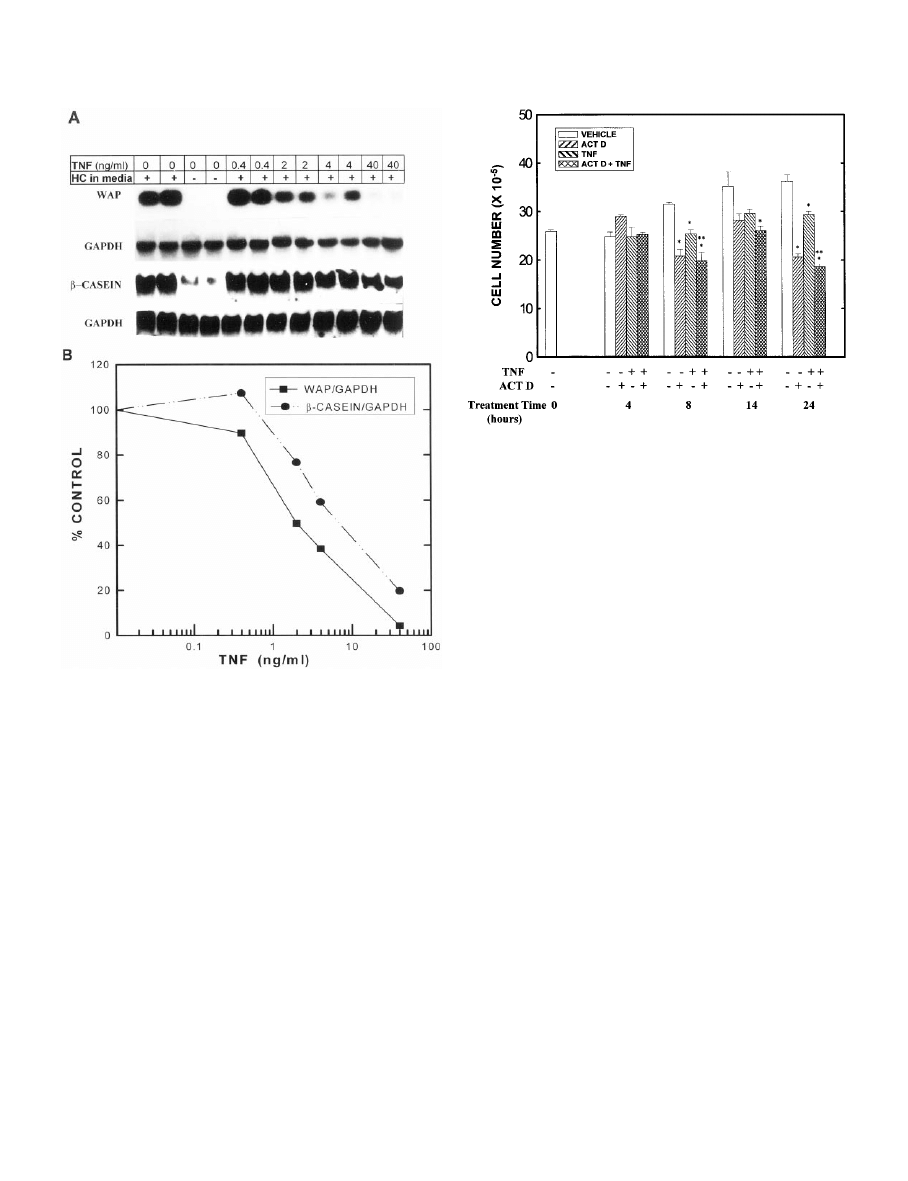
IG
. 2. Effects of various concentrations of TNF on the accumulation
of WAP and
-casein mRNAs. MEC in primary culture were grown in
complete medium containing 0 – 40 ng/ml TNF for 10 days, digested
out of the RBM, and total RNA was isolated. A, Northern blots of
WAP,
-casein, and their respective GAPDH mRNAs. RNA isolated
from duplicate samples from the same experiment was loaded in
adjacent wells. B, Quantitation by scanning densitometry of the du-
plicate lanes from the experiment shown in A, normalized to GAPDH
and calculated as a percentage of the appropriate vehicle control.
These experiments represent two separate experiments for each
probe from MEC pooled from 12–15 rats in each experiment, each
performed with a duplicate set of dishes and RNA isolation. An ad-
ditional group of MEC was grown for 10 days in medium lacking
hydrocortisone (HC) as a negative control. Quantitation by scanning
densitometry of MEC grown in medium lacking HC revealed values
of 1.8% and 13.9% of the vehicle control values for WAP and
-casein,
respectively.
F
IG
. 3. Effects of TNF and actinomycin D on viable MEC number.
MEC in primary culture were grown in optimal medium for 7 days.
On day 7, the medium was changed to optimal medium containing
TNF (0 or 40 ng/ml) and/or actinomycin D (0 or 1
g/ml), and the cells
were cultured for an additional 4, 8, 14, or 24 h. Viable cell number
was determined using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphe-
nyltetrazolium bromide assay. Each bar represents the mean
⫾
SEM
of triplicate wells. *, Statistically significant difference from the ap-
propriate vehicle control; **, statistically significant difference from
the TNF group.
TNF REGULATION OF GENE EXPRESSION IN MEC
2561
required role in WAP gene transcription (23), omission of
hydrocortisone from the culture medium virtually sup-
pressed WAP mRNA levels. Using a 10-day treatment pe-
riod, we next examined the concentration dependence of this
inhibitory effect of TNF. These experiments showed a 50%
inhibition of WAP mRNA levels at 2 ng/ml TNF and close
to maximal inhibition at 40 ng/ml (Fig. 2).
TNF also inhibited the accumulation of
-casein mRNA in
a time- and concentration-dependent manner (Figs. 1 and 2);
however, the effect of TNF on
-casein was somewhat less
pronounced than that seen with WAP. Thus,
-casein mRNA
levels decreased more gradually with time of TNF treatment,
reaching the nadir between 3–10 days of treatment (Fig. 1).
Similarly, although TNF inhibited
-casein accumulation in
a concentration-dependent manner, a concentration greater
than 4 ng/ml was required to inhibit by 50%, and at the
highest concentration tested (40 ng/ml),
-casein mRNA
levels were approximately 20% of the control level (Fig. 2).
Interestingly, the maximal inhibition of WAP or
-casein
mRNA levels achieved was comparable to that seen with the
omission of hydrocortisone (Figs. 1 and 2).
Transferrin is a milk protein that is synthesized at high
levels in mammary gland from pregnant and lactating mice,
but is relatively insensitive in vitro to the lactogenic hormones
that regulate expression of WAP and casein (24, 25). Given
this difference in regulation, it was of interest to compare the
effects of TNF on transferrin expression with its effects on the
other two milk proteins. Figure 1 demonstrates that in con-
trast to the inhibitory effect of TNF on WAP and
-casein,
TNF at 40 ng/ml had no effect on transferrin mRNA levels
and, if anything, slightly stimulated transferrin mRNA levels
at 48 h; lower concentrations had no effect (not shown). This
observation suggests that TNF may be acting selectively to
modulate WAP and casein. As shown in this figure, trans-
ferrin levels were modestly reduced in MEC cultured in the
absence of glucocorticoid.
TNF decreases WAP and
-casein mRNA levels by
decreasing the stability of the transcripts and by
decreasing transcription
Actinomycin D studies. TNF could reduce the steady state
levels of WAP and
-casein mRNAs by decreasing the sta-
bility of the mRNAs, decreasing the transcription of the
genes, and/or a combination of both mechanisms. This
might occur, for example, if TNF induced a factor mediating
either of these activities. Our first approach was to use
the transcriptional inhibitor actinomycin D to determine
whether it would interfere with the ability of TNF to inhibit
the accumulation of WAP or
-casein mRNAs. In prelimi-
nary studies we found that normal MEC were extremely
sensitive to actinomycin D-induced toxicity, so to address
this question, cells were treated with vehicle or TNF for 24,
48, or 72 h, with 1
g/ml actinomycin D added only during
F
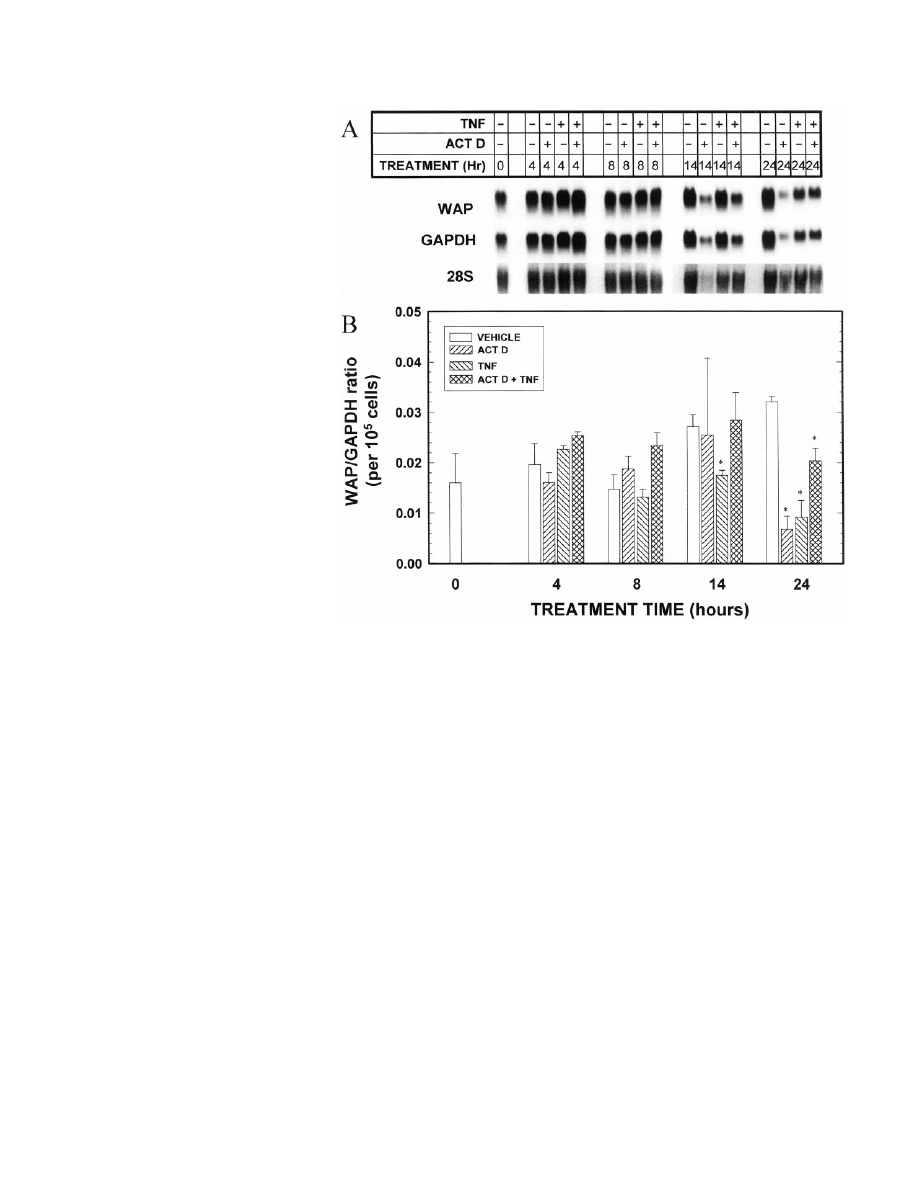
IG
. 4. Effect of concurrent exposure to
the transcriptional inhibitor actinomy-
cin D on TNF modulation of accumula-
tion of WAP mRNA. MEC in primary
culture were grown in optimal medium
for 7 days. On day 7, the medium was
changed to optimal medium containing
TNF (0 or 40 ng/ml) and/or actinomycin
D (0 or 1
g/ml), and the cells were cul-
tured for an additional 4, 8, 14, or 24 h.
MEC were then digested out of the
RBM, and total RNA was isolated. A,
Northern blot of WAP and GAPDH
mRNAs and ethidium bromide-stained
28S RNA (negative image). Three inde-
pendent Northern blots were performed
from triplicate cell cultures, and the
blots were reprobed for each milk pro-
tein as well as for GAPDH. A represen-
tative blot and 28S band are shown. Al-
though the absolute levels of GAPDH
and WAP mRNAs appear to be equally
decreased by actinomycin D at 24 h in
this experiment, when replicate exper-
iments were evaluated, actinomycin D
decreased GAPDH to 48
⫾ 5% and TNF
to 5
⫾ 1% of the 24 h control value,
respectively (n
⫽ 3). B, Quantitation by
scanning densitometry of the triplicate
experiments, normalized to GAPDH,
with the WAP/GAPDH ratio then
normalized for cell number. Each bar
represents the mean
⫾
SEM
of three ex-
periments. *, Statistically significant
difference from the appropriate vehicle
control.
2562
TNF REGULATION OF GENE EXPRESSION IN MEC
Endo
• 2001
Vol. 142
• No. 6
the last 6 h of culture. This drug concentration did not inhibit
cell growth within this time period, but inhibited [
3
H]uridine
incorporation into RNA by about 70% (data not shown). With
this protocol, actinomycin D did not alter steady state levels
of WAP,
-casein, or transferrin mRNAs in either the pres-
ence or absence of TNF (data not shown).
The lack of effect of actinomycin D in this initial study
could suggest that the inhibitory effect of TNF is not medi-
ated at the transcriptional level and/or that a TNF-induced
decrease in transcript stability does not depend on transcrip-
tion. However, it is also possible that the effect of TNF is
initiated early, and that when actinomycin D is added only
for the final 6 h of culture, it is too late for it to exert an effect,
as the gene responsible for initiating the effect of TNF may
already have been transcribed.
To address this question, a second set of experiments was
performed in which actinomycin D was added simulta-
neously with TNF, and its effects on WAP,
-casein, and
transferrin mRNAs were determined after 4, 8, 14, and 24 h
of culture. Longer time periods were not evaluated because
cell growth is rapidly inhibited by actinomycin D (Fig. 3); for
this reason also all quantitations of the milk protein mRNAs
were normalized to cell number as well as to GAPDH to
allow appropriate comparisons. Similar results were ob-
served if the mRNAs were normalized to 18S or 28S RNA
instead of to GAPDH (data not shown). Two significant
observations can be made from this experiment. First, the
inhibition of WAP mRNA accumulation by TNF was rapid
and was seen as early as 14 h after the addition of TNF and
to an even greater extent at 24 h (Fig. 4). Second, although
actinomycin D alone decreased WAP mRNA levels, when
added simultaneously with TNF, actinomycin D partially
interfered with the inhibitory action of TNF. This can be seen
at the 24 h point as well as by comparing the slower rate of
decline of WAP mRNA levels between 4 and 24 h in the TNF
plus actinomycin D group compared with that in the group
given TNF alone. As discussed in more detail below, these
data are consistent with the hypothesis that TNF induces
transcription of a factor that reduces the stability of WAP
mRNA.
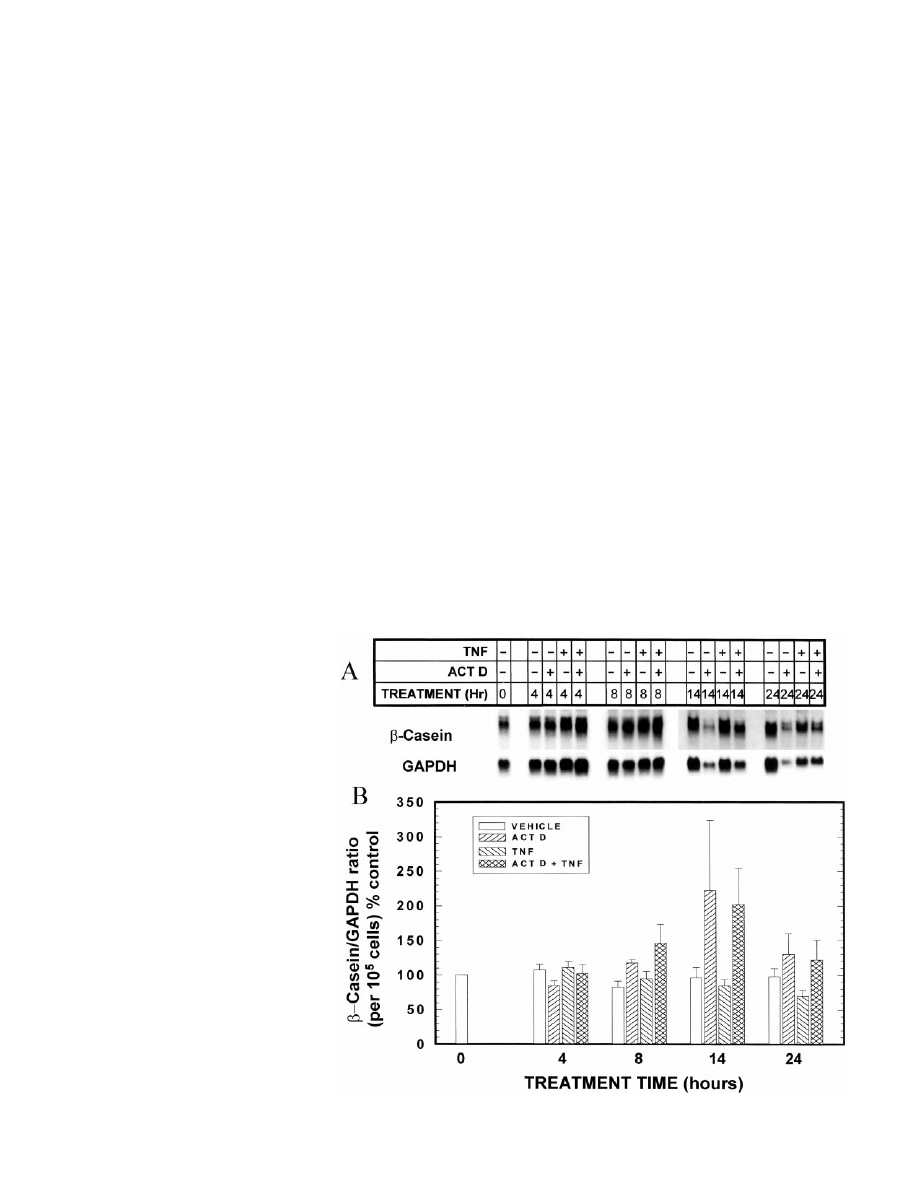
The effects of TNF and actinomycin D on
-casein were
somewhat different from those observed with WAP (Fig. 5).
Most noticeably, the inhibitory effect of TNF on
-casein
mRNA levels takes longer to occur than that with WAP
mRNA (see also Fig. 1); moreover, actinomycin D completely
blocks the inhibitory effect of TNF on
-casein mRNA levels.
Finally, as noted previously, TNF does not inhibit the accu-
mulation of transferrin mRNA (Fig. 6), and indeed, as also
shown in Fig. 1, there is a stimulation after addition of TNF
to the culture. Actinomycin D did not block this TNF-
induced stimulation.
Nuclear run-on studies. As discussed in more detail in Dis-
cussion, these data suggested that TNF inhibits accumulation
of WAP and
-casein mRNAs both by decreasing the stability
of the mRNA, as well as by inhibiting transcription. To ad-
dress the transcriptional question more directly, we at-
tempted to use the nuclear run-on assay in this MEC primary
culture model. This proved to be very difficult, however,
because nuclei isolated from MEC digested out of the RBM
were very fragile, and we were not able to obtain consistent
results. We therefore chose to use the immortalized mouse
F
IG
. 5. Effect of concurrent exposure to
the transcriptional inhibitor actinomy-
cin D on TNF modulation of accumula-
tion of
-casein mRNA. MEC in primary
culture were grown in optimal medium
for 7 days. On day 7, the medium was
changed to optimal medium containing
TNF (0 or 40 ng/ml) and/or actinomycin
D (0 or 1
g/ml), and the cells were cul-
tured for an additional 4, 8, 14, or 24 h.
MEC were then digested out of the
RBM, and total RNA was isolated. A,
Northern blot of
-casein and GAPDH
mRNAs. Three independent Northern
blots were performed from triplicate
cell cultures, and the blots were re-
probed for each milk protein as well as
for GAPDH. A representative blot is
shown. Note that this is the same RNA
sample for which the representative
28S band is shown in Fig. 4. B, Quan-
titation by scanning densitometry of the
triplicate experiments, normalized to
GAPDH, with the
-casein/GAPDH ra-
tio then normalized for cell number, and
expressed as a percentage of the time
zero vehicle control value. Each bar rep-
resents the mean
⫾
SEM
of three exper-
iments.
TNF REGULATION OF GENE EXPRESSION IN MEC
2563
mammary cell line HC11 (11), which is a clone of the
COMMA-1D cell line derived from the mammary gland of
a pregnant mouse (26). This cell line exhibits many of the
characteristics of normal MEC and has been used by many
investigators to study regulation of the
-casein gene (11,
27–30). Under the usual conditions for growth of these cells,
WAP is expressed only at very low levels.
In preliminary studies we used Northern blot analysis to
establish that 40 ng/ml TNF reduced
-casein mRNA levels
in HC11 cells to 44.3
⫾ 13.9% and 38.6 ⫾ 8.5% (mean ⫾ sem)
of the control value after 2 and 3 days of treatment, respec-
tively, thus validating the use of this cell line for these stud-
ies. In follow-up nuclear run-on experiments, we found that
TNF decreased transcription of both the
-casein and WAP
genes as early as 4 h after its addition to culture; as noted in
the primary culture model, however, TNF did not affect
transcription of transferrin, again demonstrating its selec-
tivity (Fig. 7). The transcriptional effect was lost after 48 h of
culture, suggesting that a decrease in mRNA stability may be
a more important influence on transcript levels at this time.
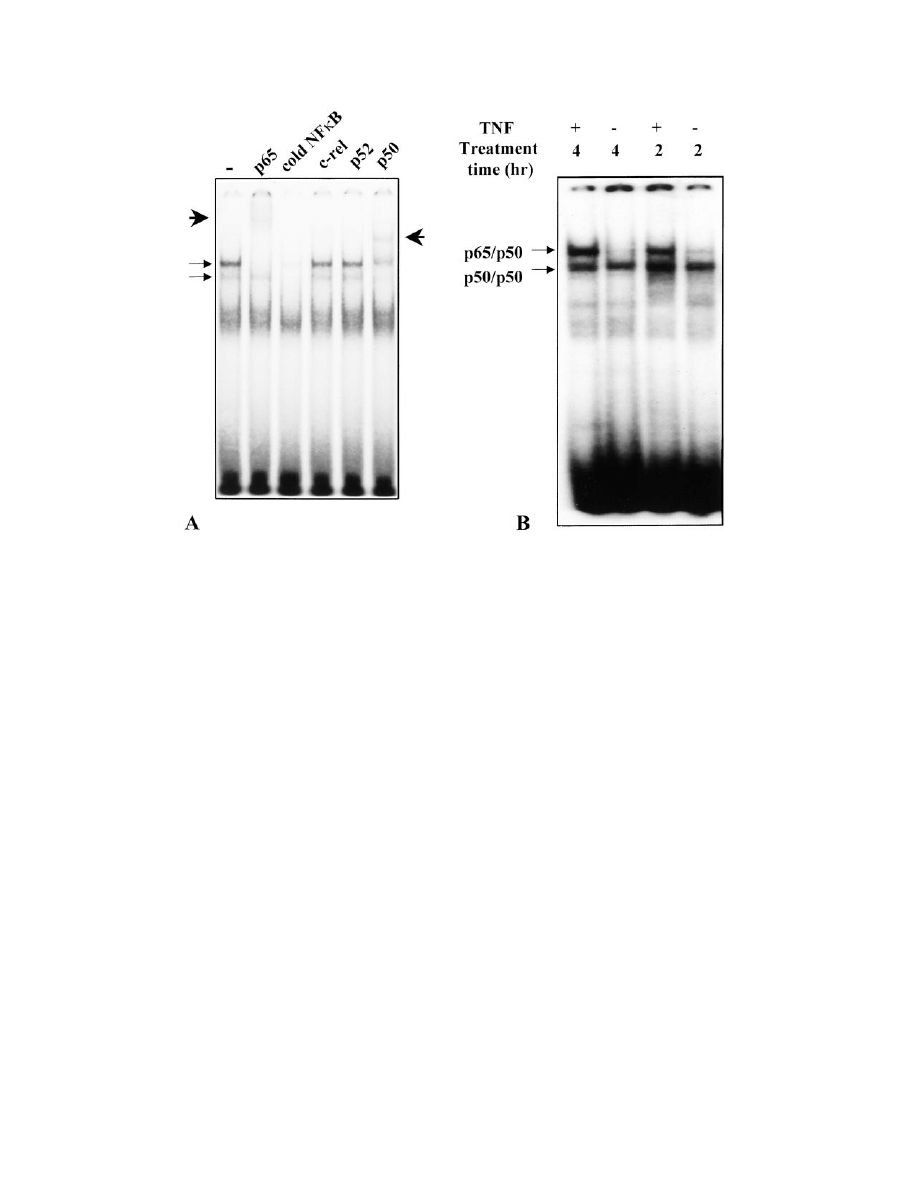
An induction of NF
B and/or an induction in MMP-9 may
contribute to the mechanism by which TNF inhibits
-casein and WAP expression
TNF has been shown to induce NF
B in many cell types
(31), and this transcription factor is a likely candidate to
mediate the effects of TNF on WAP and
-casein gene ex-
pression. As shown in Fig. 8, the p65/p50 heterodimer of
NF
B is rapidly induced by TNF in HC11 cells, suggesting
that it could mediate the transcriptional effects shown in
Fig. 7. NF
B might exert its activity directly (see Discussion)
or indirectly through induction of a specific gene product.
We chose to look at induction of MMP-9, whose transcription
F
IG
. 7. Effect of TNF on transcription of WAP,
-casein, and trans-
ferrin genes in HC11 mouse MEC (nuclear run-on assay). HC11 cells
were cultured in lactogenic medium for 3 days, then fresh lactogenic
medium containing 0 or 40 ng/ml TNF was added, and the cells were
cultured for an additional 4 or 48 h. Nuclei were prepared, and in vitro
labeled transcripts were hybridized to probes for WAP,
-casein,
transferrin, and GAPDH immobilized on nylon membrane. The figure
shown is representative of four samples run in two independent
experiments.
F
IG
. 6. Effect of concurrent exposure to
the transcriptional inhibitor actinomy-
cin D on TNF modulation of accumula-
tion of transferrin mRNA. MEC in pri-
mary culture were grown in optimal
medium for 7 days. On day 7, the me-
dium was changed to optimal medium
containing TNF (0 or 40 ng/ml) and/or
actinomycin D (0 or 1
g/ml), and the
cells were cultured for an additional 4,
8, 14, or 24 h. MEC were then digested
out of the RBM, and total RNA was iso-
lated. A, Northern blot of transferrin
and GAPDH mRNAs. Three indepen-
dent Northern blots were performed
from triplicate cell cultures, and the
blots were reprobed for each milk pro-
tein as well as for GAPDH. A represen-
tative blot is shown. Note that this is the
same RNA sample for which the repre-
sentative 28S band is shown in Fig. 4. B,
Quantitation by scanning densitometry
of the triplicate experiments, normal-
ized to GAPDH, with the transferrin/
GAPDH ratio then normalized for cell
number and expressed as a percentage
of the time zero vehicle control value.
Each bar represents the mean
⫾
SEM
of
three experiments. *, Statistically sig-
nificant difference from the appropriate
vehicle control.
2564
TNF REGULATION OF GENE EXPRESSION IN MEC
Endo
• 2001
Vol. 142
• No. 6
is known to be activated by NF
B (32–34), as our previous
studies demonstrated that TNF induced secretion of MMP-9
protein in MEC concurrent with a stimulation of branching
morphogenesis (6). We hypothesized that a local disruption
in the interaction between the extracellular matrix and the
MEC, a change in the processing of growth factors or their
receptors, and/or a change in the release of matrix-associated
growth factors as a result of an induction of MMP-9 activity,
would inhibit transcription of the milk protein genes.
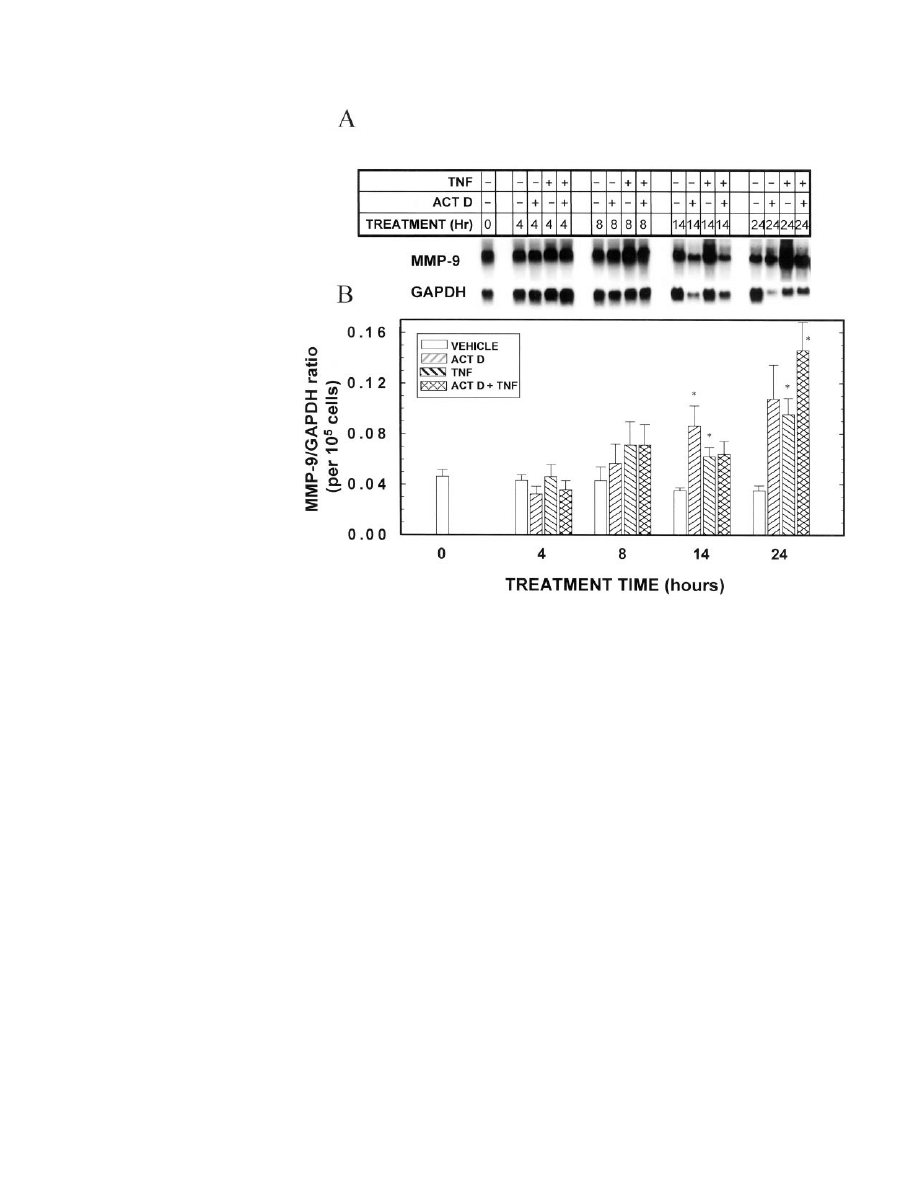
Figure 9 demonstrates that induction of MMP-9 mRNA
can be seen as early as 14 h after addition of TNF to MEC in
primary culture, although the induction is more dramatic at
the 24 h point. Unexpectedly, actinomycin D did not block
the ability of TNF to induce MMP-9 mRNA levels. Moreover,
by itself, actinomycin D increased MMP-9 mRNA, an effect
that was evident after 14 and 24 h of treatment. It should be
noted that the data have been normalized for cell number, as
actinomycin D inhibited cell growth after approximately 8 h
in culture. At the protein level, enhancement of the gelatinase
activity of MMP-9 by TNF could be seen by 14 h (Fig. 10),
although not earlier (data not shown), and reflected the
mRNA levels. Interestingly, however, in actinomycin
D-treated cultures, the activity of the MMP-9 protein differed
from that of its RNA. Specifically, actinomycin D blocked
TNF stimulation of MMP-9 gelatinase activity at 14 and 24 h,
and by itself had no effect on MMP-9 activity.
Discussion
The current experiments support our previous studies,
which demonstrated that TNF inhibits the accumulation of
casein protein in primary cultures of MEC (3–5), and extend
the data to indicate that this effect is exerted at both tran-
scriptional and posttranscriptional levels. Moreover, the cur-
rent data demonstrate that the expression of at least two milk
protein genes,
-casein and WAP, is specifically inhibited by
TNF, whereas that of another milk protein, transferrin, is not
repressed by this treatment.
The ability of actinomycin D to partially (WAP) or com-
pletely (
-casein) block the inhibitory effect of TNF on the
mRNA levels of these genes is consistent with the possibility
that TNF inhibits their transcription. Indeed, a transcrip-
tional mechanism was confirmed in the nuclear run-on stud-
ies as well as in other experiments in which TNF was found
to inhibit
-casein (30) (Zhang, H., and M. M. Ip, unpub-
lished) and WAP (Zhang, H., and M. M. Ip, unpublished)
promoter activity in transiently transfected HC11 cells. In
addition, however, we cannot rule out an effect on mRNA
stability, because the actinomycin D results are also consis-
tent with the postulate that TNF induces transcription of
factors that decrease the stability of WAP and/or
-casein
transcripts. Interestingly, the ability of actinomycin D alone
to significantly decrease WAP mRNA levels suggests that
F
IG
. 8. Effect of TNF on binding of HC11 nuclear extracts to an NF
B consensus sequence. A, HC11 cells were cultured in lactogenic medium
for 4 days, then fresh lactogenic medium containing 40 ng/ml TNF was added, and the cells were cultured for an additional 12 h. Nuclear extracts
were prepared, incubated with a 50-fold excess of unlabeled NF
B probe (cold NFB) or with antibodies against p65 (Rel A), c-Rel, p52, or p50,
as described in Materials and Methods, and then analyzed by EMSA. The arrows indicate the bands that are competed by the unlabeled NF
B.
From the supershift (arrowheads) and/or loss of intensity of these specific bands after antibody treatment, the upper and lower specific bands
are identified as the p65/p50 heterodimer and the p50 homodimer of NF
B, respectively. B, HC11 cells were cultured in lactogenic medium
for 4 days, then fresh lactogenic medium containing 0 or 40 ng/ml TNF was added, and the cells were cultured for 2 or 4 h. Nuclear extracts
were prepared and analyzed by EMSA. The positions of the two specific NF
B DNA-binding complexes are indicated. This figure is representative
of four independent samples. The intensities of the two specific bands varied with different batches of HC11 cells.
TNF REGULATION OF GENE EXPRESSION IN MEC
2565
transcription is normally required to stabilize WAP mRNA.
TNF might then act to directly inhibit the transcription of the
responsible gene(s) or to induce the expression of other genes
that decrease the stability of the WAP transcript. Finally, the
observation in the nuclear run-on experiments that TNF in-
hibited transcription of the WAP and
-casein genes after 4 h
of TNF treatment, but not after 48 h, suggests that both
transcription and transcript stability are important compo-
nents in the mechanism by which TNF exerts its effects.
Several possible mechanisms may explain the ability of
TNF to inhibit the expression of the WAP and
-casein genes.
A likely candidate is the transcription factor NF
B, which has
been shown to be induced by TNF in many cell types (31),
including the HC11 mammary epithelial cell line (herein and
Ref. 30) and normal MEC in primary culture (39). It could be
proposed that NF
B acts to directly inhibit transcription by
binding to one of the NF
B-like sequences in either the WAP
or
-casein promoters or may indirectly modulate the activ-
ity of one of the known transcriptional regulators. For ex-
ample, Geymayer and Doppler (30) demonstrated that the
NF
B p65/p50 heterodimer indirectly interferes with the
ability of STAT5 (signal transducer and activator of tran-
scription-5) to activate transcription of the
-casein gene
coincident with a reduced phosphorylation of STAT5. Neg-
ative cross-talk between STAT5 and NF
B has also been
reported in other models as well (35). This may suggest that
TNF-induced NF
B directly inhibits transcription of the
-casein gene by interfering with STAT5 activity.
An important role for the extracellular matrix (ECM) in
regulating transcription of the WAP and
-casein genes was
previously established. For example, the ECM-induced for-
mation of alveolar MEC colonies appears to be required for
the expression of WAP in vitro (36). Moreover, an ECM-
responsive element has been identified in the
-casein pro-
moter (BCE1) (37). MMPs are a family of enzymes that play
a critical role in remodeling of the ECM. Recently, we ob-
served that MMP-9, an enzyme that is expressed in both rat
and mouse mammary glands (Lee, P.-P. H., and M. M. Ip,
unpublished), is secreted by the mammary epithelium in
response to TNF (6). Moreover, MMP-9 activity is required
(6) for the extensive TNF-induced three-dimensional branch-
ing morphogenesis that occurs when MEC in primary culture
are cultured within a reconstituted basement membrane (3,
5, 6). The studies reported herein demonstrate that expres-
sion of MMP-9 mRNA as well as the activity of secreted
MMP-9 protein are rapidly induced in MEC in response to
TNF, and it is tempting to speculate that the subsequent
disruption of the ECM may contribute to the inhibition of
expression of both
-casein and WAP. An alternative pos-
sibility is that MMP-9 stimulates the processing of cytokines/
growth factors and/or their receptors or the release of ma-
trix-bound growth factors, thus altering the hormonal milieu
surrounding the MEC in such a way as to inhibit functional
differentiation.
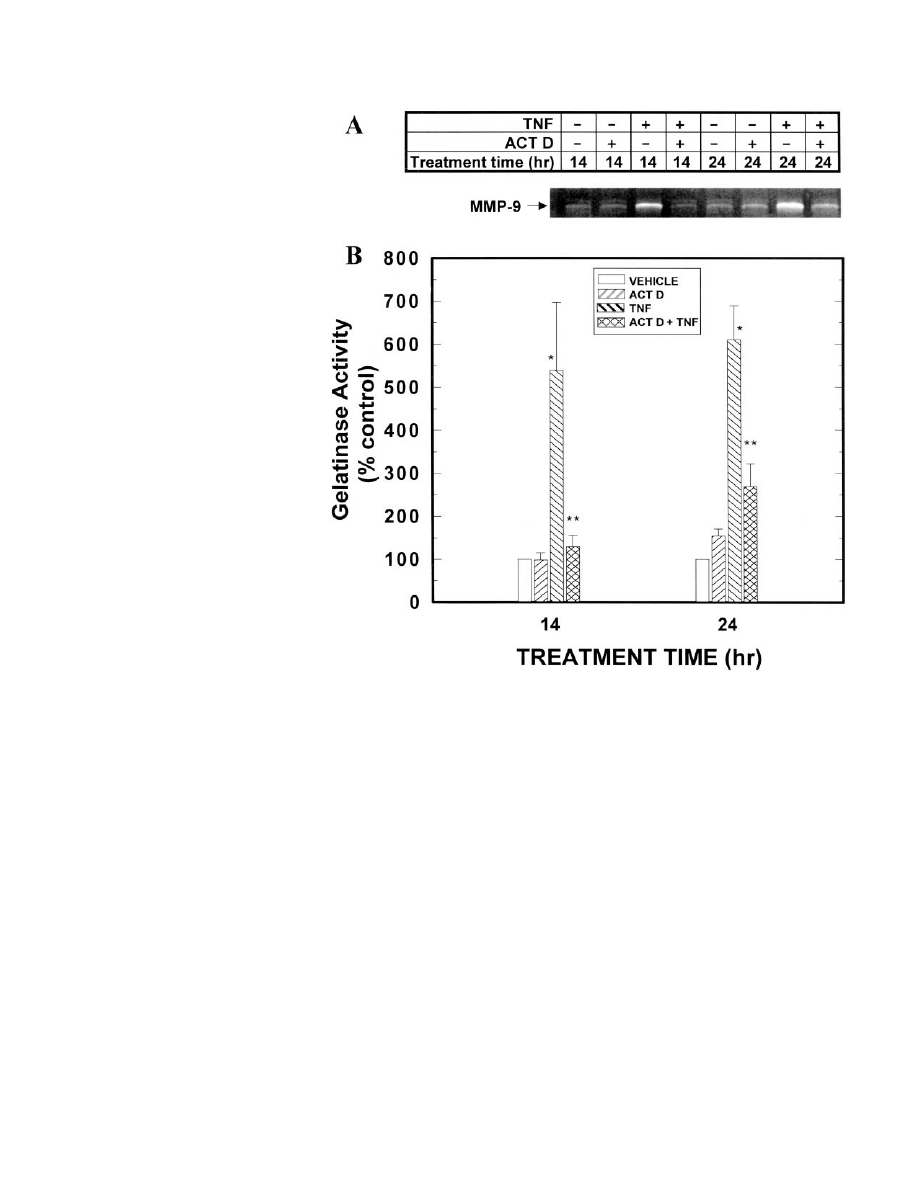
Of interest was the observation that TNF stimulation of
MMP-9 mRNA was similar in the absence or presence of the
transcriptional inhibitor actinomycin D; in contrast, gela-
tinase activity of secreted TNF-induced MMP-9 protein was
F
IG
. 9. Effects of TNF and actinomycin
D on MMP-9 mRNA levels. MEC in pri-
mary culture were grown in optimal
medium for 7 days. On day 7, the me-
dium was changed to optimal medium
containing TNF (0 or 40 ng/ml) and/or
actinomycin D (0 or 1
g/ml), and the
cells were cultured for an additional 4,
8, 14, or 24 h. MEC were then digested
out of the RBM, and total RNA was iso-
lated. A, Northern blot of MMP-9
mRNA. Three independent Northern
blots were performed from triplicate
cell cultures, and each blot was probed
for MMP-9, the milk proteins (Figs.
4 – 6), and GAPDH. A representative
blot is shown. Note that this is the same
RNA sample for which the representa-
tive 28S band is shown in Fig. 4. B,
Quantitation by scanning densitometry
of the triplicate experiments, normal-
ized to GAPDH, with the MMP-9/
GAPDH ratio then normalized for cell
number.
Each
bar
represents
the
mean
⫾
SEM
of three experiments. *,
Statistically significant difference from
the appropriate vehicle control.
2566
TNF REGULATION OF GENE EXPRESSION IN MEC
Endo
• 2001
Vol. 142
• No. 6
completely blocked by actinomycin D. This demonstrates
that MMP-9 can be regulated at multiple levels. First, the
inability of actinomycin D to block TNF stimulation of
MMP-9 mRNA suggests that in MEC, TNF does not regulate
transcription of MMP-9, but, rather, increases the stability of
the MMP-9 transcript. A similar observation was reported
with transforming growth factor-
, which was shown to
exert its stimulatory effect on MMP-9 mRNA in human pros-
tate cancer cell lines by increasing the stability of the message
(38). This would suggest that TNF-induced NF
B does not
increase transcription of MMP-9 in MEC. Second, the fact
that actinomycin D did block the gelatinase activity of
MMP-9 in the conditioned medium suggests that transcrip-
tional regulation is involved in the translation, processing,
stability, and/or secretion of this enzyme. Taken together
with the
-casein and WAP data in Figs. 4 and 5, it is possible
to make some tentative conclusions with respect to the po-
tential role that MMP-9 may play in the regulation of ex-
pression of these two genes. Specifically, the ability of acti-
nomycin D to block TNF-induced MMP-9 activity directly
correlated with the activity of this transcriptional inhibitor to
block the TNF-mediated decrease in WAP and
-casein tran-
scripts, thus raising the possibility that TNF could exert its
effects on these milk protein genes in part by stimulation of
MMP-9. Unfortunately, we did not have sufficient MMP-9-
neutralizing antibody to address this question more directly.
Finally, it should be noted that TNF also stimulates MMP-9
mRNA levels in HC11 cells (data not shown), and although
these cells are grown on plastic, the cells are allowed to
become superconfluent before the addition of lactogenic me-
dium and, as a consequence, may make their own extracel-
lular matrix. It is thus conceivable to invoke an MMP-9-
mediated mechanism for the regulation of WAP and
-casein
transcription, although we believe that this is just one of the
ways in which TNF regulates expression of these genes.
In summary, the work described herein demonstrates that
TNF is a key regulator of functional differentiation in MEC
and extends our previous studies, which demonstrated that
TNF stimulates the proliferation and branching morphogen-
esis of the mammary epithelium (3–7). In this paper we
report that TNF inhibits WAP and
-casein expression by
both transcriptional and posttranscriptional mechanisms.
Current studies in the laboratory are focused on identifying
the TNF-responsive regions in the promoters of both of these
genes and determining whether NF
B plays a functionally
significant role in their regulation or whether other tran-
scriptional regulators may mediate the effect of TNF. In any
case, a further understanding of how TNF modulates the
F
IG
. 10. Effects of TNF and actinomy-
cin D on gelatinase activity of MMP-9 in
conditioned medium from MEC treated
with TNF and/or actinomycin D. MEC
in primary culture were grown in opti-
mal medium for 7 days. On day 7, the
medium was changed to optimal me-
dium containing TNF (0 or 40 ng/ml)
and/or actinomycin D (0 or 1
g/ml), and
the cells were cultured for an additional
14 or 24 h. Conditioned media were col-
lected and evaluated by zymography. A,
Zymogram showing MMP-9 activity in
conditioned medium from normal MEC
in primary culture in response to treat-
ment with vehicle, TNF, or actinomycin
D, as indicated. Identity of the 97-kDa
(nonactivated) and 95-kDa (activated)
MMP-9 bands was confirmed by West-
ern blot (6). The results shown are rep-
resentative of eight separate samples
for each group. B, Quantitation by scan-
ning densitometry of the zymograms.
Loading of sample was normalized for
cell number; specifically, conditioned
medium from 20,000 cells was loaded in
each lane. The bar in the vehicle control
group represents the mean of eight sep-
arate samples; the bars in the three
treatment groups represent the mean
⫾
SEM
of eight separate samples for each
group. *, Statistically significant differ-
ence from the actinomycin D alone
group; **, statistically significant dif-
ference from the TNF alone group.
TNF REGULATION OF GENE EXPRESSION IN MEC
2567

transcription of milk protein genes not only will have im-
plications for mammary gland biology, but may also shed
light on the mechanism of TNF action in other cell types as
well.
Acknowledgments
The authors are grateful to Mr. Larry Mead for preparation of the
figures, to Laura Lee for the control NF
B/EMSA studies, to Dr. Jeffrey
Rosen for providing us with the rat
-casein and WAP probes, to Dr.
Lynn Matrisian for the rat MMP-9 probe, to Drs. M. Griswold and S.
Sylvester for providing us with the rat transferrin probe, to Asahi Chem-
ical Industry Co. for providing us with human recombinant TNF
␣, and
to Ms. Jane Ehrke and Drs. Haitao Zhang and Linda Varela for their
critical review of this manuscript.
References
1. Terranova PF, Hunter VJ, Roby KF, Hunt JS 1995 Tumor necrosis factor-
␣ in
the female reproductive tract. Proc Soc Exp Biol Med 209:325–342
2. Hunt JS, Chen HL, Miller L 1996 Tumor necrosis factors: pivotal components
of pregnancy. Biol Reprod 54:554 –562
3. Ip MM, Shoemaker SF, Darcy KM 1992 Regulation of rat mammary epithelial
cell proliferation and differentiation by tumor necrosis factor
␣. Endocrinology
130:2833–2844
4. Varela LM, Ip MM 1996 Tumor necrosis factor-
␣: a multifunctional regulator
of mammary gland development. Endocrinology 137:4915– 4924
5. Varela LM, Darcy KM, Ip MM 1997 The epidermal growth factor receptor is
not required for tumor necrosis factor-
␣ action in normal mammary epithelial
cells. Endocrinology 138:3891–3900
6. Lee P-PH, Hwang J-J, Murphy G, Ip MM 2000 Functional significance of
MMP-9 in TNF-induced proliferation and branching morphogenesis of mam-
mary epithelial cells. Endocrinology 141:3764 –3773
7. Stangle NC, Kollias G, Ip MM, 1999 Disrupted mammary gland development
in TNF
␣ knockout mice. Proc Am Assoc Cancer Res 40:161
8. Grell M, Douni E, Wajant H, Lo¨hden M, Clauss M, Maxeiner B, Georgo-
poulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P
1995 The
transmembrane form of tumor necrosis factor is the prime activating ligand of
the 80 kDa tumor necrosis factor receptor. Cell 83:793– 802
9. Hobbs AA, Richards DA, Kessler DJ, Rosen JM 1982 Complex hormonal
regulation of rat casein gene expression. J Biol Chem 257:3598 –3605
10. Rosen JM, Rodgers JR, Couch CH, Bisbee CA, David-Inouye Y, Campbell
SM, Yu-Lee L-Y
1986 Multihormonal regulation of milk protein gene expres-
sion. Ann NY Acad Sci 478:63–76
11. Ball RK, Friis RR, Schoenenberger CA, Doppler W, Groner B 1988 Prolactin
regulation of
-casein gene expression and of a cytosolic 120-kd protein in a
cloned mouse mammary epithelial line. EMBO J 7:2089 –2095
12. Ganguly R, Majumder PK, Ganguly N, Banerjee MR 1982 The mechanism
of progesterone-glucocorticoid interaction in regulation of casein gene expres-
sion. J Biol Chem 257:2182–2187
13. Jahn GA, Djiane J, Houdebine L-M 1989 Inhibition of casein synthesis by
progestagens in vitro: modulation in relation to concentration of hormones that
synergize with prolactin. J Steroid Biochem 32:373–379
14. Lee P-PH, Darcy KM, Shudo K, Ip MM 1995 Interaction of retinoids with
steroid and peptide hormones in modulating morphological and functional
differentiation of normal rat mammary epithelial cells. Endocrinology
136:1718 –1730
15. Darcy KM, Shoemaker SF, Lee P-PH, Ganis BA, Ip MM 1995 Hydrocortisone
and progesterone regulation of the proliferation, morphogenesis and func-
tional differentiation of normal rat mammary epithelial cells in three dimen-
sional primary culture. J Cell Physiol 163:365–379
16. Hahm HA, Ip MM 1990 Primary culture of normal rat mammary epithelial
cells within a basement membrane matrix. I. Regulation of proliferation by
hormones and growth factors. In Vitro Cell Dev Biol 26:791– 802
17. Darcy KM, Zangani D, Lee P-PH, Ip MM 2000 Isolation and culture of normal
rat mammary epithelial cells. In: Ip MM, Asch BB (eds) Methods in Mammary
Gland Biology and Breast Cancer Research. Kluwer Academic/Plenum, New
York, pp 163–175
18. Darcy KM, Black JD, Hahm HA, Ip MM 1991 Mammary organoids from
immature virgin rats undergo ductal and alveolar morphogenesis when grown
within a reconstituted basement membrane. Exp Cell Res 196:49 – 65
19. Brown T, Mackey K 1997 Analysis of RNA by northern and slot blot hybrid-
ization. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith
JA, Struhl K (eds) Current Protocols in Molecular Biology. Wiley & Sons, New
York, vol 1:4.9.1– 4.9.16
20. Lamers WH, Hanson RW, Meisner HM 1982 cAMP stimulates transcription
of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver
nuclei. Proc Natl Acad Sci USA 79:5137–5141
21. McKnight GS, Palmiter RD 1979 Transcriptional regulation of the ovalbumin
and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem
254:9050 –9058
22. Olnes MI, Kurl RN 1994 Isolation of nuclear extracts from fragile cells: a
simplified procedure applied to thymocytes. BioTechniques 17:828 – 829
23. Li S, Rosen JM 1994 Glucocorticoid regulation of rat whey acidic protein gene
expression involves hormone-induced alterations of chromatin structure in the
distal promoter region. Mol Endocrinol 8:1328 –1335
24. Lee EYH, Barcellos-Hoff MH, Chen L-H, Parry G, Bissell MJ 1987 Transferrin
is a major mouse milk protein and is synthesized by mammary epithelial cells.
In Vitro Cell Dev Biol 23:221–226
25. Chen L-H, Bissell MJ 1987 Transferrin mRNA level in the mouse mammary
gland is regulated by pregnancy and extracellular matrix. J Biol Chem
262:17247–17250
26. Danielson KG, Oborn CJ, Durban EM, Butel JS, Medina D 1984 Epithelial
mouse mammary cell line exhibiting normal morphogenesis in vivo and func-
tional differentiation in vitro. Proc Natl Acad Sci USA 81:3756 –3760
27. Hynes NE, Taverna D, Harwerth IM, Ciardiello F, Salomon DS, Yamamoto
T, Groner B
1990 Epidermal growth factor receptor, but not c-erbB-2, activation
prevents lactogenic hormone induction of the
-casein gene in mouse mam-
mary epithelial cells. Mol Cell Biol 10:4027– 4034
28. Goodman HS, Rosen JM 1990 Transcriptional analysis of the mouse
-casein
gene. Mol Endocrinol 4:1661–1670
29. Schmitt-Ney M, Doppler W, Ball RK, Groner B 1991
-Casein gene promoter
activity is regulated by the hormone- mediated relief of transcriptional re-
pression and a mammary-gland-specific nuclear factor. Mol Cell Biol
11:3745–3755
30. Geymayer S, Doppler W 2000 Activation of NF-
B p50/p65 is regulated in the
developing mammary gland and inhibits STAT5-mediated
-casein gene ex-
pression. FASEB J 14:1159 –1170
31. Wallach D, Kovalenko AV, Varfolomeev EE, Boldin MP 1998 Death-inducing
functions of ligands of the tumor necrosis factor family: a Sanhedrin verdict.
Curr Opin Immunol 10:279 –288
32. Bond M, Fabunmi RP, Baker AH, Newby AC 1998 Synergistic upregulation
of metalloproteinase-9 by growth factors and inflammatory cytokines: an
absolute requirement for transcription factor NF-
B. FEBS Lett 435:29–34
33. Yoshizaki T, Sato H, Furukawa M, Pagano JS 1998 The expression of matrix
metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane pro-
tein 1. Proc Natl Acad Sci USA 95:3621–3626
34. Farina AR, Tacconelli A, Vacca A, Maroder M, Gulino A, Mackay AR 1999
Transcriptional up-regulation of matrix metalloproteinase-9 expression during
spontaneous epithelial to neuroblast phenotype conversion by SK- N-SH neu-
roblastoma cells, involved in enhanced invasivity, depends upon GT-box and
nuclear factor kappaB elements. Cell Growth Differ 10:353–367
35. Luo GY, Yu-Lee LY 2000 Stat5b inhibits NF
B-mediated signaling. Mol En-
docrinol 14:114 –123
36. Chen L-H, Bissell MJ 1989 A novel regulatory mechanism for whey acidic
protein gene expression. Cell Regul 1:45–54
37. Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ
1992 A novel transcriptional enhancer is involved in the prolactin- and ex-
tracellular matrix-dependent regulation of
-casein gene expression. Mol Biol
Cell 3:699 –709
38. Sehgal I, Thompson TC 1999 Novel regulation of type IV collagenase (matrix
metalloproteinase-9 and -2) activities by transforming growth factor-
1 in
human prostate cancer cell lines. Mol Biol Cell 10:407– 416
39. Varela LM, Stangle-Castor NC, Shoemaker SF, Shea-Eaton WK, Ip MM, TNF
␣
induces NF
B/p50 in association with the growth and morphogenesis of normal
and transformed rat mammary epithelial cells. J Cell Physiol, in press
2568
TNF REGULATION OF GENE EXPRESSION IN MEC
Endo
• 2001
Vol. 142
• No. 6
Wyszukiwarka
Podobne podstrony:
Abolicja podatkowa id 50334 Nieznany (2)
4 LIDER MENEDZER id 37733 Nieznany (2)
katechezy MB id 233498 Nieznany
metro sciaga id 296943 Nieznany
perf id 354744 Nieznany
interbase id 92028 Nieznany
Mbaku id 289860 Nieznany
Probiotyki antybiotyki id 66316 Nieznany
miedziowanie cz 2 id 113259 Nieznany
LTC1729 id 273494 Nieznany
D11B7AOver0400 id 130434 Nieznany
analiza ryzyka bio id 61320 Nieznany
pedagogika ogolna id 353595 Nieznany
Misc3 id 302777 Nieznany
cw med 5 id 122239 Nieznany
D20031152Lj id 130579 Nieznany
mechanika 3 id 290735 Nieznany
więcej podobnych podstron