See discussions, stats, and author profiles for this publication at:
https://www.researchgate.net/publication/51568451
The Role of Spinal Manipulation, Soft-Tissue
Therapy, and Exercise in Chronic Obstructive
Pulmonary Disease: A Review of the Literature
and Proposal of an Anatomical Explanation
Article
in
Journal of alternative and complementary medicine (New York, N.Y.) · August 2011
Impact Factor: 1.59 · DOI: 10.1089/acm.2010.0517 · Source: PubMed
CITATIONS
12
READS
91
2 authors:
10
PUBLICATIONS
77
CITATIONS
94
PUBLICATIONS
1,116
CITATIONS
All in-text references
underlined in blue
are linked to publications on ResearchGate,
letting you access and read them immediately.
Available from: Roger Engel
Retrieved on: 08 May 2016

The Role of Spinal Manipulation, Soft-Tissue Therapy,
and Exercise in Chronic Obstructive Pulmonary Disease:
A Review of the Literature and Proposal
of an Anatomical Explanation
Roger Engel, DO, DC,
1
and Subramanyam Vemulpad, PhD
2
Abstract
The premise that lung function can regulate chest wall mobility is an accepted concept. Descriptions of the
primary and accessory respiratory structures do not usually include spinal components as a part of these
classifications. The case for including these components as a part of the respiratory mechanism and their role in
the development of dyspnea and chest wall rigidity in chronic obstructive pulmonary disease (COPD) is re-
viewed. Mechanical impairment of the chest wall is a contributing factor in the prognosis of COPD. Reducing
this impairment improves prognosis. Because spinal manipulation and soft-tissue therapy increase joint mobility
and decrease muscle hypertonicity, respectively, applying these interventions to the chest wall in COPD could
reduce chest wall rigidity, thereby improving breathing mechanics. Improvements in breathing mechanics re-
duce the work of the respiratory muscles and delay the onset of dyspnea. Exercise capacity is reliant on the
ability to overcome activity-limiting dyspnea, which usually occurs prior to maximum exercise capacity being
reached. Delaying the onset of dyspnea permits more exercise to be performed before dyspnea develops. Spinal
manipulation and soft-tissue therapy have the potential to deliver such a delay. Because exercise tolerance is
considered to be a strong predictor of quality of life and survival in COPD, any increase in exercise capacity
would therefore improve prognosis for the disease.
Introduction
T
he idea that lung function
can regulate chest wall
mobility is not a new concept.
1
Most descriptions of
normal respiration recognize the necessity for adequate chest
wall movements.
In the 1980s, Forkert found that the relative distribution of
inspired gas to a lung region was dependent on the mobility
of the overlying chest wall, concluding that regional lung
function was dependent on regional rib cage excursion.
2
More recently, O’Donnell showed that localized restriction of
the chest wall during exercise induced severe dyspnea in
otherwise healthy individuals.
3
The concept is not limited to
healthy individuals. For example, even in the absence of lung
or pleural disease, people with rheumatoid arthritis develop
changes in rib cage mobility, which alters breathing me-
chanics and results in a decrease in lung volume and the
development of dyspnea.
4
In ankylosing spondylitis, a sim-
ilar decrease in spinal mobility results in a loss of chest wall
mobility and a fall in lung vital capacity.
5
At the other end of the spectrum, increasing chest wall
mobility can also affect lung function. A number of studies
have shown that increasing thoracic joint mobility improves
lung function in the short term, in normal individuals.
6–8
The scenario is applicable in the presence of respiratory
disease as well. Chest percussion and vibration assist spu-
tum removal and improve lung ventilation in chronic ob-
structive pulmonary disease (COPD) and bronchiectasis.
9,10
Paraspinal muscle inhibition, rib raising, myofascial release,
and thoracic lymphatic pump have been used with some
success in the management of COPD
11
and pneumonia in the
elderly.
12,13
High-frequency chest wall compression im-
proves respiratory function in patients with neuromuscular
disease,
14
COPD,
15,16
and cystic fibrosis.
17
Spinal and costal
joint manipulation has some benefit for lung function and
quality of life in patients with COPD.
18–20
1
Department of Chiropractic, Macquarie University, Sydney, New South Wales, Australia.
2
Faculty of Science, Macquarie University, Sydney, New South Wales, Australia.
THE JOURNAL OF ALTERNATIVE AND COMPLEMENTARY MEDICINE
Volume 17, Number 9, 2011, pp. 797–801
ª Mary Ann Liebert, Inc.
DOI: 10.1089/acm.2010.0517
797
Following a biomechanical model, descriptions used to
explain these results include impeded inspiratory muscle
action,
2,21,22
rib cage restriction and stiffness,
4,18,20
and better
pulmonary secretion clearance resulting from improved air-
flow velocities.
23
Other explanations refer to the short-term
beneficial effects on breathing frequency resulting from re-
laxation exercises
24,25
and improvements in respiratory cili-
ary function following mechanical chest vibration.
26
Missing from these explanations is a description of the
changes that occur in some of the spinal structures that make
up the chest wall, as mobility is altered. The objectives of this
article are to review the effect of spinal manipulation, soft-
tissue therapy, and exercise on increasing lung function in
COPD, and to propose an anatomical basis for this effect.
Functional Anatomy of the Chest Wall in COPD
In healthy individuals, an increase in respiratory demand
is met by normal compensatory responses. Most descriptions
of the respiratory system, in this context, focus on the pri-
mary and secondary respiratory structures. In COPD,
breathing mechanics become distorted beyond the body’s
ability to manage respiratory demand with normal com-
pensatory responses using these structures. This results in
additional structures becoming involved. In this article, these
additional structures will be referred to as the ‘‘reserve’’ re-
spiratory structures, as they are always at hand, but not
traditionally included within the classifications of primary or
accessory respiratory structures. They include spinal mus-
cles, joints of the thoracic spine, and connective tissues as-
sociated with these structures.
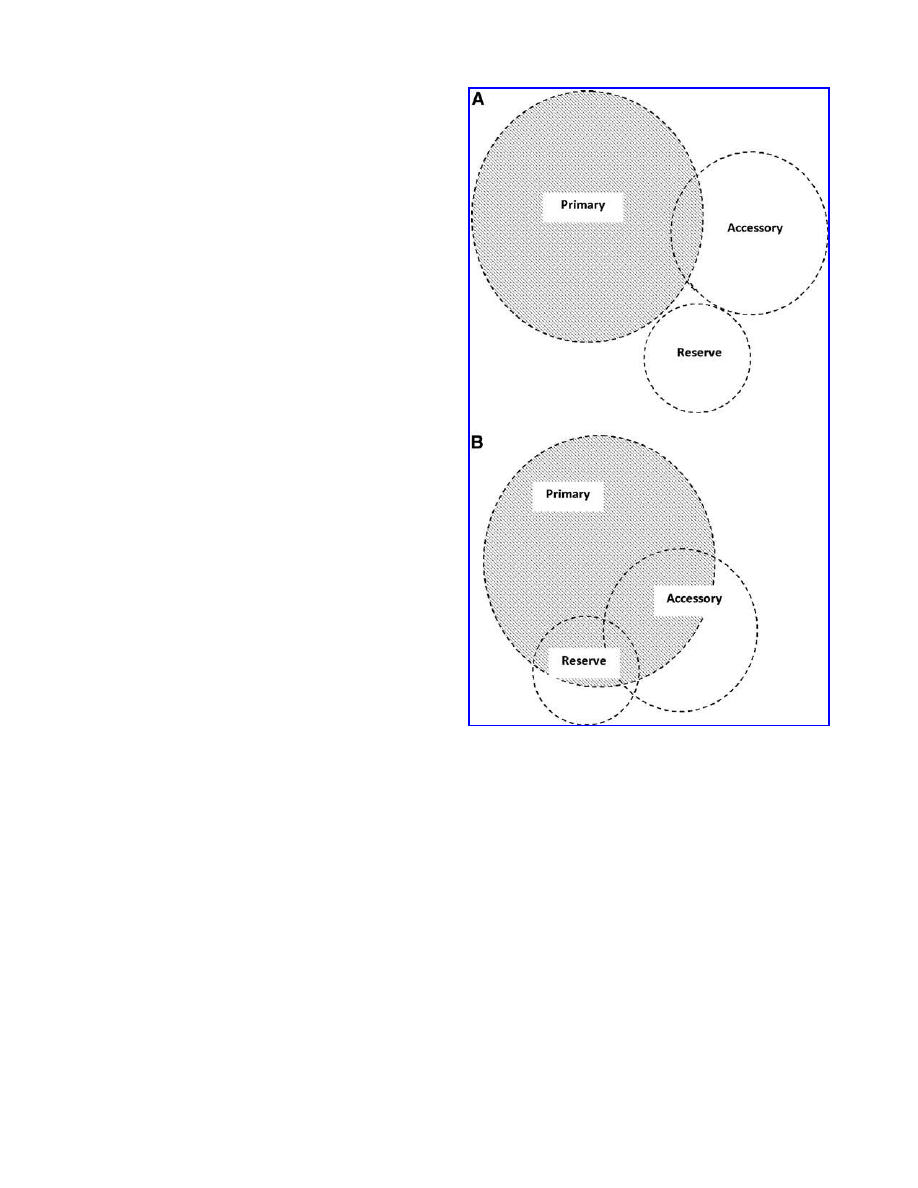
Possible role of ‘‘reserve’’ respiratory structures
in ‘‘normal’’ breathing
During normal breathing at rest, the reserve respiratory
structures are not active (Fig. 1A). Under increased load
conditions, such as deep inspiration or forced expiration,
they may become active as part of normal breathing.
The configuration of the erector spinae muscle creates
multiple links between the posterior rib cage and the lumbar
spine and sacrum.
27,28
Acting as a group, contraction of the
erector spinae causes extension of the spine; individually,
subgroups have the ability to depress several ribs during
forced expiration.
The erector spinae aponeurosis is made up from tendons of
longissimus thoracis pars thoracis and iliocostalis lumborum
pars thoracis. As movement of the aponeurosis is independent
from the rest of erector spinae,
27
these muscles are able to act
as independent stabilizers of the posterior thoracic cage.
Multifidus is responsible for the segmental ‘‘rocking’’
component of extension in the spine.
27
This ability enables it to
assist in creating additional rib elevation in the later stages of
deep inspiration by creating movement in the costovertebral
and costotransverse joints through extension of the vertebrae.
The thoracolumbar fascia links the anterior abdominal wall
and the muscles of the lower back, via the lateral raphe.
27
This
configuration enables it to assimilate multidirectional forces
arising from the abdomen, lumbar muscles, and lumbar spine
and highlights its dynamic role in breathing.
Thoracic vertebrae articulate with more than one rib on
either side. Each rib is connected to two adjacent vertebrae
and their intervening intervertebral disc at multiple points.
As a result, each vertebra operates as the functional center of
a number of ribs. The constant distractive force created by
the intervertebral disc’s ability to resist deformation,
27
to-
gether with the inherent elastic nature of the ribs, produces
tension in the posterior joints of the chest wall (i.e., costo-
vertebral and costotransverse joints). These forces are bal-
anced by the capsular, sternocostal, and intra-articular
ligaments anteriorly. Together with the muscles, these
structures govern the level of chest wall recoil, or rigidity, as
the case may be.
Role of ‘‘reserve’’ respiratory structures in COPD
In COPD, there is a loss of lung elastic recoil, which results
in an increase in airway resistance, reducing dynamic
FIG. 1.
Primary, accessory, and ‘‘reserve’’ respiratory
structures involved in breathing at rest. Shaded areas rep-
resent involvement of the respective structures. A. Normal
individuals. B. Individuals with chronic obstructive pulmo-
nary disease. The proposal in (B) is tentative in that the ex-
tent of involvement of Accessory and Reserve structures is
unknown at this time.
798
ENGEL AND VEMULPAD

pulmonary compliance.
29
As a result, additional air is left in
the lungs at the end of expiration, leading to an increase in
end-expiratory volume. Chest wall expansion occurs, forcing
the inspiratory muscles to operate at nonoptimal lengths,
reducing their maximal contractile force.
30
The reduced
contractile force means the workload of these muscles in-
creases substantially during breathing. They adapt to the
situation by remodeling,
31
shortening in length and in-
creasing in tone.
As attempts to reduce end-expiratory volume continue,
air-trapping occurs, resulting in lung hyperinflation.
32–34
Hyperinflation pushes the sternum anteriorly and causes a
loss in thoracolumbar spine mobility, not dissimilar to what
occurs in ankylosing spondylitis or rheumatoid arthritis. The
erector spinae is stretched, pulling the aponeurosis taut,
further limiting movement of the lower ribs. As a result, the
ability of the quadratus lumborum to act as a stabilizer of
the diaphragm during deep inspiration
28
is compromised.
The need to recruit the abdominal muscles in order to im-
prove respiratory function increases the pull on the thor-
acolumbar fascia via the lateral raphe. This has an additional
impact on the lower rib cage’s ability to expand.
Within 4–6 weeks, the diaphragm adapts by dropping
sarcomeres and shortening its operational length.
35,36
This
restores its force-generating capacity but reduces its ability to
undergo displacement.
37
As a result, expansion of the lower
rib cage, which usually occurs as part of diaphragmatic
movement early in normal inspiration, is compromised. In-
spiration becomes less efficient while breathing becomes
more dependent on rib cage inspiratory muscle activity.
38
However, inspiratory muscle activity is already compro-
mised due to the changes brought about by hyperinflation.
In severe cases, contraction of the diaphragm produces a
decrease rather than an increase in the transverse diameter of
the lower rib cage.
39,40
Additional rib elevation is provided by multifidus. How-
ever, this action is set against a backdrop of increasing chest
wall rigidity.
Compared to normal breathing, the reserve, accessory,
and primary respiratory structures are all active in patients
with COPD, even when breathing at rest (Fig. 1B).
The role of ‘‘reserve’’ respiratory structures in dyspnea
Dyspnea is one of the main symptoms in COPD.
9
Its origin
is multifactorial, with part of the cause attributable to
changes in chest wall mechanics.
22,41
As hyperinflation con-
tinues, the force needed by the respiratory muscles to
counterbalance the inward recoil of the lung and chest wall
at the end of expiration becomes substantial.
21
This addi-
tional effort accelerates the onset of inspiratory muscle fa-
tigue and dyspnea.
42,43
Rationale for Increasing Chest Wall Mobility
Increasing chest wall rigidity reduces chest wall move-
ments during breathing. Any contribution that these move-
ments might have in promoting lung elasticity would be
diminished. As lung elasticity is a function of lung capacity,
increasing chest wall rigidity has a detrimental effect on lung
capacity. In a similar manner, decreasing chest wall rigidity
could have a beneficial effect on lung capacity. In support of
this view, the level of mechanical impairment of the chest
wall has been identified as a contributing factor in the
prognosis of COPD.
41,44
Spinal manipulation, soft-tissue therapy, and exercise
Spinal manipulation increases joint mobility while soft-
tissue therapy decreases muscle hypertonicity.
45,46
Applying
spinal manipulation and soft-tissue therapy to a single ver-
tebral joint complex would increase joint motion and de-
crease muscle tone locally. Applying these interventions at
multiple levels would alter the overall contribution the
posterior elements make toward chest wall mobility. In
the case of COPD, an increase in the mobility of any of the
posterior components of the chest wall would lessen chest
wall rigidity. The overall impact on chest wall rigidity would
be enhanced as a reciprocal flow-on effect occurred in the
anterior components of the chest wall. Decreasing chest wall
rigidity permits an increase in inspiratory muscle lengths,
improving their efficiency and reducing the level of muscle
fatigue. If independently reducing contractile respiratory
muscle effort improves dyspnea in COPD,
22
then decreasing
chest wall rigidity should produce a similar effect.
In COPD, exercise intolerance is considered to be a strong
predictor of quality of life and survival.
47
Increasing exercise
tolerance would therefore improve these outcomes. Exercise
capacity in COPD is reliant on the ability to overcome activity-
limiting dyspnea, which usually occurs prior to maximum
exercise capacity being reached.
21,22
As mentioned earlier,
spinal manipulation and soft-tissue therapy increase joint
mobility and decrease muscle hypertonicity, respectively.
45,46
Increasing chest wall mobility through spinal manipulation
and soft-tissue therapy could have a role in reducing dyspnea
levels and increasing exercise capacity, thereby improving
prognosis of the condition. This warrants further investigation.
Conclusions
When normal respiratory mechanics become compro-
mised beyond the body’s ability to manage respiratory de-
mand with normal compensatory responses, structures not
usually associated with normal breathing are recruited to
assist with breathing. These ‘‘reserve’’ respiratory structures
include spinal muscles and joints and connective tissues as-
sociated with these structures. Improving the mobility of
these ‘‘reserve’’ structures may produce a flow-on effect in
other respiratory structures, resulting in an overall decrease
in chest wall rigidity and a delay in respiratory muscle fa-
tigue. The role of spinal manipulation and soft-tissue therapy
in reducing chest wall rigidity and improving dyspnea in
COPD merits consideration and further research.
Acknowledgments
The authors would like to thank Sharyn Eaton for helpful
comments during the preparation of this article.
Disclosure Statement
No competing financial interests exist.
References
1. Guyton AC, Hall JE. Textbook of Medical Physiology, 11th
ed. Philadelphia: Elsevier, 2006:474.
MANUAL THERAPY AND EXERCISE IN COPD
799
2. Forkert L. Effect of regional chest wall restriction on regional
lung function. J Appl Physiol 1980;49:655–662.
3. O’Donnell DE, Hong HH, Webb KA. Respiratory sensation
4. Begin R, Randoux V, Cantin A, Menard HA. Stiffness of the
rib cage in a subset of rheumatoid patients. Lung
1988;166:141–148.
5. Fisher LR, Cawley MI, Holgate ST. Relation between chest
6. Miller JA, Bulbulian R, Sherwood WH, Kovach M. The effect
7. Gosling C, Williams KA. Comparison of the effects of tho-
racic manipulation and rib raising on lung function of
asymptomatic individuals. J Osteopath Med 2004;7:103.
8. Engel R, Vemulpad S. The effect of combining manual
9. Australian Lung Foundation. The COPDX Plan: Australian
and New Zealand Guidelines for the Management of Chronic
Obstructive Pulmonary Disease 2010. Online document at:
www.copdx.org.au/ Accessed on October 19, 2010.
10. Yang M, Yuping Y, Yin X, et al. Chest physiotherapy for
pneumonia in adults. Cochrane Database Syst Rev 2010;
2:CD006338.
11. Noll DR, Johnson JC, Baer RW, Snider EJ. The immediate
12. Noll DR, Shoves J, Bryman PN, Masterson EV. Adjunctive
osteopathic manipulative treatment in the elderly hospital-
ized with pneumonia: A pilot study. J Am Osteopath Assoc
1999;99:143–146.
13. Noll DR, Shores JH, Gamber RG, et al. Benefits of osteo-
14. Lange DJ, Lechtzin N, Davey W, et al. High-frequency chest
wall oscillation in ALS: An exploratory randomized, con-
trolled trial. Neurology 2006;67:991–997.
15. Piquet J, Brochard L, Isabey D. High frequency chest wall
oscillation in patients with chronic air-flow obstruction. Am
Rev Respir Dis 1987;136:1355–1359.
16. Sivasothy P, Brown L, Smith IE, Shneerson JM. Effect of
manually assisted cough and mechanical insufflations on
cough flow of normal subjects, patients with chronic ob-
structive pulmonary disease (COPD), and patient with re-
spiratory muscle weakness. Thorax 2001;56:438–444.
17. Arens R, Gozal D, Omlin KJ, et al. Comparison of high fre-
quency chest compression and conventional chest physio-
therapy in hospitalized patients with cystic fibrosis. Am J
Respir Crit Care Med 1994;150:1154–1157.
18. Howell RK, Allen TW, Kapler RE. The influence of osteo-
19. Masarsky CS, Weber M. Chiropractic management of
chronic obstructive pulmonary disease. J Manip Physiol
Ther 1988;11:505–510.
20. Noll DR, Degenhardt BF, Johnson JC, Burt SA. Immediate
21. O’Donnell DE, Laveneziana P. Dyspnea and activity limi-
tation in COPD: Mechanical factors. J COPD 2007;4:225–236.
22. O’Donnell DE, Ora J, Webb KA, et al. Mechanisms of ac-
tivity-related dyspnea in pulmonary diseases. Respir Physiol
Neurobiol 2009;176:116–132.
23. King M, Phillips DM, Gross D, et al. Enhanced tracheal
mucus clearance with high frequency chest wall compres-
sion. Am Rev Respir Dis 1983;128:511–515.
24. Renfroe KL. Effect of progressive relaxation on dyspnea and
25. Gift AG, Moore T, Soeken K. Relaxation to reduce dyspnea
and anxiety in COPD patients. Nurs Rev 1992;41:242–246.
26. Thomas J, DeHueck A, Kleiner M, et al. To vibrate or not to
vibrate: Usefulness of the mechanical vibrator for clearing
bronchial secretions. Physiother Can 1995;47:120–125.
27. Bogduk N. Clinical anatomy of the lumbar spine, 4th ed.
Philadelphia: Churchill Livingstone, 2005:97–121.
28. Standring S, ed. Grays Anatomy: The Anatomical Basis of
Clinical Practice, 39th ed. London: Elsevier, 2005:765–767.
29. Aliverti A, Stevenson N, Dellaca R, et al. Regional chest wall
volumes during exercise in chronic obstructive pulmonary
disease. Thorax 2004;59:210–216.
30. Hill NS. Noninvasive ventilation for chronic obstructive
pulmonary disease. Respir Care 2004;49:72–87.
31. Guyton AC, Hall JE. Textbook of Medical Physiology, 11th
ed. Philadelphia: Elsevier, 2006:82.
32. Decramer M, Jiang TX, Demedts M. Effects of acute hyper-
inflation on chest wall mechanics in dogs. J Appl Physiol
1987;63:1493–1498.
33. Decramer M. Effects of hyperinflation on the respiratory
muscles. Eur Respir J 1989;2:299–302.
34. Martinez FJ, Couser JI, Celli BR. Factors influencing venti-
35. Farkas G. Functional characteristics of the respiratory mus-
cles. Semin Respir Med 1991;12:247–257.
36. Sinderby C, Beck J, Spahija J, et al. Voluntary activation of
the human diaphragm in health and disease. J Appl Physiol
1998;85:2146–2158.
37. Rochester DF. The diaphragm in COPD: Better than ex-
pected, but not good enough. NEJM 1991;325:961–962.
38. De Troyer A. The respiratory muscles. In: Crystal EG, ed.
The Lung: Scientific Foundation. Philadelphia: Lippincott
Raven, 1997:1203–1215.
39. Gilmartin JJ, Gibson GJ. Abnormalities of chest wall motion
in patients with chronic airflow obstruction. Thorax
1984;39:264–271.
40. Gilmartin JJ, Gibson GJ. Mechanisms of paradoxical rib cage
motion in patients with chronic obstructive pulmonary dis-
ease. Am Rev Respir Dis 1986;134:684–687.
41. Pride NB, Macklem PT. Lung mechanics in disease. In:
Fishman AP, ed. Handbook of Physiology, Section 3, vol III,
Part 2: The Respiratory System. Bethesda: American Phy-
siological Society, 1986:659–692.
42. Leblanc P, Summers E, Inman MD, et al. Inspiratory muscles
during exercise: A problem of supply and demand. J Appl
Physiol 1988;64:2482–2489.
43. Harver A, Mahler DA, Daubenspeck JA. Targeted inspira-
tory muscle training improves respiratory muscle function
800
ENGEL AND VEMULPAD

and reduces dyspnea in patients with chronic obstructive
pulmonary disease. Ann Intern Med 1989;111:117–124.
44. Ranieri VM, Giuliani R, Cinnella G, et al. Physiologic effects
of positive end-expiratory pressure in patients with chronic
obstructive pulmonary disease during acute ventilatory
failure and controlled mechanical ventilation. Am Rev Re-
spir Dis 1993;147:5–13.
45. Bogduk N, Mercer S. Selection and application of treatment.
In: Refshauge K, Gass E, eds. Musculoskeletal Physiother-
apy: Clinical Science and Evidence-Based Practice, 2nd ed.
Oxford: Butterworth-Heinemann, 2004:253.
46. Bialosky JE, Bishop MD, Robinson ME, George SZ. The
mechanisms of manual therapy in the treatment of muscu-
loskeletal pain: A comprehensive model. Manual Ther
2009;14:531–538.
47. Martinez FJ, Foster G, Curtis JL, et al. for the NETT Research
Group. Predictors of mortality in patients with emphysema
and severe airflow obstruction. Am J Respir Crit Care Med
2006;173:1326–1334.
Address correspondence to:
Roger Engel, DO, DC
Department of Chiropractic
Macquarie University
Balaclava Road, North Ryde, Sydney
New South Wales 2109
Australia
E-mail: roger.engel@mq.edu.au
MANUAL THERAPY AND EXERCISE IN COPD
801

Wyszukiwarka
Podobne podstrony:
JACM Engel Vemulpad 2011 1 1
2011 2 KOSZE
higiena dla studentów 2011 dr I Kosinska
Plan pracy na 2011 pps
W 8 Hormony 2010 2011
wm 2011 zad 2
Zawal serca 20 11 2011
PRK 23 10 2011 org
PIW 4z 2011
pmp wykład podmioty 2011 2012
perswazja wykład2 2011 Zasady skutecznej perswazji Petty & Cacioppo
2011 Leki przeciwgrzybicze Kopiaid 27453 ppt
NIEDOKRWISTOŚCI SEM 2011 2012
więcej podobnych podstron