
Prace redakcyjne
/
Editorial papers
Mikol. Lek. 2004, 11 (2): 145-151
ISSN 1232-986X
The role of antigen and antibody testing
in the diagnosis of invasive candidiasis
Znaczenie wykrywania antygenów i przeciwcia³ w diagnostyce inwazyjnej dro¿d¿ycy
Guillermo Quindós
1
, María Dolores Moragues
2
, José Pontón
1
1
Departamento de Inmunología, Microbiología y Parasitología, Facultad de Medicina y Odontología, Bilbao, Spain
2
Departamento de Enfermería I, Universidad del País Vasco-Euskal Herriko Unibertsitatea, Bilbao, Spain
The multiple problems presented by the clinical and microbiological diagnosis of invasive candidiasis have prompted the development of tests based
on detection of Candida antigens or/and antibodies against these antigens. To be valuable, these methods must accomplish the difficult task of diffe-
rentiating normal colonization of human mucous membranes by Candida species, particularly Candida albicans, from tissue invasion and candidemia
requiring antifungal therapy. In this article, we review the current status in diagnosis of invasive candidiasis based on the detection of Candida anti-
gens or/and anti-Candida antibodies.
Key words: candidiasis, diagnosis, antigen, antibody, combination
Liczne problemy zwi¹zane z kliniczn¹ i mikrobiologiczn¹ diagnoz¹ inwazyjnej kandydozy przyczyni³y siê do rozwoju testów opartych na wykrywaniu antyge-
nów Candida lub/i przeciwcia³ skierowanych przeciwko tym antygenom. Aby by³y wartociowe, metody te musz¹ spe³niæ trudne zadanie odró¿nienia zwyk³ej
kolonizacji ludzkich b³on luzowych przez gatunki Candida, szczególnie Candida albicans, od inwazji tkanek i kandydemii wymagaj¹cych terapii przeciwgrzybi-
czej. W artykule zosta³ przedstawiony obecny stan diagnostyki inwazyjnej kandydozy w oparciu o wykrywanie antygenów Candida lub/i przeciwcia³ skierowa-
nych przeciwko Candida.
S³owa kluczowe: kandydoza, diagnoza, antygen, przeciwcia³o, kombinacja testów
145
Streszczenie
Abstract
Introduction
Initial studies on serological methods for the diagnosis of
invasive candidiasis began about 50 years ago, when blood
culture detection techniques often failed to recover Candida
organisms (1, 2) and only antibody detection could provide
useful data for the clinician (3). During the following years,
other techniques for the detection of anti-Candida antibo-
dies, Candida antigens and metabolites were developed,
but none of them have got a widespread clinical use. Today,
the situation has changed; blood culture allows a better de-
tection of candidemia (4, 5) and new and sensitive nucleic
acid-based diagnostic tests are being introduced in the labo-
ratory for diagnosing invasive candidiasis (6, 7). Despite these
advances, the problems associated with the laboratory diag-
nosis of invasive candidiasis are far from solved and it will be
difficult to develop a single test with sufficient sensitivity
and specificity to make a definitive diagnosis. Physicians may
need to rely on cumulative information from serial specimens
and diagnostic techniques to have evidence enough to be-
gin antifungal therapy for invasive candidiasis (4, 8-10) and
serological methods may also be used as markers when mo-
nitoring the efficacy of antifungal therapy (8, 11-13). In this
article, we review the current status in diagnosis of invasive
candidiasis based on the detection of Candida antigens
or/and anti-Candida antibodies.
146
Guillermo Quindós, María Dolores Moragues, José Pontón
Mikol. Lek. 2004, 11 (2)
Detection of antigens
A wide variety of assays have been developed for the de-
tection of circulating Candida antigens, including latex ag-
glutination, ELISA, immunoblotting, dot immunoassay, lipo-
somal immunoassay, and RIA (14). Antigens detected by
these assays include mannan and mannoproteins, glucan,
HSP90, enolase, and other immunodominant cytoplasmic
antigens.
Mannan usually circulates in the form of immune com-
plexes which are rapidly cleared by the liver and kidneys.
Mannan concentrations in serum from patients with inva-
sive candidiasis are found in the low nanogram per millili-
ter range (9, 15). Moreover, antigenic differences between
the mannans of different species of Candida, can make
that a test devised to detect Candida albicans mannan
may not be appropriate to detect mannan from Candida
krusei or Candida glabrata (16, 17). These facts ma-
ke mannan detection difficult by serological assays, the-
refore dissociation of the complexes by heat, protease,
acid or NaOH treatments and testing of sequential serum
samples are strategies used to improve the sensitivity of as-
says (9). Mannan antigenemia preceded significant rises
in antibodies against mannan and cytoplasmic antigens
by one to three weeks and it has been found to comple-
ment blood culture for the diagnosis of invasive candidia-
sis in neutropenic cancer patients (18, 19). These au-
thors (20) did not detect mannoprotein in the serum spe-
cimens from 15 patients with hematological malignancies
and proven or probable hepatosplenic candidiasis. Howe-
ver, mannan detection has been detected in cerebrospi-
nal fluid from patients with Candida meningitis and this
test may be valuable the diagnosis of meningeal candidia-
sis (21).
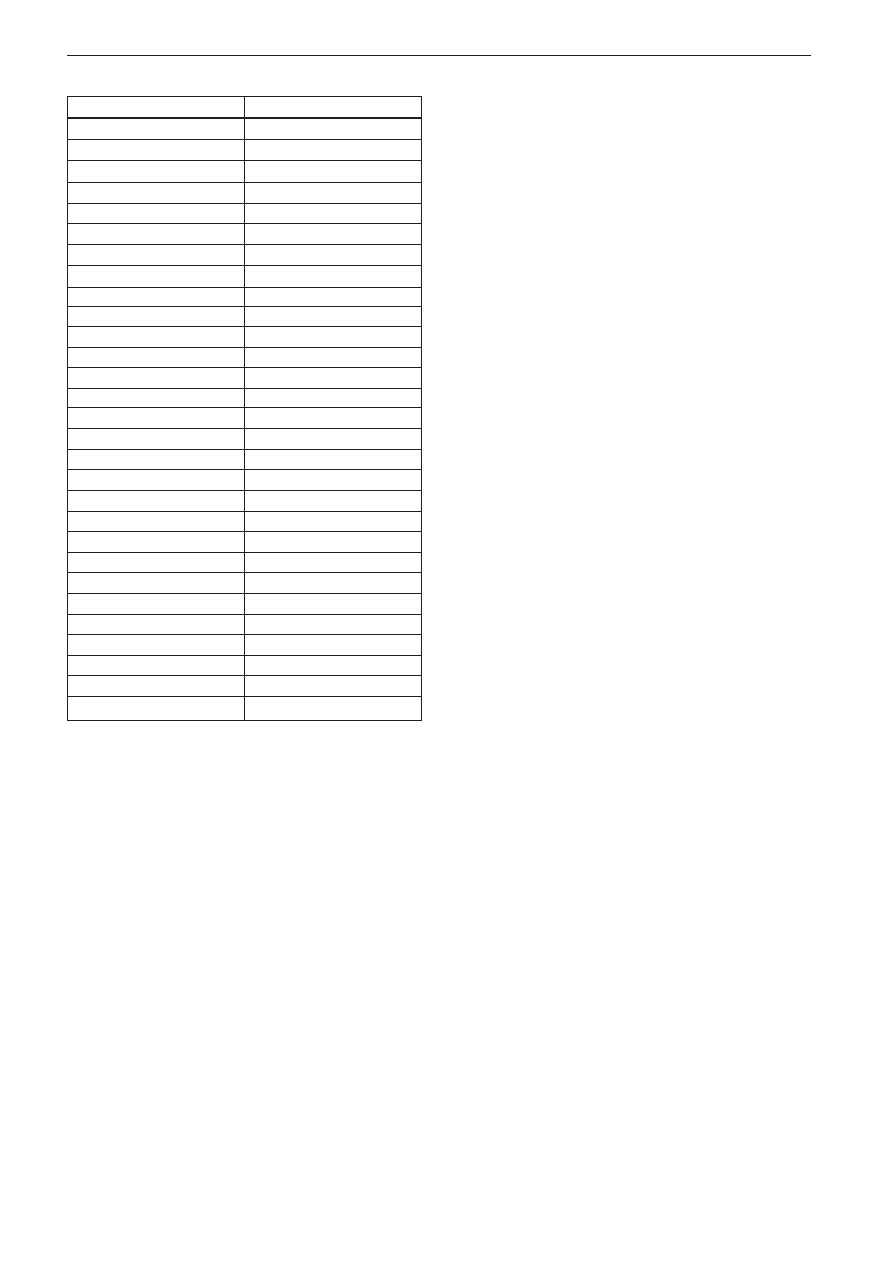
Fig. 1. Detection of antibodies to Candida albicans germ
tube by an indirect immunofluorescence assay.
A. Candida albicans IFA IgG kit (Vircell Laborato-
ries, Spain)
B. Microscopic appearance. Only germ tubes are
fluorescent stained
B
A
147
The role of antigen and antibody testing in the diagnosis of invasive candidiasis
Some tests for mannan detection have been commer-
cialized, thus making possible a broad evaluation (22).
LA-Candida Antigen Detection System (ImmunoMycolo-
gics, USA), based on latex particles coated by polyclonal
anti-mannan antibodies to detect the antigen in sera pre-
treated with heat and protease, has been evaluated by
different groups who found it to be insensitive (11, 23,
24). ICON Candida Assay system (Hybritec Inc., USA)
detects mannan with polyclonal antibodies in an ELISA
format, after dissociation of immune complexes with pro-
tease and heat treatment. Pfaller et al. (25) observed for
this test a sensitivity of 86% and a specificity of 92% in
the diagnosis of invasive candidiasis in a mainly immuno-
competent patient population. Detectable mannanemia
preceded diagnosis by other methods, such as blood
culture, histology, etc., in only 36% of patients. However,
the test tended to complement the diagnosis made on
the basis of positive blood culture.
Cand-Tec test (Ramco Laboratories Inc., USA) is a la-
tex agglutination assay to detect an uncharacterized heat-
labile antigen of C. albicans that does not contain man-
nan and is detected without immune complex dissocia-
tion steps. Although there is a vast literature on the
performance of the Cand-Tec test, there is no consensus
about its value for the diagnosis of invasive candidiasis
because variable results have been reported in different
studies (8, 24, 26-30). Misaki et al. (31) compared
a new presentation, Cand-Tec microtiter system (Cand-
-Tec MT, Ramco, Japan) which expresses Candida anti-
gen level as the cutoff index value by a colorimetric analy-
sis, with the original Cand-Tec for the diagnosis of invasi-
ve candidiasis in 25 patients with hematological diseases.
The sensitivity and specificity of Cand-Tec MT were
100% and 80%, respectively. The cutoff index value de-
creased in 75% of patients responding to antifungal the-
rapy.
Directigen (Becton Dickinson, USA) is a liposomal im-
munoassay to detect enolase antigenemia (32, 33) with
a sensitivity in serum spiked with antigen of 0.5 to 1 mg/l.
The assay was positive in cancer patients with invasive
candidiasis by C. albicans and Candida tropicalis, de-
tecting enolase in 85% of patients with deep tissue infec-
tion and in 64% of patients with fungemia (specificity of
96%). The study documented the transient nature of eno-
lase antigenemia and daily sampling was proposed in fe-
brile patients with neutropenia.
An ELISA was developed to detect C. albicans proteina-
ses, which reach serum concentrations of 0.13 mg/ml and
have a half-life of approximately 60 min (34, 35). How-
ever, the assay showed a low sensitivity and specificity
for the diagnosis of invasive candidiasis. The low sensitivi-
ty may be related to the presence of anti-proteinase anti-
bodies in patients sera which bind the antigen and facili-
tate clearance. The proteinaceous nature of the antigen
precluded a simple disassociation of immune complexes,
as can be achieved with mannan (22). However, Na and
Song (36) developed a monoclonal antibody-based ELISA
inhibition technique to detect a C. albicans secreted
aspartic proteinase and reported a sensitivity of 93.9%
and a specificity of 96% for the diagnosis of invasive can-
didiasis.
Monoclonal antibody EBCA1 coated on latex beads in
the Pastorex Candida test (Bio-Rad, France) has been
used to detect systemic candidiasis. This antibody reacts
with mannans from most common Candida species, such
as C. albicans, C. tropicalis, Candida dubliniensis,
Candida famata, C. glabrata, Candida guilliermondii,
Candida lusitaniae, and Candida parapsilosis (37,38),
but not with mannan from C. krusei (37). Several authors
(30, 33, 39, 40) have reported a sensitivity range of 26-
-60% and a specificity of 100% for the diagnosis of invasive
candidiasis in a mainly immunocompetent adults and chil-
dren. Although sera were treated to dissociate the immu-
nocomplexes with EDTA and heat, the test showed a low
sensitivity in patients who had high titers of anti-mannan an-
tibodies. In an attempt to increase the sensitivity of mannan
detection, an ELISA was developed using EBCA1 (Platelia
Candida Ag, Bio-Rad). Mannanemia was detected on an
average of 6 days before isolation of C. albicans. How-
ever, false-positive reactions have been observed in diffe-
rent groups of patients, as in intensive care patients treated
with hydroxyethyl starch (14).
There is a wide consensus that antigen detection may
be useful for the diagnosis of invasive candidiasis in im-
munodeficient patients as antigen concentration is inde-
pendent of the immunological status of the patient, and
may give an early indication of infection. However, anti-
gen detection may not automatically be the serological
Table I: Cell wall antigens of Candida albicans. Modified from Pontón et al. (14)
Antigen described
Antigen type ()
11C11
II
12B12
I
155 kDa
I
15C9
II
16B1F10
I
180 kDa
I
200 kDa
I
21E6
IV
260
I
2G8
I
3B7
IV
3D9
I
3G6
IV
3H8
III
4E1
I
AF-1
IV
Ag 1.183
II
B9E
III
C3d receptor
II
C6
IV
G3B
IV
Hwp1
I
Mannans
III
Mp58
III
N3B
III
PA10F
II
D9F
IV
14.8
IV
Enolase
IV

148
Guillermo Quindós, María Dolores Moragues, José Pontón
Mikol. Lek. 2004, 11 (2)
test of choice in immunodeficient patients as it may de-
pend on the type of antigen and the mechanisms needed
for its release in infected tissues. Common features ob-
served in all antigen detection assays are low levels of cir-
culating antigens and a transient nature of antigenemia
(10, 18, 19, 41-44). Therefore, antigen detection tests
for the diagnosis of invasive candidiasis would require the
use of sensitive assays and frequent sampling, possibly
on a daily basis in patients at high risk.
Detection of antibodies
Antibody detection-based diagnostic methods must ac-
complish the difficult task of differentiating Candida coloni-
zation of mucous membranes or superficial infection from
candidemia and invasive candidiasis requiring antifungal
therapy (14). Two main limitations are evident, the specifici-
ty of tests may be low because antibody titers can be high in
colonized patients, and tests may present a low sensitivity
because the antibody response may be delayed, reduced
or absent. These limitations can be overcome by improving
the specificity of the tests through the appropriate selection
of antigens (purified molecules, recombinant antigens, etc.)
and sensitivity can be increased with more sensitive techni-
ques, such as the ELISA. A useful test must combine impro-
vements in both sensitivity and specificity, since nothing
is gained from a more sensitive test unless a more specific
antigen is also used (45, 46). There is a general belief that
antibody tests are both insensitive and non-specific (9, 46),
but there is an important evidence suggesting that detection
of antibodies in highly immunodeficient patients, such as
neutropenic bone marrow transplant recipients or liver
transplant recipients, is possible and useful for the diagno-
sis of invasive candidiasis (47-50).
Six different types of antigens have been described in
the C. albicans cell wall (51). Type I antigens are truly
germ tube-specific and are expressed on the germ tube
cell wall surface only. Type II antigens are expressed on
both the germ tube cell surface and within the blastoconi-
dium cell wall. Presence of type II antigens within the bla-
stoconidium cell wall would explain the induction of anti-
bodies to germ tubes in both patients and animals infec-
ted with Candida species unable to produce germ tubes
in serum (52-54). Type III antigens are expressed on
both the blastoconidium and germ tube surface. Type IV
antigens are expressed within the cell wall of both blasto-
conidia and germ tubes (tab. I). Type V antigens are
expressed on the blastoconidium cell surface and within
the germ tube cell wall and type VI antigens are expres-
sed on the blastoconidium surface only.
Mannan is an abundant and highly immunogenic type
III antigen located on the Candida cell wall surface (55)
and anti-mannan antibodies have been detected in many
studies (14). A sensitivity of 64% and a specificity of 97%
in the diagnosis of invasive candidiasis in immunocompe-
tent patients, have been reported for anti-mannan antibo-
dy detection by using a commercially available ELISA
(Platelia Candida Ab, Bio-Rad) in sequential serum sam-
ples (42, 43, 56). Other studies have shown lower dia-
gnostic values (sensitivity, 59% to 91%; specificity, 18%
to 63%) using this and other commercial tests (such as
Candiquant, Biomerica, USA; and Candida albicans ELISA,
Virotech GmbH, Germany) (57).
A mannoprotein of 230-250 kDa located on the germ
tube cell wall surface is recognized by sera from patients
with invasive candidiasis. Our group (14, 58, 59) has de-
veloped an indirect immunofluorescence assay to detect
antibodies (CAGTA) against this antigen present in C. al-
bicans germ tubes that has been useful in the diagnosis
of invasive candidiasis in different groups of patients, inc-
luding intravenous heroin users (60-62), bone marrow
transplant recipients (63), patients with hematological dis-
orders and intensive care patients (12, 63-67). The test
has shown an overall sensitivity of 77-89% and a specifi-
city of 91-100% (48, 58, 59, 68). These results are in
contrast to those obtained when antibodies to C. albi-
cans blastoconidia were detected, since detection of an-
tibodies to C. albicans blastoconidia, which are mainly
directed against mannans, is more sensitive than detec-
tion of CAGTA, but less specific. Sera sequentially drawn
from patients at risk of developing invasive candidiasis
showed CAGTA before the microbiological diagnosis was
made, especially in patients with tissue proven invasive
candidiasis and detection of CAGTA seemed to comple-
ment and even anticipate blood culture (63). Detection of
CAGTA in patients with invasive infections caused by
Candida species other than C. albicans (C. tropicalis,
C. parapsilosis, C. glabrata, C. dubliniensis, C. guil-
liermondii and C. krusei) may also be positive, although
titers are lower than in candidiasis by C. albicans (12,
52, 54, 59, 63, 64, 67, 69, 70). In addition, detection
of CAGTA may be useful for the therapeutic monitoring of
patients with invasive candidiasis, since the administra-
tion of antifungal therapy usually results in decreasing ti-
ters of CAGTA (12, 61, 67, 70).
The test Candida albicans IFA IgG (Vircell Laborato-
ries, Spain) has been recently commercialized for CAGTA
detection (fig. 1). This test has been compared in a retro-
spective study to the standard test in 172 sera from 51
hematological and intensive care patients (123 sera from
32 patients with invasive candidiasis and 49 sera from 19
patients without evidence of infection by Candida). Can-
dida albicans IFA IgG test showed a sensitivity of 84%
and a specificity of 95%, while the standard test showed
a sensitivity of 78% and a specificity of 100%. Results
with both techniques presented a high correlation (R2=
0.951 by patients, R2=0.899 by sera). The commercially
available Candida albicans IFA IgG test was similar to the
test generally used for the detection of CAGTA and provi-
ded faster and easier diagnosis of invasive candidiasis in
the clinical microbiology laboratory (67).
CAGTA present in patients with invasive candidiasis
are likely to be directed against both C. albicans type
I and II antigens (59). Since the germ tube cell wall also
contains mannan, to detect CAGTA the sera have to be
adsorbed, a process that is time consuming and requires
large amounts of heat-killed blastoconidia. A test based
on the detection of antibodies against type I antigens may
not require the adsorption of the sera and would therefo-
re facilitate serodiagnosis of invasive candidiasis. In this
regard, by using an ELISA and purified C. albicans type
I and II antigens, Bikandi et al. (14) detected antibodies
in sera from patients with invasive candidiasis with a sen-
sitivity of 78% and a specificity of 68% without removing
the anti-mannan antibodies from the sera. In a similar stu-
dy, Berdin et al. (71) developed an ELISA to detect IgG

149
The role of antigen and antibody testing in the diagnosis of invasive candidiasis
antibodies against a C. albicans type I antigen in patients
with invasive candidiasis and reported a sensitivity of 78%
and a specificity of 82%.
Candida antigens with enzymatic activity (enolase,
aspartic proteinase and metallopeptidase) have also been
used as targets for antibody detection with controversial re-
sults. Antibody response against enolase has shown a sen-
sitivity of 50 to 92% and a specificity of 86-95% for the dia-
gnosis of invasive candidiasis in immunocompetent patients
and a sensitivity of 53% and a specificity of 78% in immuno-
deficient patients (30, 72, 73). Detection of antibodies aga-
inst a C. albicans secreted aspartic proteinase in patients
with invasive candidiasis had a sensitivity of 70% and a spe-
cificity of 76% (35, 36). Antibodies against a C. albicans
metallopeptidase detected by ELISA showed a sensitivity of
83% and a specificity of 97% (74).
Although in most studies only anti-Candida IgG anti-
bodies were detected, other immunoglobulin classes have
also been investigated in some studies. Aubert et al. (75)
used an immunocapture technique to detect IgM, IgA
and IgE anti-Candida antibodies against somatic antigens
in immunocompetent patients and found that IgA was
a particularly valuable marker of invasive candidiasis. De-
tection of IgA CAGTA showed a higher sensitivity than IgG
or IgM detection in the diagnosis of invasive candidiasis
(58, 60). Gutierrez et al. (33) detected IgM antibodies
to C. albicans whole cells by indirect immunofluorescen-
ce in patients with first time candidemia and reported
a 100% sensitivity and specificity. Kostiala et al. (76) de-
tected rises in titers of IgG and IgA antibodies by ELISA in
sera sequentially drawn from patients with candidemia.
Since the main limitations of antibody detection (low sen-
sitivity and specificity) are especially manifested when only
a single serum sample is studied, detection of antibodies by
commercially available test kits with multiple sequential sera
from the patient at risk for developing invasive candidiasis
should be performed. This will allow a kinetic study of the anti-
body response in the patient who will provide a more reliable
reflection of the development of the infection (12, 42, 77).
Combinations of tests
Combinations of tests for detection of antibodies and an-
tigens (29,44,56,78), antigens and non antigenic compo-
nents such as D-arabinitol, mannose, (1®3)-b-D-glucan,
etc. (8, 30, 79), and antibodies, antigens and non antigenic
components (11, 80, 81) may be useful to overcome the
deficiencies of individual tests. The need for a combination
of tests is particularly evident when a specific antigen and an-
tibodies to this antigen are detected in the same patient, sin-
ce the antibodies may facilitate the clearance of the antigen.
Interestingly, detection of mannan seemed to complement
anti-mannan antibody detection, since patients with antige-
nemia did not have significant levels of anti-mannan antibo-
dies or vice versa, and detection of anti-mannan antibodies
was inversely correlated to the antigenemia. For combined
results of both tests, a sensitivity of 80% to 95% and a speci-
ficity of 53% to 93% have been reported (42-44, 56, 82).
The accuracy of diagnosis of candidemia was increased
when several combinations of tests were used, including
(1®3)-b-D-glucan and mannan detection (8, 30) and enola-
se and (1®3)-b-D-glucan detection (30). Platenkamp et al.
(80) reported a sensitivity of 77% and a specificity of 100%
when a combination of antibody, antigen and D-arabinitol de-
tection was used to differentiate invasive candidiasis from
Candida colonization in immunodeficient patients. However,
Bougnoux et al. (81) did not find the combination of anti-
body, antigen and D-arabinitol assays useful to differentiate
disseminated from peripheral candidiasis.
Conclusions
Substantial progress has been made in diagnosis of in-
vasive candidiasis with the development of a variety of me-
thods for the detection of antibodies and antigens. How-
ever, no single test has found widespread clinical use due
to the difficulties in obtaining consistently reliable serological
diagnosis in all patients with invasive candidiasis and due to
the limited number of commercial assays available. There
is a consensus in the literature that diagnosis based on
a single specimen lacks sensitivity. Therefore, it is neces-
sary to test sequential samples taken while the patient is at
greatest risk for developing invasive candidiasis to optimize
the diagnosis. Testing of serum samples should be started
at patient admission to obtain baseline data and considera-
tion should be given to the fact that different assays may be
needed for the diagnosis of invasive candidiasis. Further-
more, patients should be screened taking into account the
different expected kinetics of each assay (weekly for anti-
body and (1®3)-b-D-glucan detection, and at least twice
a week for antigen and D-arabinitol detection). Results ob-
tained from a panel of diagnostic tests in association with
blood culture findings and clinical aspects of the patient,
will likely be the most useful strategy for early diagnosis of
patients with invasive candidiasis and monitoring of thera-
peutic response.
Authors have been financed by the project IE019 (Diamolfun subproject)
from the Departamento de Industria, Comercio y Turismo del Gobierno Va-
sco-Eusko Jaurlaritza.
References
1. Bodey G.P.: Fungal infections complicating acute leukemia. J. Chro-
nic. Dis., 1966, 19, 667-687.
2. Myerowitz R.L., Pazin G.J., Allen C.M.: Disseminated candidiasis.
Changes in incidence, underlying diseases and pathology. Am.
J. Clin. Pathol., 1977, 68, 29-38.
3. Taschdjian C.L., Seelig M.S., Kozinn P.J.: Serological diagnosis of
candidal infections. CRC Crit. Rev. Clin. Lab. Sci., 1973, 4, 19-60.
4. Walsh T.J., Pizzo P.A.: Laboratory diagnosis of candidiasis. [In:] Can-
didiasis. Pathogenesis, diagnosis and treatment. ed. G.P. Bodey.
2nd ed., Raven Press, New York, 1993, 109-135.
5. Reimer L.G., Wilson M.L., Weinstein M.P.: Update on detection of
bacteremia and fungemia. Clin. Microbiol. Rev., 1997, 10, 444-465.
6. Morace G., Pagano L., Sanguinetti M., Posteraro B., Mele L., Equitani
F., DAmore G., Leone G., Fadda G.: PCR-restriction enzyme analysis
for detection of Candida DNA in blood from febrile patients with he-
matological malignancies. J. Clin. Microbiol., 1999, 37, 1871-1875.
7. Chen S.C.A., Halliday C.L., Meyer W.: A review of nucleic acid-based
diagnostic tests for systemic mycoses with emphasis on polymerase
chain reaction-based assays. Med. Mycol., 2002, 40, 333-357.
8. Kohno S., Mitsutake K., Maesaki S., Yasuoka A., Miyazaki T., Kaku M.,
Koga H., Hara K.: An evaluation of serodiagnostic tests in patients
with candidemia: beta-glucan, mannan, Candida antigen by Cand-
Tec and D-arabinitol. Microbiol. Immunol., 1993, 37, 207-212.
9. Jones J.M.: Laboratory diagnosis of invasive candidiasis. Clin. Micro-
biol. Rev., 1990, 3, 32-45.
10. Walsh T.J., Chanock S.J.: Diagnosis of invasive fungal infections: ad-
vances in nonculture systems. Curr. Clin. Top. Infect. Dis., 1998, 18,
101-153.

150
Guillermo Quindós, María Dolores Moragues, José Pontón
Mikol. Lek. 2004, 11 (2)
11. Bisbe J., Miró J.M., Torres J.M., Latorre X., Alia C., Amaral M., Estivill
D., Mallolas J., Trilla A., Soriano E.: Diagnostic value of serum antibo-
dy and antigen detection in heroin addicts with systemic candidia-
sis. Rev. Infect. Dis., 1989, 11, 310-315.
12. Iruretagoyena J.R., Regúlez P., Quindós G., Pontón J.: Antibodies to
Candida albicans germ tube in two intensive care patients with inva-
sive candidiasis. Rev. Iberoam. Micol., 2000, 17, 93-96.
13. Sigmundsdóttir G., Christensson B., Björklund L.J., Håkansson K.,
Pehrson C., Larsson L.: Urine D-arabinitol/L-arabinitol ratio in diagno-
sis of invasive candidiasis in newborn infants. J. Clin. Microbiol.,
2000, 38, 3039-3042.
14. Pontón J., Moragues M.D., Quindós G.: Non-culture-based diagno-
stics. [In:] Candida and candidiasis. ed. R.A. Calderone. ASM Press,
Washington, D.C., 2002, 395-425.
15. Reiss E., Morrison C.: Nonculture methods for diagnosis of dissemi-
nated candidiasis. Clin. Microbiol. Rev., 1993, 6, 311-323.
16. Nakamura A., Ishikawa N., Suzuki H.: Diagnosis of invasive candidia-
sis by detection of mannan antigen by using the avidin-biotin enzy-
me immunoassay. J. Clin. Microbiol., 1991, 29, 2363-2367.
17. Pavliak V., Sandula J.: Cross-reactivity of pathogenic Candida man-
nans studied by enzyme-linked immunosorbent assay (ELISA) and
precipitin methods. Mycoses, 1988, 31, 34-39.
18. Girmenia C., Martino SP., De Bernardis F., Cassone A.: Assessment
of detection of Candida mannoproteinemia as a method to differen-
tiate central venous catheter-related candidemia from invasive disea-
se. J. Clin. Microbiol., 1997, 35, 903-906.
19. Girmenia C., Micozzi A., Cartoni C., De Bernardis F., Cassone A., Mar-
tino P.: Detection of Candida mannoproteinemia in patients with
neutropenic enterocolitis. Eur. J. Clin. Microbiol. Infect. Dis., 1999,
18, 55-58.
20. Girmenia C., Martino P., De Bernardis F., Boccanera M., Cassone A.:
Lack of circulating Candida mannoprotein antigen in patients with
focal hepatosplenic candidiasis. Med. Microbiol., 2004, 53, 103-106.
21. Lunel F.M.V., Voss A., Kuijper E.J., Gelinck L.B.S., Hoogerbrugge
P.M., Liem K.L., Kullberg B.J., Verweij P.E.: Detection of the Candida
antigen mannan in cerebrospinal fluid specimens from patients su-
spected of having Candida meningitis. J. Clin. Microbiol., 2004, 42,
867-870.
22. Rüchel R.: Diagnosis of invasive mycoses in severely immunosup-
pressed patients. Ann. Hematol, 1993, 67, 1-11.
23. Kappe R.: Coexistence of free antigens, free antibodies and immune
complexes in sera from patients with suspected deep-seated candi-
diasis. Mycoses, 1989, 32, 24-32.
24. Phillips P., Dowd A., Jewesson P., Radigan G., Tweeddale M.G., Clar-
ke A., Geere I., Kelly M.: Non value of antigen detection immunoas-
says for diagnosis of candidemia. J. Clin. Microbiol., 1990, 28,
2320-2326.
25. Pfaller M.A., Cabezudo I., Buschelman B., Bale M., Howe T., Vitug M.,
Linton H.J., Densel M.: Value of the hybritech ICON Candida assay in
the diagnosis of invasive candidiasis in high-risk patients. Diagn. Mi-
crobiol. Infect. Dis., 1993, 16, 53-60.
26. Gentry L.O., Wilkinson I.D., Lea A.S., Price M.F.: Latex agglutination
test for detection of Candida antigen in patients with disseminated
disease. Eur. J. Clin. Microbiol., 1983, 2, 122-128.
27. Tokunaga S., Ohkawa M., Nakashima T., Hisazumi H.: Candida anti-
gen detection by a latex agglutination test in candiduria patients.
Urol. Int., 1992, 49, 163-166.
28. Sánchez, M.L., Pfaller M.A., Cabezudo I., Bale M., Buschelman B.:
Diagnosis of disseminated candidiasis in hospitalized patients using
the Cand-Tec latex agglutination assay. Mycopathologia, 1992, 118,
153-162.
29. Smith K.K., Qadri S.M.H.: Rapid diagnosis of systemic candidiasis
using commercial antigen and/or antibody detection kits. J. Micro-
biol. Methods, 1992, 16, 231-237.
30. Mitsutake K., Miyazaki T., Tashiro T., Yamamoto Y., Kakeya H., Otsubo
T., Kawamura S., Hossain M.A., Noda T., Hirakata Y., Kohno S.: Enola-
se antigen, mannan antigen, Cand-Tec antigen, and b-glucan in pa-
tients with candidemia. J. Clin. Microbiol., 1996, 34, 1918-1921.
31. Misaki H., Iwasaki H., Ueda T.: A comparison of the specificity and
sensitivity of two Candida antigen assay systems for the diagnosis of
deep candidiasis in patients with hematologic diseases. Med. Sci.
Monit., 2003, 9, MT1-MT7.
32. Walsh T.J., Hathorn J.W., Sobel J.D., Merz W.G., Sanchez V., Maret
S.M., Buckley H.R., Pfaller M.A., Schaufele R., Sliva C., Navarro E.,
Lecciones J., Chandrasekar P., Lee J., Pizzo P.A.: Detection of circu-
lating Candida enolase by immunoassay in patients with cancer and
invasive candidiasis. N. Engl. J. Med., 1991, 324, 1026-1031.
33. Gutierrez J., Maroto C., Piédrola G., Martín E., Pérez J.A.: Circulating
Candida antigens and antibodies: useful markers of candidemia.
J. Clin. Microbiol., 1993, 31, 2550-2552.
34. Rüchel R., Böning B.: Detection of Candida proteinase by enzyme
immunoassay and interaction of the enzyme with alpha-2-macroglo-
bulin. J. Immunol. Meth., 1983, 61, 107-116.
35. Rüchel R., Böning-Stutzer B., Mari A.: A synoptical approach to the
diagnosis of candidosis relying on serological antigen and antibody
tests, on culture, and on evaluation of clinical data. Mycoses, 1988,
31, 87-106.
36. Na B.K., Song C.Y.: Use of monoclonal antibody in diagnosis of can-
didiasis caused by Candida albicans: detection of circulating aspar-
tyl proteinase antigen. Clin. Diagn. Lab. Immunol., 1999, 6, 924-929.
37. George E., Garrigues M.L., Poirot J.L., Masini J.P., Meyohas M.C.:
Diagnostic des candidoses systémiques par un test au latex. Résul-
tats dune année de suivi sérologique chez des patients à risque.
J. Mycol. Med., 1991, 1, 25-28.
38. Rimek D., Singh J., Kappe R.: Cross-reactivity of the Platelia Candida
antigen detection enzyme immunoassay with fungal antigen ex-
tracts. J. Clin. Microbiol., 2003, 41, 3395-3398.
39. Herent P., Stynen D., Hernando F., Fruit J., Poulain D.: Retrospective
evaluation of two latex agglutination tests for detection of circulating
antigen during invasive candidosis. J. Clin. Microbiol., 1992, 3,
2158-2164.
40. Rao D.S., Ghosh A., Singhi S., Chakrabarti A.: Mannan antigen detec-
tion in the diagnosis of patients with invasive candidiasis. Indian.
J. Med. Res., 2002, 116, 13-20.
41. Reboli A.C.: Diagnosis of invasive candidiasis by a dot immunobin-
ding assay for Candida antigen detection. J. Clin. Microbiol., 1993,
31, 518-523.
42. Sendid B., Tabouret M., Poirot J.L., Mathieu D., Fruit J., Poulain D.:
New enzyme immunoassays for sensitive detection of circulating
Candida albicans mannan and antimannan antibodies: useful com-
bined test for diagnosis of systemic candidiasis. J. Clin. Microbiol.,
1999, 37, 1510-1517.
43. Yera H., Sendid B., Francois N., Camus D., Poulain D.: Contribution of
serological tests and blood culture to the early diagnosis of systemic
candidiasis. Eur. J. Clin. Microbiol. Infect. Dis., 2001, 20, 864-870.
44. Sendid B., Jouault T., Coudriau R., Camus D., Odds F., Tabouret M.,
Poulain D.: Increased sensitivity of mannanemia detection tests by
joint detection of alpha- and beta-linked oligomannosides during
experimental and human systemic candidiasis. J. Clin. Microbiol.,
2004, 42, 164-171.
45. De Repentigny L., Reiss E.: Current trends in immunodiagnosis of
candidiasis and aspergillosis. Rev. Infect. Dis., 1984, 6, 301-312.
46. De Repentigny L., Kaufman L., Cole G. T., Kruse D., Latgé J.-P., Mat-
thews R. C.: Immunodiagnosis of invasive fungal infections. J. Med.
Vet. Mycol., 1994, 32, Suppl. 1, 239-252.
47. Navarro D., Monzonis E., López-Ribot J.L., Sepúlveda P., Casanova
M., Nogueira J.M., Martínez J.P.: Diagnosis of systemic candidiasis
by enzyme immunoassay detection of specific antibodies to myce-
lial phase cell wall and cytoplasmic candidal antigens. Eur. J. Clin.
Microbiol. Infect. Dis., 1993, 12, 839-846.
48. Villalba R., González A.I., Linares M.J., Casal M., Torres A.: Detection
of antibodies to Candida albicans germ tube as a possible aid in dia-
gnosing systemic candidiasis in bone marrow transplant patients.
Eur. J. Clin. Microbiol. Infect. Dis., 1993, 12, 347-349.
49. Hoppe J.E., Friess D., Niethammer D.: Orointestinal yeast coloniza-
tion of paediatric oncologic patients during antifungal prophylaxis:
results of quantitative culture and Candida serology and comparison
of three polyenes. Mycoses, 1995, 38, 41-49.
50. Klingspor L., Stintzing G., Tollemar J.: Deep Candida infection in child
liver transplant recipients: serological diagnosis and incidence. Acta
Paediatr., 1995, 84, 424-428.
51. Pontón J., Marot-Leblond A., Ezkurra P.A., Barturen B., Robert R., Se-
net J.M.: Characterization of Candida albicans cell wall antigens with
monoclonal antibodies. Infect Immun., 1993, 61, 4842-4847.

151
The role of antigen and antibody testing in the diagnosis of invasive candidiasis
52. Quindós G., Pontón J., Cisterna R., Mackenzie D.W.R.: Value of de-
tection of antibodies to Candida albicans germ tube in the diagnosis
of systemic candidosis. Eur. J. Clin. Microbiol. Infect. Dis., 1990, 9,
178-183.
53. Regúlez P., Arilla M.C., Bikandi J., Quindós G., Cisterna R., Pontón J.:
Identification of antigens reacting with anti-Candida albicans germ
tube antibodies. Eur. J. Epidemiol., 1992, 8, 356-361.
54. Bikandi J., San Millán R., Regúlez P., Moragues M.D., Quindós G., Po-
ntón J.: Detection of antibodies to Candida albicans germ tubes du-
ring experimental infections by different Candida species. Clin.
Diagn. Lab. Immun., 1998, 5, 369-374.
55. Pontón J., Omaetxebarria M.J., Elguezabal N., Alvarez M., Moragues
M.D.: Immunoreactivity of the fungal cell wall. Med. Mycol., 2001,
39 Suppl. 1, 101-110.
56. Sendid B., Poirot J.L., Tabouret M., Bonnin A., Caillot D., Camus D.,
Poulain D.: Combined detection of mannanaemia and antimannan
antibodies as a strategy for the diagnosis of systemic infection cau-
sed by pathogenic Candida species. J. Med. Microbiol., 2002, 51,
433-442.
57. Persat F., Topenot R., Piens M.A., Thiebaut A., Dannaoui E., Picot S.:
Evaluation of different commercial ELISA methods for the serodia-
gnosis of systemic candidosis. Mycoses, 2002, 45, 455-460.
58. Quindós G., Pontón J., Cisterna R.: Detection of antibodies to Candi-
da albicans germ-tube in the diagnosis of systemic candidiasis. Eur.
J. Clin. Microbiol., 1987, 6, 142-146.
59. Pontón J., Quindós G., Arilla M.C., Mackenzie D.W.R.: Simplified ad-
sorption method for detection of antibodies to Candida albicans
germ tubes. J. Clin. Microbiol., 1994, 32, 217-219.
60. Quindós G., Alvarez M., Regúlez P., Pontón J., Cisterna R.: Diagnóstico
de las candidiasis sistémicas en adictos a drogas por vía parenteral
mediante detección de anticuerpos anti-micelio. Med. Clín. (Barc),
1988, 90, 451-455.
61. Quindós G., Rowe I., Higgens C., Pontón J., Cisterna R., Mackenzie
D.W.R.: Candidal infection of the bone. Assessment of serologic te-
sts in diagnosis and management. Diagn. Microbiol. Infect. Dis.,
1990, 13, 297-302.
62. Linares M.J., Javier M.R., Villanueva J.L., Solís F., Torre-Cisneros J.,
Rodríguez F., Kindelán J.M., Casal M.: Detection of antibodies to
Candida albicans germ tubes in heroin addicts with systemic candi-
diasis. Clin. Microbiol. Infect., 2001, 7, 224-226.
63. García-Ruiz J.C., Arilla M.C., Regúlez P., Quindós G., Álvarez A., Po-
ntón J.: Detection of antibodies to Candida albicans germ tubes for
the diagnosis and therapeutic monitoring of invasive candidiasis in
patients with hematologic malignancies. J. Clin. Microbiol., 1997,
35, 3284-3287.
64. Regúlez P., Arilla M.C., García-Ruiz J.C., Moragues M.D., Quindós
G., Pontón J.: Estudio comparativo de dos técnicas para el dia-
gnóstico de la candidiasis invasiva. Enf. Infecc. Microbiol. Clín.,
1995, 13, 229-235.
65. Ibáñez-Nolla J., Nolla-Salas M., León M.A., García F., Marrugat J., So-
ria G., Díaz R.M., Torres-Rodríguez J.M.: Early diagnosis of candidia-
sis in non-neutropenic critically ill patients. J. Infect., 2004.
66. Ibáñez-Nolla J., Torres-Rodríguez J.M., Nolla M., León M.A., Mendez
R., Soria G., Diaz R.M., Marrugat J.: The utility of serology in diagno-
sing candidosis in non-neutropenic critically ill patients. Mycoses,
2001, 44, 47-53.
67. Moragues M.D., Ortíz N., Iruretagoyena J.R., García Ruíz J.C., Amutio
E., Rojas A., Mendoza J., Quindós G., Pontón J.: Evaluación de una
nueva técnica comercializada (Candida albicans IFA IgG) para el
diagnóstico de la candidiasis invasora. Enferm. Infec. Microbiol.
Clín., 2004, 22, 83-88.
68. Torres-Rodríguez J.M., Madrenys-Brunet N., Nolla-Salas J., Carceller
A., Tur C.: Candiduria in non-neutropenic critically-ill surgical pa-
tients. Detection of IgA, IgG and IgM antibodies to Candida albicans
by germ tube immunofluorescence. Mycoses, 1997, 40, 439-444.
69. Quindós G., Reid C.D.L., Mackenzie D.W.R.: Utilidad de la sero-
logía en el diagnóstico de una septicemia porCandida tropicalis.
Rev. Clín. Esp., 1993, 193, 70-72.
70. Salesa R., Moragues M.D., Sota R., Pemán J., Quindós G., Pontón J.:
Specific antibody response in a patient with Candida dubliniensis
fungemia. Rev. Iberoam. Micol., 2001, 18, 42-44.
71. Berdin B., Boux de Casson-Raimbeau F., Marot-Leblond A., Robert R.,
Senet J.M.: Etude préliminaire évaluant lintérêt de lutilisation dan-
tigène purifié (Ag3D9, Ag48) pour le sérodiagnostic des candidoses
profondes par méthode immuno-enzymatique ELISA (Enzyme Lin-
ked Immunosorbent Assay). J. Mycol. Méd., 1995, 5, 140-144.
72. Deventer A.J.M. van, Vliet H.J.A. van, Hop W.C.J., Goessens W.H.F.:
Diagnostic value of anti-Candida enolase antibodies. J. Clin. Micro-
biol., 1994, 32, 17-23.
73. El Moudni B., Rodier M-H, Daniault G., Jacquemin J.L.: Improved im-
munodiagnosis of human candidiasis by enzyme-linked immunosor-
bent assay using a Candida albicans 52-kilodalton metallopepti-
dase. Clin. Diagn. Lab. Immunol., 1998, 5, 823-825.
74. Mitsutake K., Kohno S., Miyazaki T., Miyazaki H., Maesaki S., Koga H.:
Detection of Candida enolase antibody in patients with candidiasis.
J. Clin. Lab. Anal., 1994, 8, 207-210.
75. Aubert D., Puygauthier-Toubas D., Leon P., Pignon B., Foudrinier F.,
Marnef F., Boulant J., Pinon J.M.: Characterization of specific anti-
Candida IgM, IgA and IgE: Diagnostic value in deep-seated infec-
tions. Mycoses, 1996, 39, 169-176.
76. Kostiala I., Kostiala A.A.I., Larinkari U., Valtonen V.V., Miettinen A.: An-
tibodies against antigens of Candida albicans in patients with funga-
emia and bacteraemia, studied by ELISA, precipitation, passive hae-
magglutination and immunofluorescence techniques. J. Med. Micro-
biol., 1981, 14, 483-492.
77. Deventer A.J.M. van, Goessens W.H.F., Zeijl J.H. van, Mouton J.W.,
Michel M.F., Verbrugh H.A.: Kinetics of anti-mannan antibodies use-
ful in confirming invasive candidiasis in immunocompromissed pa-
tients. Microbiol. Immunol., 1996, 40, 125-131.
78. Klingspor L., Stintzing G., Fasth A., Tollemar J.: Deep Candida infec-
tion in children receiving allogeneic bone marrow transplants: inci-
dence, risk factors and diagnosis. Bone Marrow Transplant., 1996,
17, 1043-1049.
79. Chryssanthou E., Klingspor L., Tollemar J., Petrini B., Larsson L., Chri-
stensson B., Ringdén O.: PCR and other non-culture methods for dia-
gnosis of invasive Candida-infections in allogenic bone marrow and
solid organ transplant recipients. Mycoses, 1999, 42, 239-247.
80. Platenkamp G.-J., Duin A.M. van, Porsius J.C., Schouten H.J.A., Zon-
dervan P.E., Michel M.F.: Diagnosis of invasive candidiasis in pa-
tients with and without signs of immune deficiency: a comparison of
six detection methods in human serum. J. Clin. Pathol., 1987, 40,
1162-1167.
81. Bougnoux M.-E., Hill C., Moissenet D., de Chauvin M.F., Bonnay M.,
Vicens-Sprauel I., Pietri F., McNeil M., Kaufman L., Dupouy-Camet J.,
Bohuon C., Andremont A.: Comparison of antibody, antigen, and me-
tabolite assays for hospitalized patients with disseminated or peri-
pheral candidiasis. J. Clin. Microbiol., 1990, 28, 905-909.
82. Sendid B., Caillot D., Baccouch-Humbert B., Klingspor L., Grandjean
M., Bonnin A., Poulain D.: Contribution of the Platelia Candida-speci-
fic antibody and antigen tests to early diagnosis of systemic Candi-
da tropicalis infection in neutropenic adults. J. Clin. Microbiol., 2003,
41, 4551-4558.
Address for correspondence:
Guillermo Quindós
Departamento de Immunología
Microbiología y Parasitología
Fascultad de Medicina y Odontología
Universidad del País Vasco-Euskal Herriko Unibertsitatea
Apartado 699, E-48080 Bilbao, Spain
Tel.: (+34-94) 6012854
Fax: (+34-94) 4649266
e-mail: oipquang@lg.ehu.es
Received: 31-03-2004
Approved: 12-05-2004
Wyszukiwarka
Podobne podstrony:
diagnostyka inwazyjna
Metody wykrywania antygenu D
Diagnostyka inwazyjna w chorobie niedokrwiennej serca
ANTYGENY I PRZECIWCIAŁA
podstawy diagnostyki inwazyjnej w kardiologii
Przeciwcia a przeciwcytrulinowe w diagnostyce RZS
Diagnostyka inwazyjna w chorobach układu krążenia, specjalizacja-TESTY, notatki
Ćw 08 Antygeny i przeciwciała Odczyny aglutynacyjne czynne
diagnostyka inwazyjna
Znaczenie analizy białek PMR w diagnostyce zapalnych układu nerwowego
Otępienie semantyczne znaczenie wywiadu i oceny neurpsychologicznej w diagnostyce różnicowej 2009
Obok napisz wyrazy o znaczeniu przeciwnym, ^ MATERIAŁY (edukacja, terapia i zabawa)
więcej podobnych podstron