
102
Vol.22 No.1 HONG Hua et al: Fabrication and Properties of Multilayer Chitosan
Fabrication and Properties of Multilayer
Chitosan Membrane Loaded with Tinidazole
HONG Hua, CHI Ping, LIU Changsheng*
(Engineering Research Center of Biomedical Materials under Ministry of Education,
East China University of Science and Technology, Shanghai 200237,China)
Abstract: With the aim of providing effective periodontal disease therapeutic method, multilayer
membranes which were loaded with drug for guided tissue regeneration were prepared using an immerse-
precipitation phase inversion technique. Single layer, bi-layer and tri-layer membranes were fabricated with
chitosan used as carrier and tinidazole as medicine model which was loaded on the membrane. The infl uence
of layer on structure and properties of membrane were studied by SEM, UV spectrophotometer and mechanical
test. Drug release properties of three types of layer membranes were also investigated. The results showed that
release rate could be slown down in both bi-layer and tri-layer membranes (to 11 days and 14 days respectively)
and tri-layer membrane lasted the longest. After a process of rapid release, the concentration of tinidazole which
was released by the membrane was maintained at an effi cient dosage level. Compared with single layer and bi-
layer membranes, we found tri-layer membrane could play a role in controlling low-rate drug release especially
at the early stage of release, and keep an effi cient dosage at affected part for a long period of time. The loss of
drug which loaded on membrane decreased from 84.6% for single layer to 13.04% for tri-layer. The mechanical
strength of three types of membrane were detected and showed that it could meet the requiremens of clinical
practice. The membranes especially with tri-layer could be more valuable in application.
Keywords:
chitosan; membrane; multilayer; drug loss; release rate
(Received: June 18, 2005; Accepted: Sept.21,2006)
HONG Hua (洪华): E-mail: hhua64@163.com
*Corresponding author: LIU Changsheng (刘昌胜):Prof.;Ph
D;E-mail:csliu@sh163.net
Supported by the State Outstanding Young Talents Foundation
( No.20425621)
1 Introduction
During the cure process of pericoronitis, the tissue
atrophy which were not expected to occur in many
clinical cases. Gottlow
[1]
pointed out, the main reason
for tissue atrophy was there was not enough space
for tissue regeneration between the root of teeth and
peridontium. Placing carrier membrane was one of
the resolvent. So many types of membranes had been
investigated and used in the treatment of periodontium
disease such as pericoronitis and periodontal ligament
defect. Non-biodegradable membranes which made of
polytetrafl uoroethylene had been used to facilitate tissue
regeneration. For the material was non-biodegradable,
a second surgical procedure was necessary to remove
the membrane. This additional surgical trauma was
a negative effect both to the patient and to the newly
regenerated tissue. To avoid them, the biodegradable
chitosan was considered as the barrier membrane
material. Chitosan was a N-deacetylated product of
chitin which was one of the most polysaccharides
in nature and had good physical, biological and
biodegradable properties. It had been widely used for its
excellent characteristic in biomedical fi eld. In addition
to its biological properties, structural characteristics of
chitosan made it a good scaffold for cell attachment
[2,3]
.
In drug delivery application, chitosan could promote
absorption of drugs and proteins through biological
tissue
[4,5]
.Chitosan was reported to enhance periodontal
tissue regeneration
[6-8]
. In this paper, the chitosan was
chosen as the drug carrier due to its film ability and
degradability. Tinidazole,1-(2-ethylsulfonylethyl)-2-
methyl-5-nitroimidazole, was an anti-parasitic drug
popularly used throughout the world as treatment for
a variety of anaerobic infections in the acute ulcerous
gingivitis, respiratory tract, skin and soft tissues
[9,10]
.
Special anaerobic bacterium was the dominated one
on tooth root infections
[11]
. Tinidazole played as drug
model in the study. For membrane drug delivery
system, it was common that drug release rate boomed
at the early stage which was not benefi cial for disease
cure and it should be avoided. This would be one of
problems improved in this study. With regard to the
immerse-precipitation phase inversion technique, there
was a wet phase separation step which can result in
drug loss loaded on the membrane. Following it could
low the drug efficiency due to part of the tinidazole
dissolved into the immersiong liquid. Because chitosan
can only dissolve in the dilute acid, the process of
preparing chitosan membrane included the step of
DOI 10.1007/s11595-005-1102-6
Journal of Wuhan University of Technology-Mater. Sci. Ed. Feb. 2007
103
alkali neutralization. With the aim of decreasing loss
of drug loaded on the membrane and slowing down the
release rate especially at early stage, we fabricated the
multi-layer membranes including bi-layer and tri-layer
membrane which only one layer, up layer for bi-layer
membrane and central layer for tri-layer membrane,
was loaded with drug. The release characteristic of
various membrane were also investigated and the
surface morphology and mechanical properties were
characterized.
2 Experimental
2.1 Preparing of asymmetric
gradational-
changed porous chitosan membrane
2.1.1 Materials
Chitosan, medical grade with molecular weight
30 KD and 85% degree of deacetylated, was purchased
from Shanghai Kabo Industrial Trade Company.
Polyglycol 6 000, analysis grade, with the average
molecular weight 5 500-7 000 and crystal point
54-57 ℃, white wax-liked solid, was purchased from
Shanghai Chemical Reagent Company.
Glacial Acetic Acid,analysis grade, was purchased
from Shanghai Feida Industrial Trade Co.
Tinidazole, white powder, was purchased from
Zhejiang Kelisian Medicine Manufacture Company.
2.1.2 Cast solution without drug
2.0×10
-2
g/mL chitosan solution was prepared by
dissolving chitosan powder in 1.0%(v/v) glacial acetic
acid aqueous solution overnight and fi ltered by 2
#
sand
core fi ller to get rid of any insoluble material. Filtered
4.0×10
-2
g/mL polyglycol 6 000 aqueous solution
dissolved in water was added to chitosan solution with
a ratio of 50/50 by weight. The mixture was then stirred
for 4 h and defoamed by leaving it quietly for about 1 h
at room temperature.
2.1.3 Cast solution with drug
Tinidazole aqueous solution with concentration
around 2.0 μg/mL were added into the acetic acid
solution with 1.0%(v/v), and the chitosan were
dissolved into the mixing solution to form 2.0×10
-2
g/mL chitosan solution with drug. Following process
was accordance with the preparing of the cast without
drug.
2.1.4 The single layer membrane preparing
30 mL cast solution with drug was poured into
Petri dish (Ф12 cm), which must be washed carefully,
and spread slowly to form an even liquid film. The
solution was pre-vaporized in oven at 50 ℃ for about
60 min. to form membrane, then the membrane was
immersed into the sodium hydroxide solution(1
mol/L)for 24 h to neutralize redundant acetic acid
the resulting membrane was rinsed repeatedly with
deionized water to remove residual NaOH and freezed
dry. The end membrane were kept in desiccator for
characterization.
2.1.5 The bi-layer membrane preparing
15 mL chitosan cast solution without drug was
poured into Petri dish and then placed at a dry oven at
50 ℃ to form the fi rst layer of bi-layer membrane. And
10 mL chitosan cast solution with drug was cast on the
fi rst layer and it was placed in the dry oven at 50 ℃ to
prevaporized for a certain time. Then the membrane
was immersed into the sodium hydroxide solution(1
mol/L )for 24 h the resulting membrane was rinsed
repeatedly with the deionized water to remove the
remaining NaOH and freezed dry. The freeze-dried
membrane were kept in desiccator for characterization.
2.1.6 The tri-layer membrane preparing
10 mL chitosan cast solution without drug was cast
into Petri dish and then placed at the dry oven at 50 ℃
to form the down layer of the tri-layer. 10 mL chitosan
cast solution with drug was cast on the down layer after
it was dry to form the middle layer of the tri-layer. And
then 10 mL cast solution without drug was cast onto the
previous membrane also after it had been dry again to
form the upper layer of the tri-layer. Subsequently the
membrane was immersed into the sodium hydroxide
solution(1 mol/L)for 24 h the resulting membrane
was rinsed repeatedly with the deionized water to
remove the remaining NaOH and freezed dry. The
freeze-dried membrane were kept for further use.
2.2 Characterization of the membrane
2.2.1 Morphology
Scanning electron microscope, SEM, model:JSM-
6360LV made in JEOL. The membrane was prepared
in plot at the size of 1 cm×1 cm. Fixation them with
electric glue, and coated them with golden.
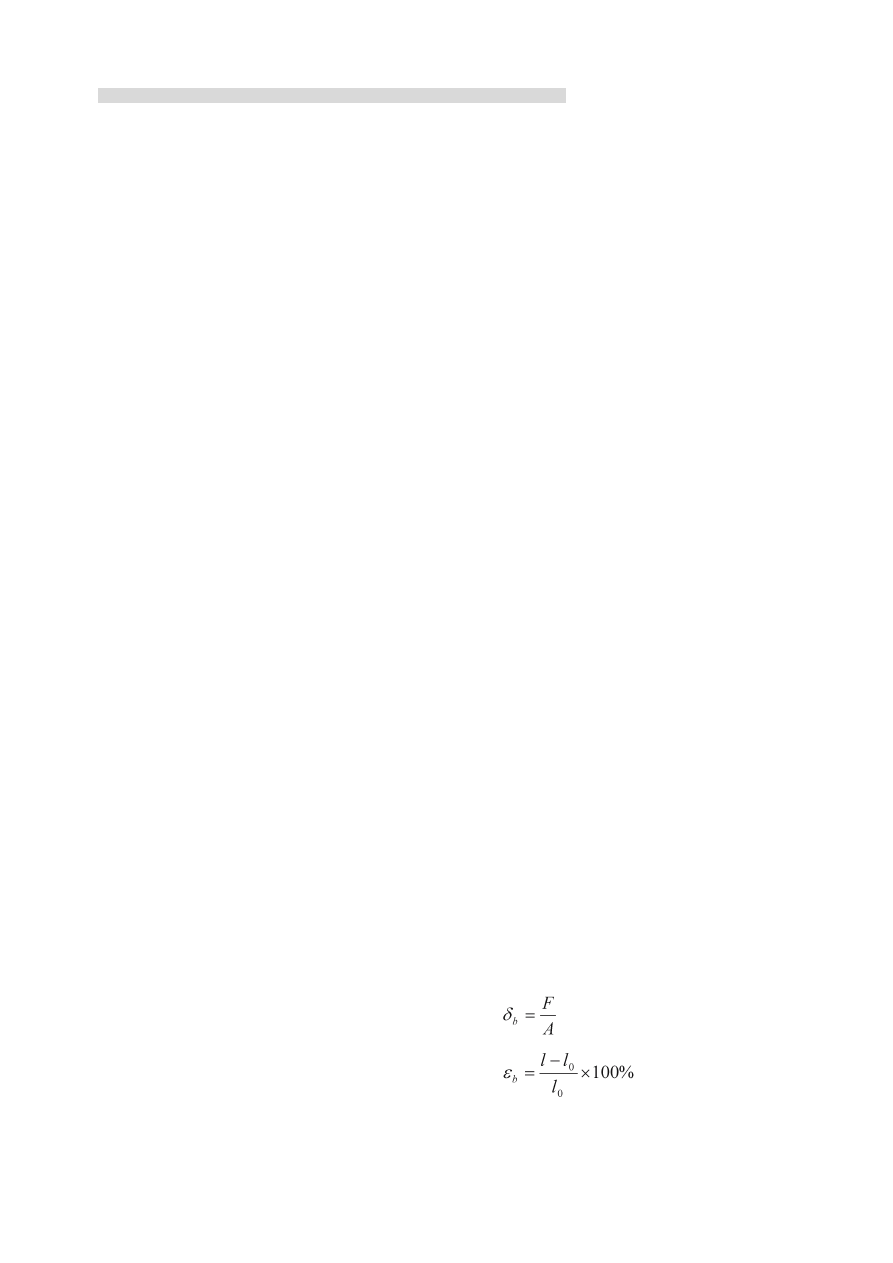
2.2.2 Tensile strength δ
b
) and the elongating rate at
break(ε
b
)
Mechanical properties of the membrane were
tested on a universal testing machine (AG-2000A,
Shimadzu Autograph, Shimadzu Co. Ltd, Japan), the
rate of tensile was 10 mm/min. The samples were cut
into 5 cm×1 cm strips. δ
b
and ε
b
were calculated as
follows:
Where F(N)was load when the membrane broken,
A (m
2
) the initial cross proportion, l
0
the initial length
of the membrane, l length between the measurement
lines when the membrane was broken.
104
Vol.22 No.1 HONG Hua et al: Fabrication and Properties of Multilayer Chitosan
2.2.3 In-vitro biodegradation of membrane
Membrane was cut into about 1×1 cm
2
film
and was pre-weighed. Biodegradation study of the
membranes was conducted in vitro by soaking the fi lms
in phosphate buffer solution (PBS) containing 1.0×10
-4
g/mL trypsin at pH 7.4 and 37 ℃ for 1,4,8,13,19,26
days, respectively. At predetermined time intervals, the
fi lm was removed from the PBS medium, washed with
distilled water and dried in an oven.
The degradable ratio was examined by weight loss
from the formula:
Weight loss =
×100%
Where W
i
was initial dry weight of the sample.
W
f
(t) the weight of sample after a certain time t of
immersion. Each experiment was repeated three times
and the average value was taken as the weight loss.
2. 3 Release properties
in vitro
2.3.1 The choice of the wavelength
Weighed tinidazole accurately which had kept at
constant weight by dried at 100 ℃ dry oven. The 100
μg·mL
-1
drug concentration solution was prepared.
After scanning from the wavelength 200-400 nm with a
ultraviolet-visible spectrophotometer, the result showed
that tinidazole had the max absorbability at 317 nm;
and the blank chitosan solution had no absorbability at
317 nm after scanning from the wavelength 200-400
nm. So the wavelength 317 nm was chosen as the
determine wavelength.
2.3.2 The solubility of drug on the membrane
Weighed membrane (m) was immersed in a
supersaturated solution of tinidazole at 37 ℃ in
culture box with rotating motion(250 rpm) for 48 h.
After the drug had been saturated in the membrane,
the membrane was picked out from the drug solution
and washed with deionized water to remove excess
drug on the surface. Then the membrane was placed
in redistilled water bath incubator with rotating
motion to be carried out drug release. The membrane
was picked out from the water every 24 h and drug
concentration C
i
of the release solution was tested at
a maximum wavelength of 317 nm for tinidazole with
the ultraviolet-visible spectrophotometer until the drug
was released completely. The solubility of drug on the
membrane was calculated as follows:
S=[ ∑(C
i
×5 mL) ] / m
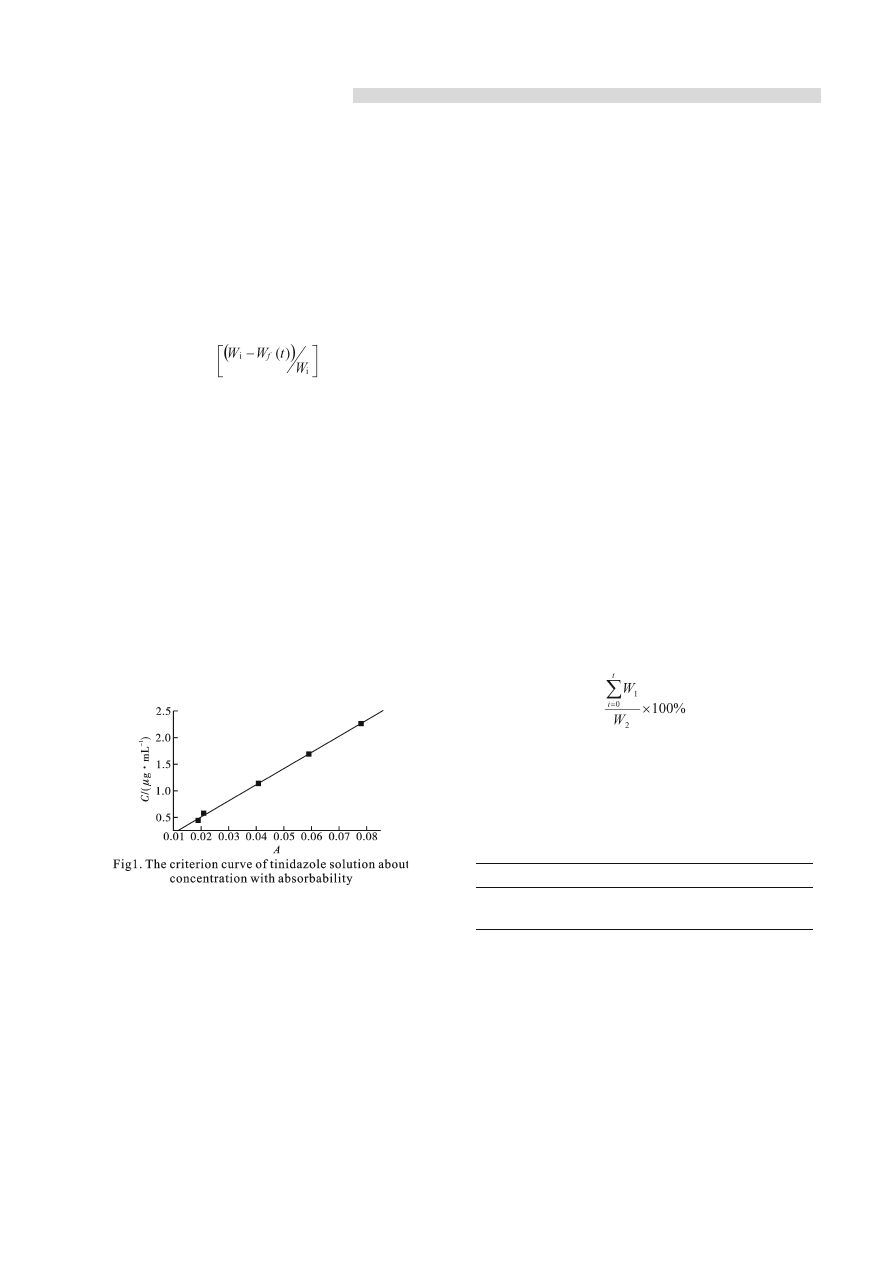
2.3.3 The criterion curve
Weigh 113.4 mg tinidazole accurately which
had been at constant weight by drying at dry oven
at 100 ℃. Tinidazole was put into 1 000 content
flask and diluted into the scale level with deionized
water. 1.00 1.25 2.50 3.75 5.00 mL drug solution was
measured accurately, was put into 250 mL content
flask respectively and was diluted into the flask scale
with shaking up. 2 mL solution was taken out for
determining the absorbability at 317 nm. The linearity
regress formula about absorbability (A) and the
concentration (C) as follows:
C=30.35×A-0.10 (r=0.996 n=5)
The result showed that for tinidazole the
concentration and the absorbability present good
linearity relationship.
2.3.4 Measurement for accumulated release rate of
tinidazole
Chitosan drug membrane which had been weighed
in advance was placed into the weighing bottle in
which there was 5 mL release media. Place it into the
constant temperature culture oven at 37 ℃, picked out
the sample at same interval period. Measurement the
absorbability of the sample via UV spectrophotometer
at 317 nm. The accumulated tinidazole in vitro was
calculated as follows:
accumulated =
Where W
1
was TNZ sample accumulated drug
release amount with t period, W
2
was TNZ sample drug
content.
3 Results and Discussion
3.1 The influence of layer on mechanical
properties
Playing a role as supporting barrier, the adequate
mechanical strength was required for chitosan drug
membrane when placed at the affected part. In the
meantime, the flexibility and adhesive were expected
for clinical application.
Table 1 Infl uence of chitosan membrane layer on the
mechanical property
Single layer
Bilayer
Trilayer
Tensile strength/MPa
Elongation rate/%
7.088
39.108
3.63
34.189
1.88
22.866
Journal of Wuhan University of Technology-Mater. Sci. Ed. Feb. 2007
105
Table 1 showed that tensile strength and
elongation rate were decreased as increasing layer
of the membrane. And the tensile strength decreased
faster than the elongation rate. The tensile strength for
the single layer membrane was 7.09 MPa, and tensile
strength for the trilayer membrane was 1.88 MPa. The
max tensile strength for human periodontal ligament
was 5.04×10
4
Pa appeared at the tooth cervix
[12]
. So the
strength of membrane made on this paper can meet the
need of practicality.
The reasons resulted in the decreasing of
membrane tensile strength and elongation rate as the
increase of the membrane layer were the focus stress.
During the complex process of membrane, each layer
covered the other one layer by layer. Generally, the
membranes were acceptable in structural integrity
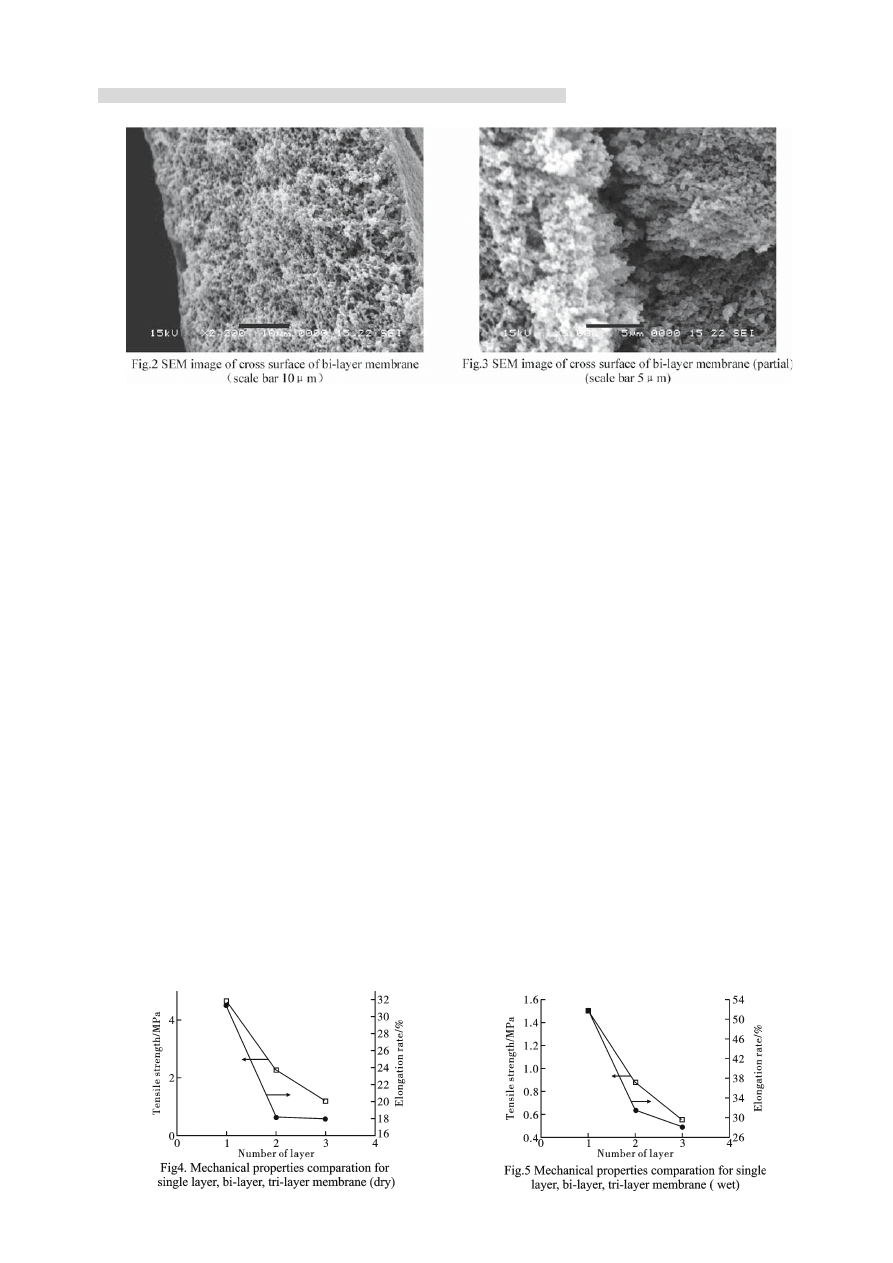
(Fig.2 ), but there was still defects in some individual
parts(Fig.3). There were some deficiency at interface
between two layers, the internal structure could not
change smoothly like a single layer one. Fig.2, the
SEM photos of cross membrane, showed that there was
internal crackle deficiency obviously at the interface
place. The stress focus resulted from the crackle
deficiency could result in membrane broken more
easily when it was extended, so the tensile strength
decreased. Tri-layer membrane experienced double
time superposition, so the interface defi ciency occurred
more easily and resulted the decrease of tensile strength
further.
3.2 The infl uence of layer on the mechanical
property of dry-wet state
For most research work about membrane, the
determination of mechanical property was focused on
wet state which was more available for preserving and
test. But the final application environment was inside
the human body which was with body fl uid. Upon this
reason, the mechanical properties of wet and dry state
were test respectively in this paper.
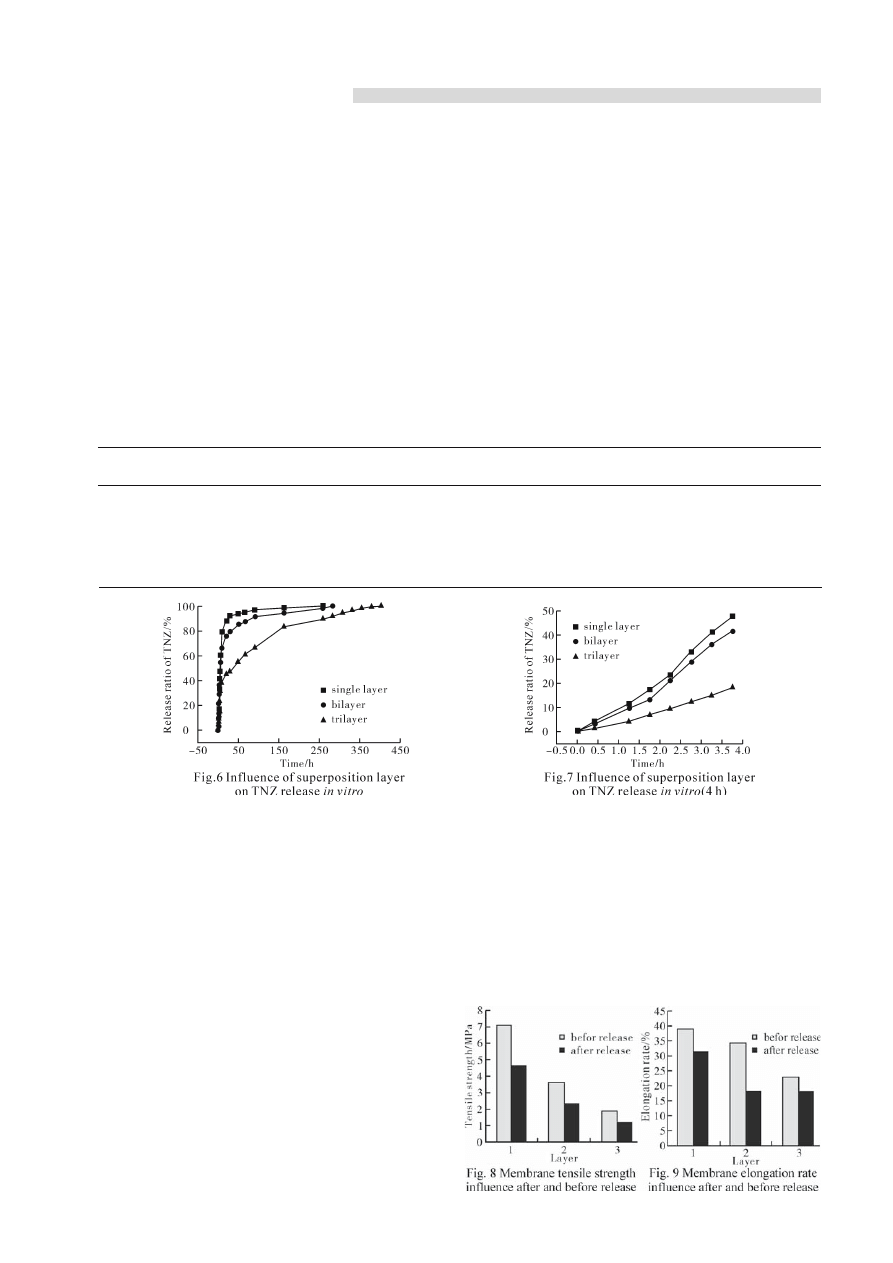
Figs.4,5 showed, for single, bi-layer and tri-layer
membrane, the change trend for the different state on
wet and dry were coherent. The tensile strength in dry
was stronger than that in wet, the elongation rate in
wet was stronger than that in dry. The reason was the
chitosan molecular arrayed more compactness in dry
membrane than that in wet one, the strength was larger
because the water molecular played a role as plastic
agent in wet. It made the chitosan molecular chain
moved about easily. So the tensile strength was higher.
3.3 Influence of layer on the property of
degradation
in vitro
Generally, the integrity of films taken from the
PBS medium was preserved. And on the surface of the
fi lms there were no signifi cant changes such as cracks,
rough and other signs of deformation.
Table 2 showed the influence of superposition
layer on weight loss. The data indicated that the
effect among different layers on weight loss were not
distinct. For each type of three ones, there was a slight
reduction in the weight loss. The fi lms still maintained
its physical form without obvious weight loss after 26
106
Vol.22 No.1 HONG Hua et al: Fabrication and Properties of Multilayer Chitosan
days soaking.
The weight loss data were in agreement with the
overall characteristic of the appearance of fi lms. That
means chitosan membrane may degradated slowly in
PBS containing trypsin. To account for length of the
paper, the study on degradation of chitosan membrane
should be discussed in detail in another paper.
3.4 Release characterization
in vitro
3.4.1 Infl uence of layer on the release property
Fig.6 was the contrast curves of three type of layer
membrane on the accumulated drug release during the
period of entirely release process. It showed the bi-
layer membrane compared with the single layer had
lower release rate, but the decrease extend was not very
obvious. Moreover, the whole time it took to fi nish the
release process for bi-layer membrane was close to that
for single membrane. For tri-layer membrane, the drug
release rate slowed down at a great extend and it was
close to the constant release especially at early stage
(Fig.7).
For single layer and bi-layer membrane, the
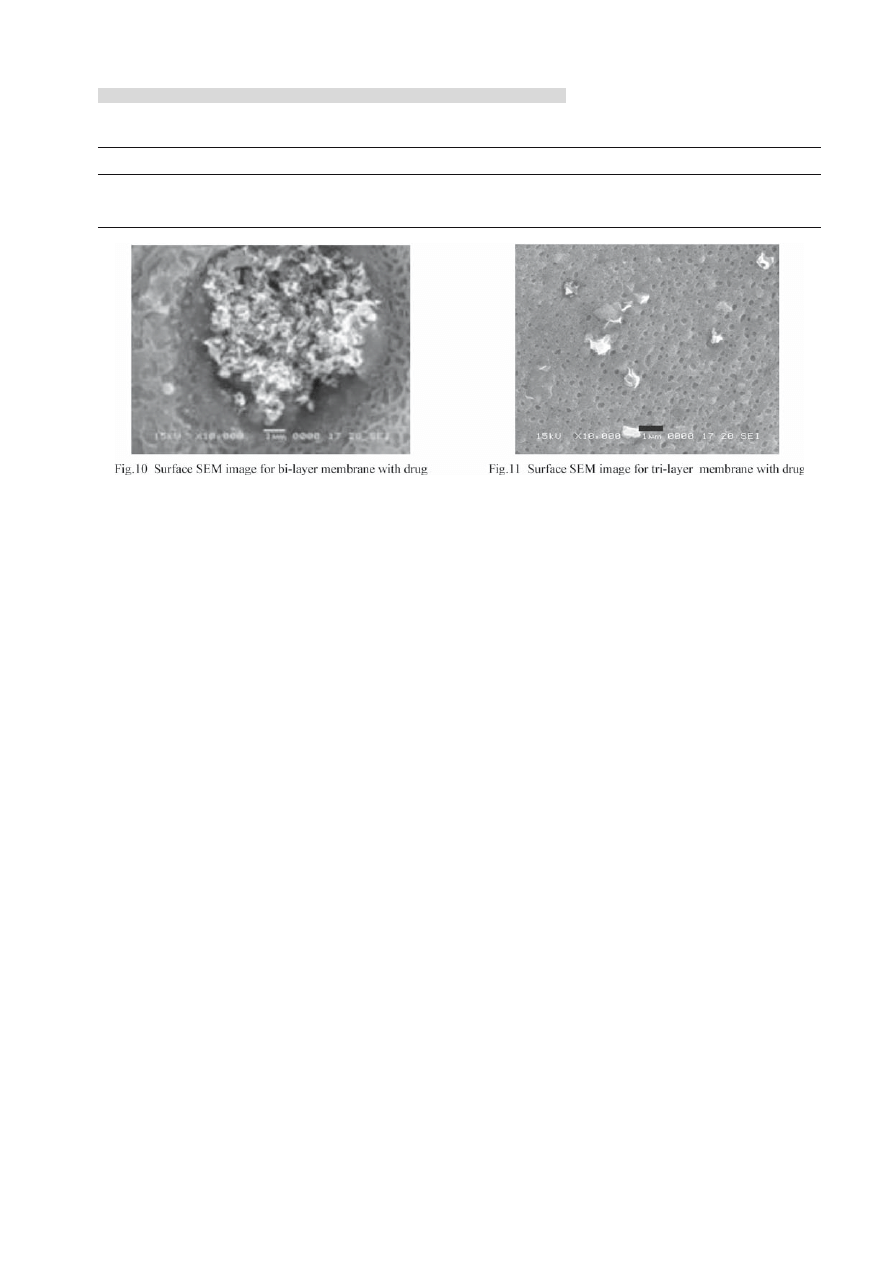
forepart release rates were fast because the loading
content on the membrane far more exceeded the drug
saturation solubility on the membrane. The drug
molecular present in form both of assemble state
dispersing in membrane and molecular state dissolving
in the membrane. There was many accumulated
tinidazole particle on the membrane surface(Fig.10)
, when the membranes were immersed into the
release media, the accumulated tinidazole particle
Table 2 Infl uence of superposition layer on weight loss/wt%
Layer
Time /day
single layer
bi layer
tri layer
1
4
8
13
19
26
1.96
5.56
9.72
12.28
18.53
24.10
5.44
8.45
9.58
11.76
18.79
23.89
1.49
6.60
9.03
11.14
18.42
23.73
would dissolve and diffuse into the media directly
and rapidly. The process of dissolving and diffusing
occurred simultaneity, so the drug release was fast
comparatively. Subsequently, tinidazole molecular in
form of molecular state among molecular state diffused
into the membrane surface and then dissolved into the
media. Tinidazole molecular in form of assemble state
among membranes dissolved into the membrane and
continued diffuse into membrane surface and dissolved
into media. It took two steps including dissolving
firstly and diffusing into media for drug, so it would
take a longer time, the release rate was slower. Among
the three types of layer membrane, tri-layer membrane
performanced well at drug release slowing down and
could meet the practical need in application.
3.4.2 Influence of release on membrane mechanical
property after one-week release in media
From the release curve of single layer membrane,
after the membrane had been immersed in the media for
one week and the drug release fi nished, the mechanical
characteristic decreased. Chitosan was degradable
biomaterial which would result in chitosan membrane
collapsing as it was used. If the membrane collapsed
before the drug release did not fi nished completely, it
would not benefit the control of drug release. So the
mechanical characteristic should be considered after
the drug release had conducted for a while. Figs.8 and
9 showed, although the tensile strength and elongation
rate decreased, the remaining strength could also meet
Journal of Wuhan University of Technology-Mater. Sci. Ed. Feb. 2007
107
Table 3 Infl uence of superposition layer on drug loss
Layer
Original drug content (mg/g)
Accumulated drug release(mg/g)
Drug loss(mg/g)
Drug loss content/%
Single layer
bilayer
trilayer
20
10
6.67
3.08
2.92
5.80
16.92
7.08
0.87
84.60
70.80
13.04
the demand for practical application because the max
tensile strength for human periodontal ligament was
5.04×10
4
Pa appeared at the tooth cervix
[12]
.
3.4.3 Infl uence of superposition layer on the drug loss
of membrane
Table 3 showed that drug loss decreased
sequencely for the single layer, bi-layer and tri-layer.
Compared with single layer membrane, the drug loss
changed a little bit for bi-layer, they all surpassed 50%,
but the drug loss decreased a lot for tri-layer, it was
28.04%.
For tri-layer membrane, the layer with drug
was in the middle, there were not many drug on the
surface (Fig.11) comparing to single layer and bi-layer
membrane (Fig.10). With blank layer covered upon
up-layer and down-layer, when tri-layer membrane
experienced the immersion process, the drug loss
decreased greatly. So tri-layer membrane could lowed
down the drug loss.
4 Conclusions
The aim of using membrane loaded drug to replace
the traditional drug delivery was for maintaining the
effective drug concentration on the periodontal box
for relatively long time. The results showed that the
concentration of tinidazole was maintained at an
effective dosage. The release rate could be slown down
to both bi-layer and tri-layer membranes (11 days and
14 days respectively) and the drug release lasted longer.
For tri-layer membrane, the release rate was close to
the constant release at the early stage. And the drug loss
loaded on membrane decreased greatly from 84.6%
for single layer to 13.04% for tri-layer. They present
mechanical strength to meet the demand of the clinical.
But the degradability of chitosan was not expected and
should be studied further. The easy method of drug
interlayer based on sandwich was feasible for slowing
down the early release rate and decreasing the drug
loss.
References
[1] Gottlow, J S Nyman, J Lindhe, et al. 1986 New Attachment
Formation in the Human Periodontium by Guided Tissue
Regeneration: Case Reports[J]. J. Clin. Periodontal,1986,13:604
-616
[2] Mukherjee DP, Tunkle AS, Roberts RA, et al. An Animal
Evaluation of a Paste of Chitosan Glutamate and Hydroxyapatite
as a Synthetic Bone Graft Material[J]. J. Biomed. Mater. Res.,
2003,67B: 603-609
[3] VandeVord PJ, Matthew HW, DeSilva SP, et al. Evaluation of
the Biocompatibility of a Chitosan Scaffold in Mice[J]. J.
Biomed. Mater. Res., 2002,59: 585-590
[4] Nsereko S, Amiji M. Localized Delivery of Paclitaxel in Solid
Tumors from Biodegradable Chitin Microparticle Formulations.
[J] Biomaterials, 2002,23: 2 723-2 731
[5] Singla AK, Chawla M. Chitosan. Some Pharmaceutical and
Biological Aspects - An Update[J]. J. Pharm Pharmacol, 2001,
53: 1 047-1 067
[6] Senel S, Kremer MJ, Kas S, et al. Enhancing Effect of Chitosan
on Peptide Drug Delivery across Buccal Mucosa[J].
Biomaterials, 2000, 21: 2 067-2 071
[7] Madihally SV, Matthew HW. Porous Chitosan Scaffolds for
Tissue Engineering[J]. Biomaterials, 1999, 20:1 133-1 142
[8] Park JS, Choi SH, Moon IS, et al. Eight Week Histological
Analysis on the Effect of Chitosan on Surgically Created One-
wall Intrabony Defects in Beagle Dogs[J]. J. Clin Periodontol,
2003, 30: 443-453
[9] V I Levina,L A Trukhacheva. Investigation of the No-donor
Activity of the Antimicrobial Drug Tinidazole[J]. Pharmaceutical
Chemistry Journal,2004,38,1:15-18
[10] S V Emshanova, A P Zuev. Composition and Technology of
Tinidazole Core Tablets[J]. Pharmaceutical Chemistry Journal,
2004,38,11:628-631
[11] Brook I, Frazier EH, Thompson DH. Aerobic and Anaerobic
Microbiology of Periapical Abscess[J]. Oral Microbiol.
Immunol., 1991,6(1):123-124
[12] Xiu Wencui. The Research of Periodontal Tissue Stress
Distribution under the Orthoraedic Force and the Control of
Orthoraedic Curve on the Tooth Moving[D]. Nanjing: Dongnan
University,2003
Wyszukiwarka
Podobne podstrony:
3 Analiza firmy 2015 (Kopia powodująca konflikty (użytkownik Maciek Komputer) 2016 05 20)
Maciek Ogorzałka
Maciek1
Maciek3
program wczesnego wspomagania MACIEK, Dokumenty(1)
osnowa nr 8 maciek
Maciek11
kez5 maciek
maciekzagadnienia
Nie Ma Takich Gór Maciek Starnawski
Maciek 30
Maciek Kubryn Ms Word
wycieczka maciek
Maciek63, studia
Referat TQM Maciek
pkm wal maciek
raport maciek
Maciek UPN
Maciek21
więcej podobnych podstron