
Structure Determination by NMR
I. Choose a biologically important question.
II. Determine if and how NMR can address the question.
III. Synthesize or extract the molecule to study.
IV. Design the NMR study.
V. Make the NMR sample(s).
VI. Acquire and process the NMR data.
VII. Extract information relevant to your question or hypothesis.
VIII. Report your findings.

Hepatitis B Virus
The Disease
• Member of the hepatocellular DNA virus
family
• 300 million people worldwide are carriers.
• Symptom of infection vary but usually
involve inflamation of the liver and sometimes
liver damage.
• 90% of the people who contract the virus
will go through an acute phase of infection
and then recover with lasting immunity.
• 10% of the people who contract the disease
do not resolve the primary infection and
become carriers.
• Those that have the chronic infection have
a 100-fold or greater risk of hepatocellular
carcinoma (liver cancer).
The Hepatitis B Virus Genome
5’
5’
+
-
RNA
Protein
Plus strand
+
5’-GGCAGAGGTGAAA-3’
3’-CCGTCTCCACTTT-5’
Direct Repeat Sequence
~3.2 kilobases

The Hepatitis B Virus
Direct Repeat Sequence
5’-GGCAGAGGTGAAA-3’
3’-CCGTCTCCACTTT-5’
I. Performs a critical role in the initiation of
viral DNA
synthesis which is not
completely understood.
II. Deletion or mutation of just one residue can
be
catastrophic to virus.
III. Small enough to be studied by NMR.
IV. Are there any unique structural features
that can give us
insight into biological
activity?
V. The sequence will have an extra base-pair
on each end.
Review of DNA Structure
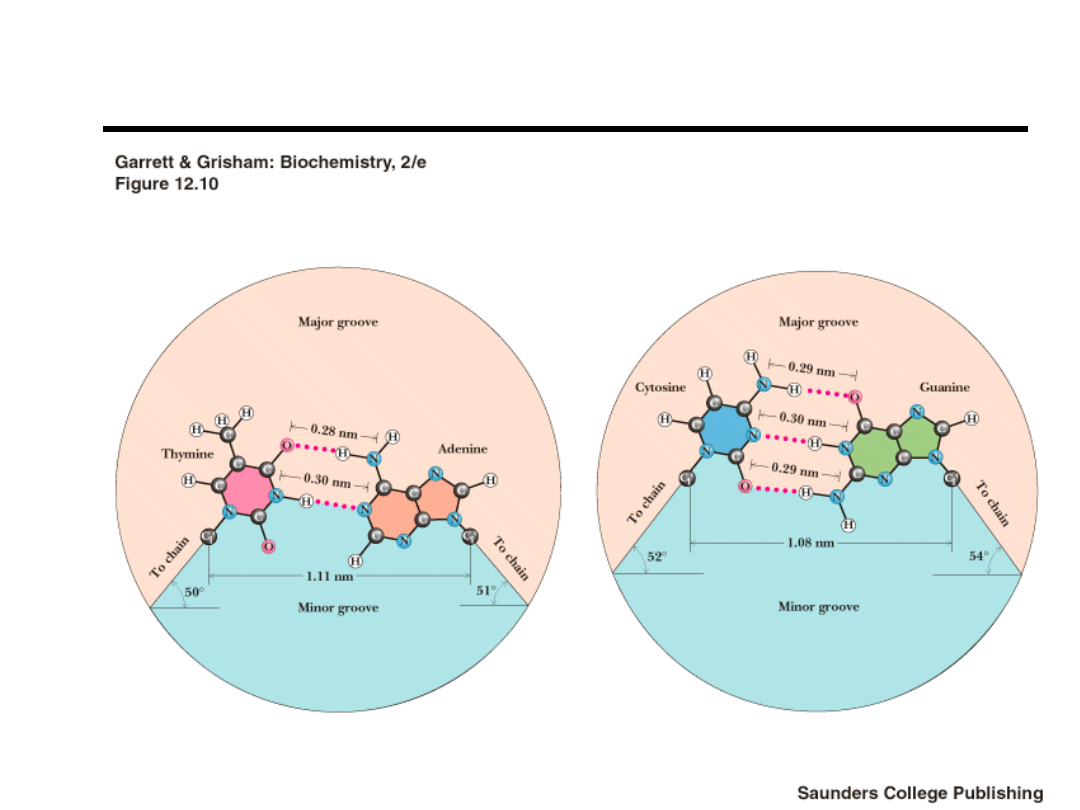
Review of DNA Structure
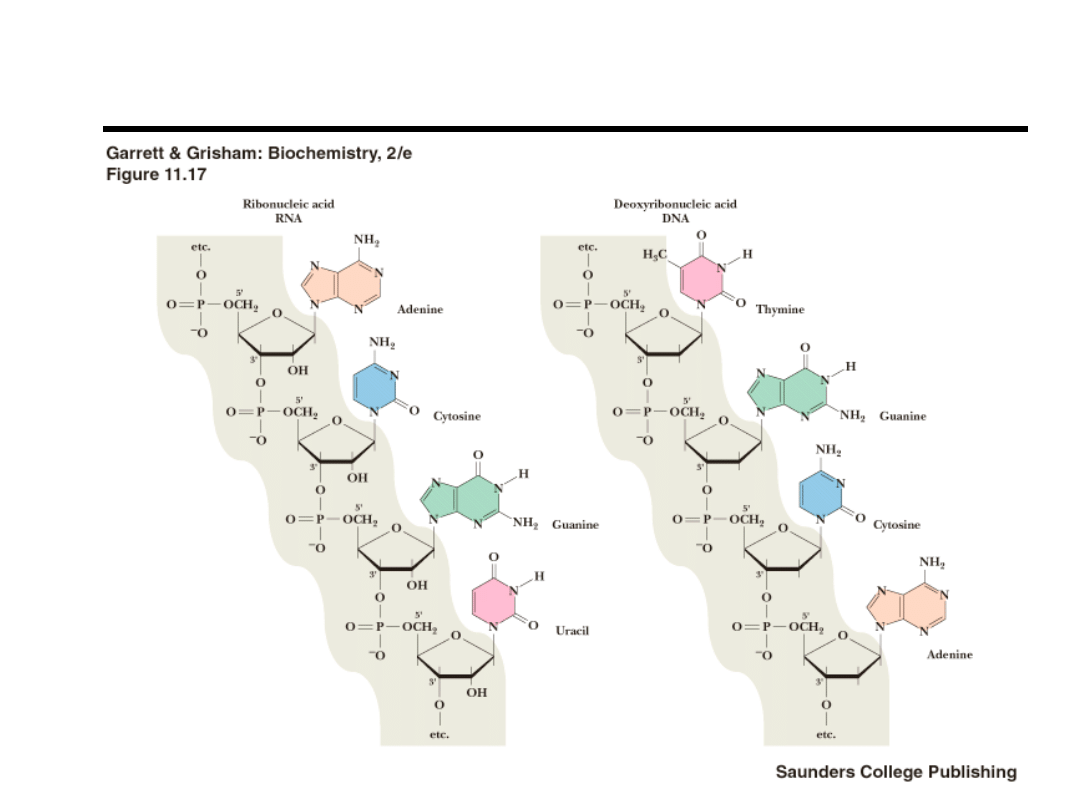
Review of DNA Structure
Review of DNA Structure

NMR Study of DR1
COSY
resonance assignments
torsion angles
sugar conformation
NOESY
resonance assignments
interproton distances
Chemical exchange
imino proton exchange rates, i.e. base pair opening

Resonance Assignments
A combination of COSY and NOESY.
Use known characteristics of molecule.
sequence, identity of terminal bases, etc.
Confirm base-pair formation.
Initially assume it has a regular structure, e.g. B-DNA.
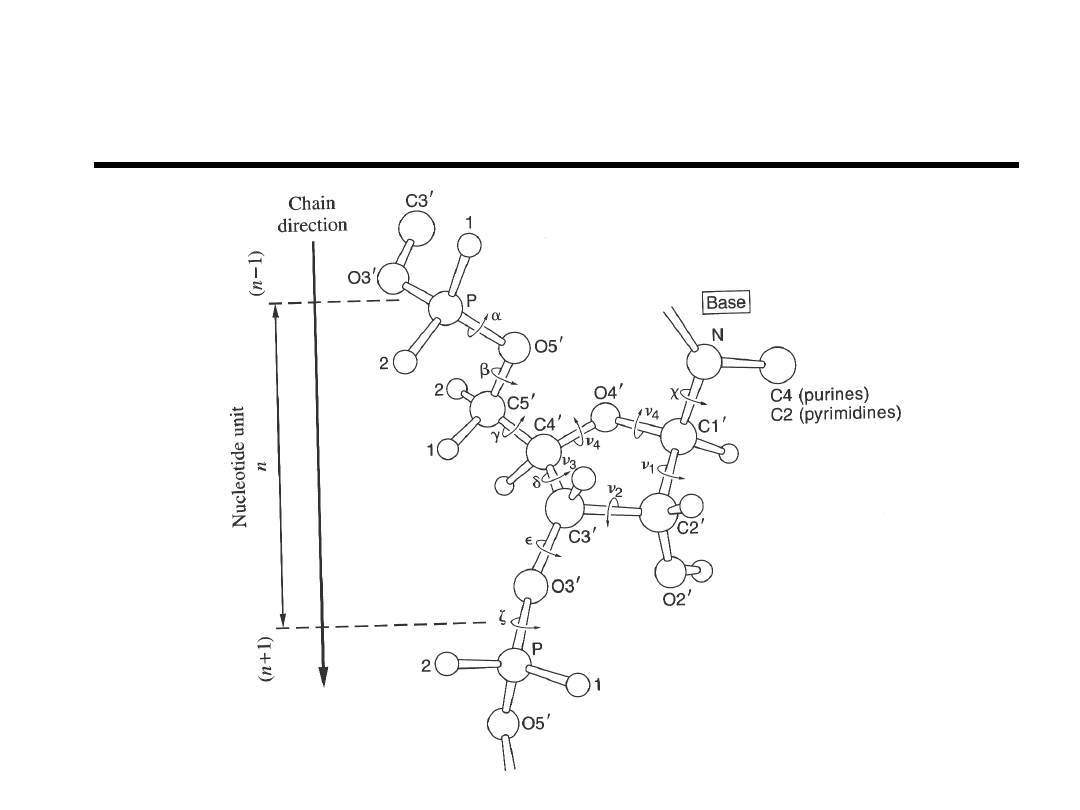
DNA/RNA Backbone Structure
Bloomfield et.al. “Nucleic Acids; Structure,
Properties, and Functions” 2000.
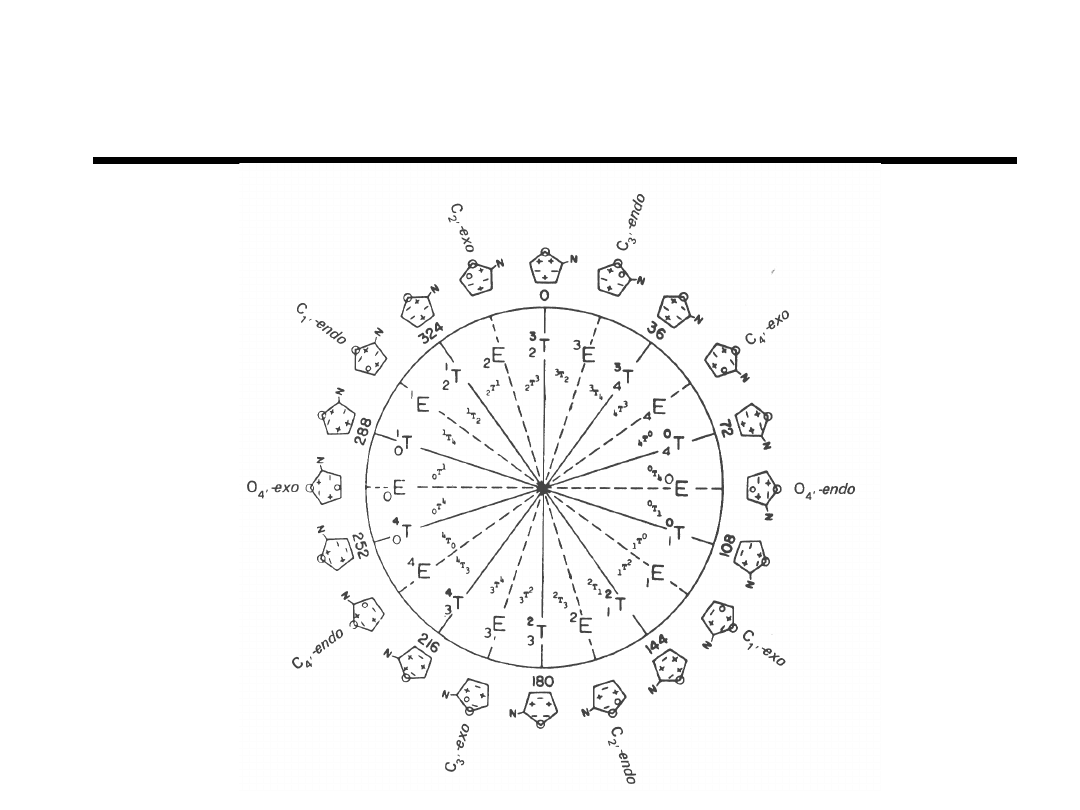
Pseudorotation Phase Cycle of
Deoxyribose
“Principles of Nucleic Acid Structure”
Saenger, pg 19 (1984).
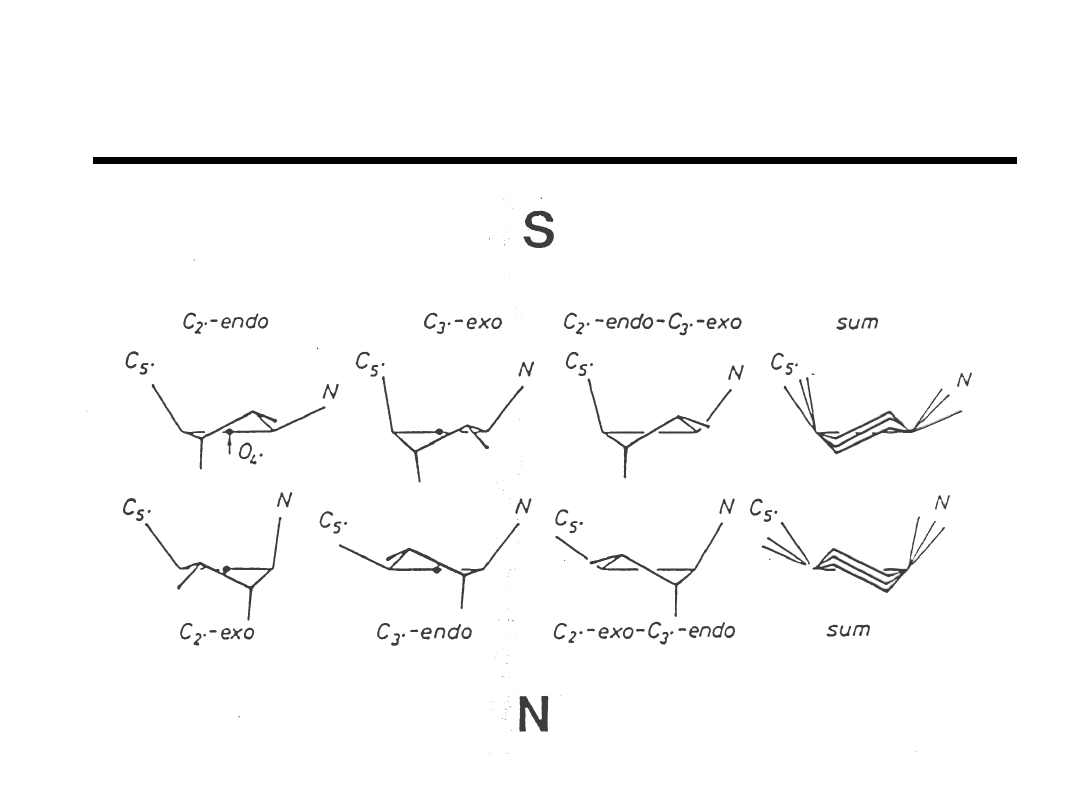
Preferred Pseudorotation Phase Angles
“Principles of Nucleic Acid Structure”
Saenger, (1984).
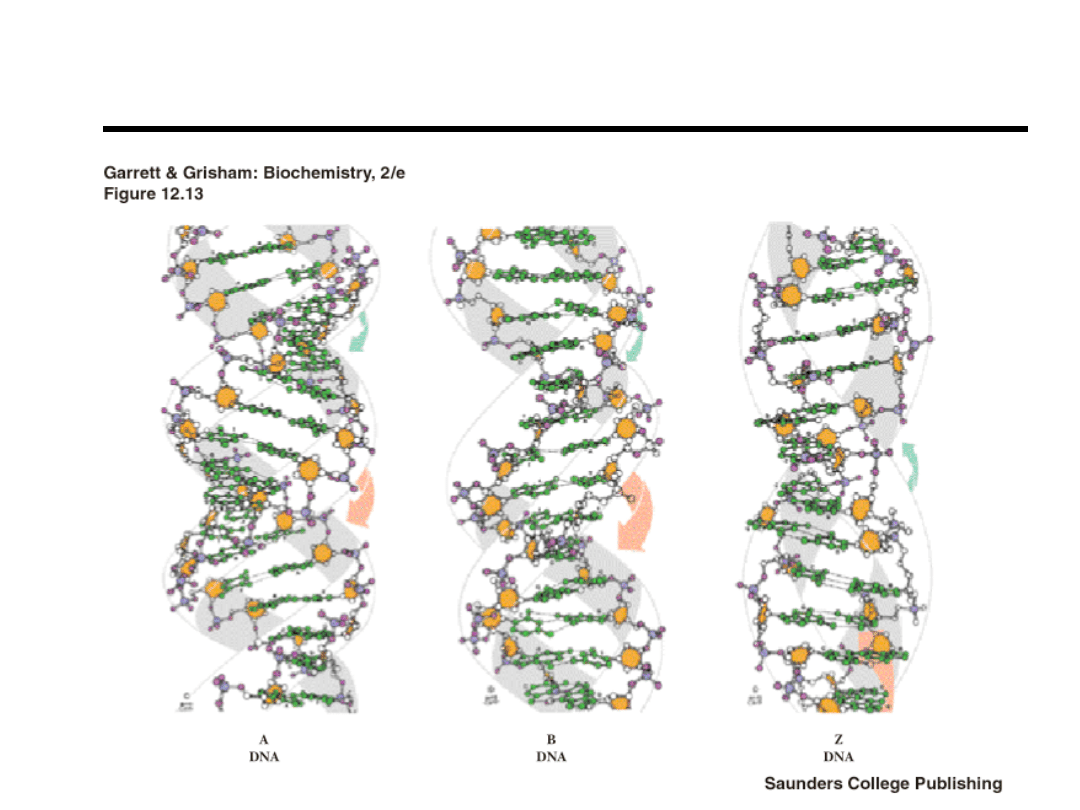
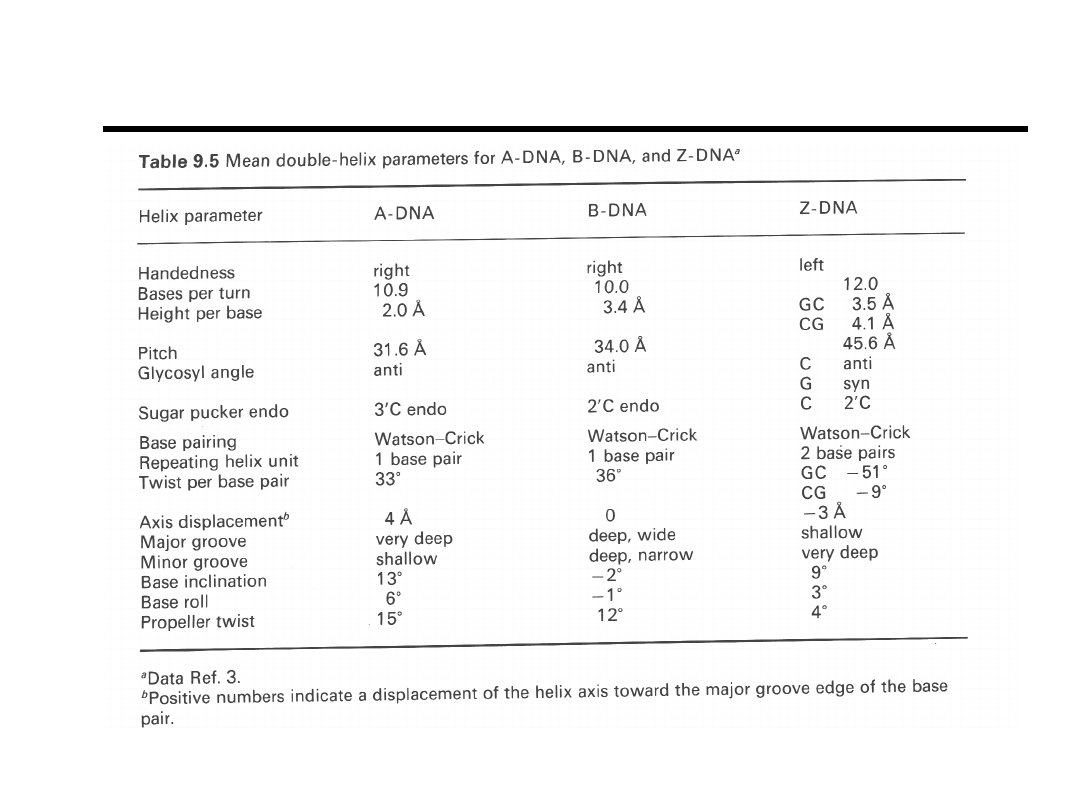
B-DNA
A-DNA, RNA
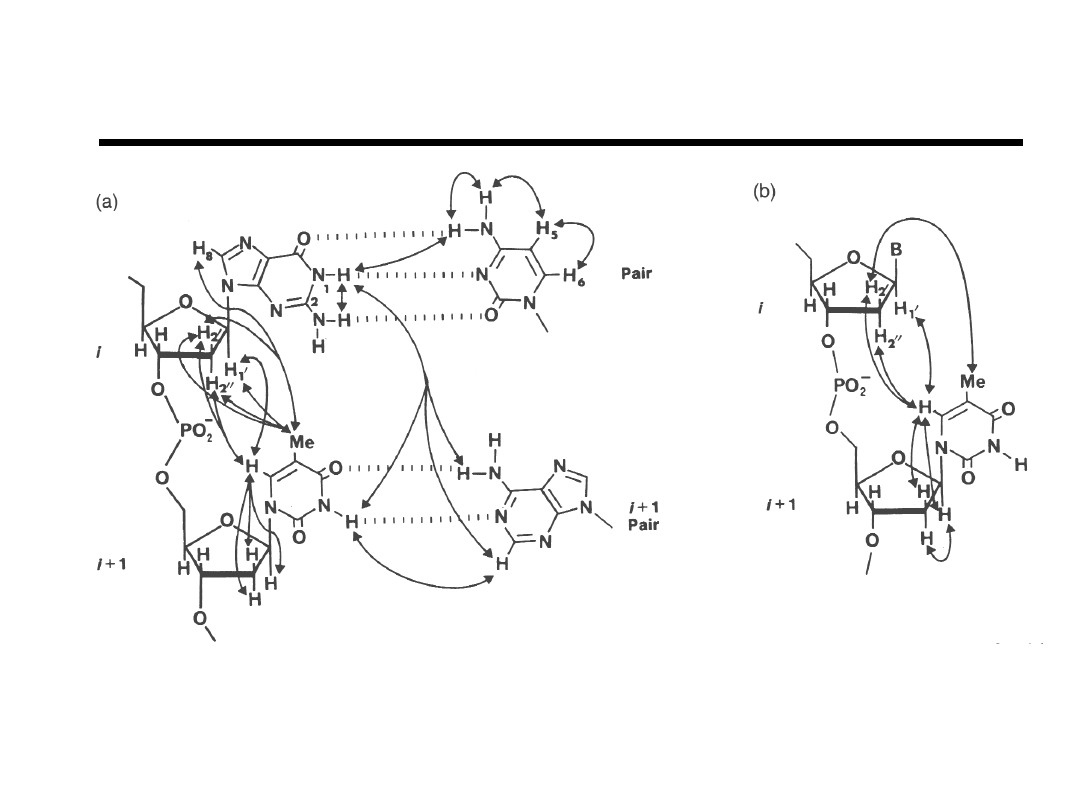
Sequential Resonance Assignments
Interproton contacts less than 4Å in (a) B-DNA and (b) A-DNA.
“Biomolecular NMR Spectroscopy” J.N.S. Evans, pg 350 (1995).
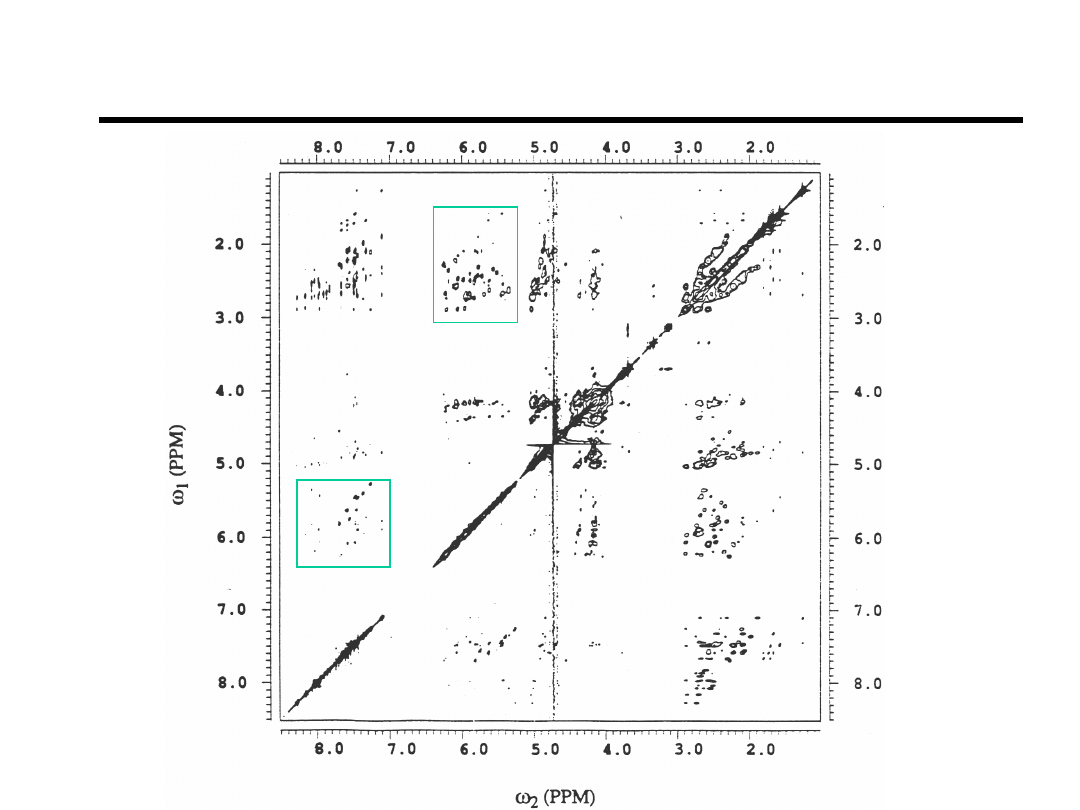
2D NOESY of DR1
H8,H6-to-H1’,H5
H1’-to-H2’H2”
Bishop et.al., Biochemistry (1994).
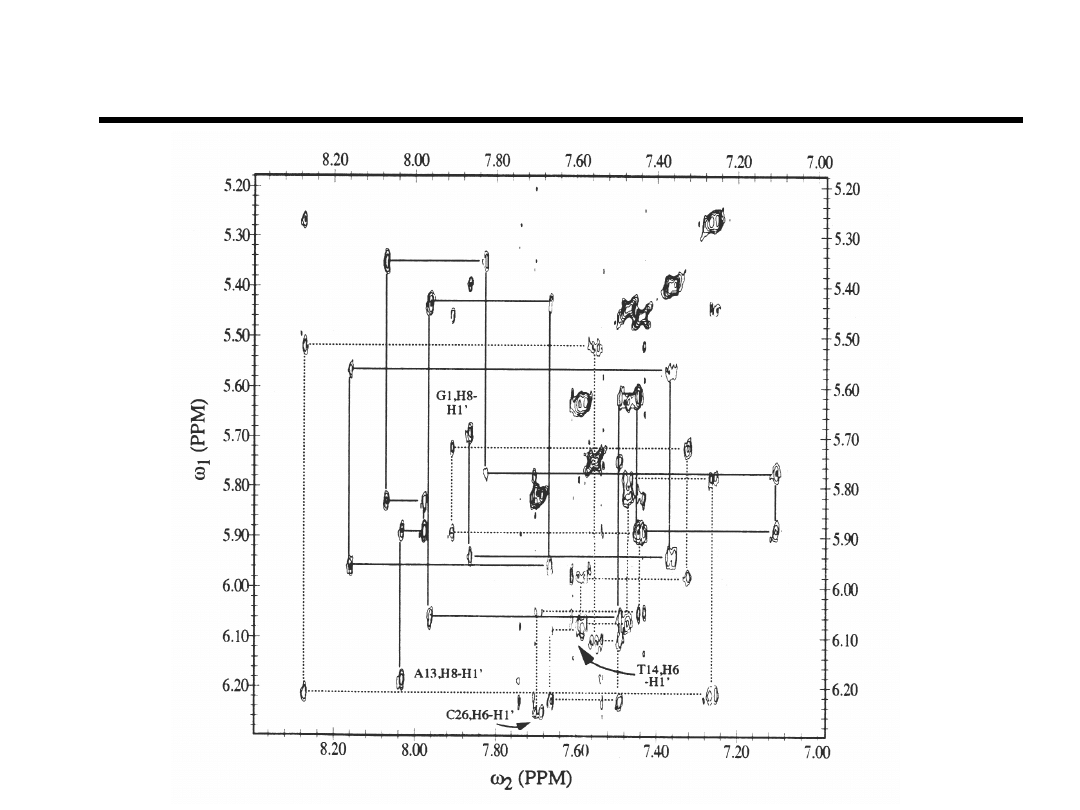
Base-to-H1’ NOESY-walk
Bishop et.al., Biochemistry (1994).
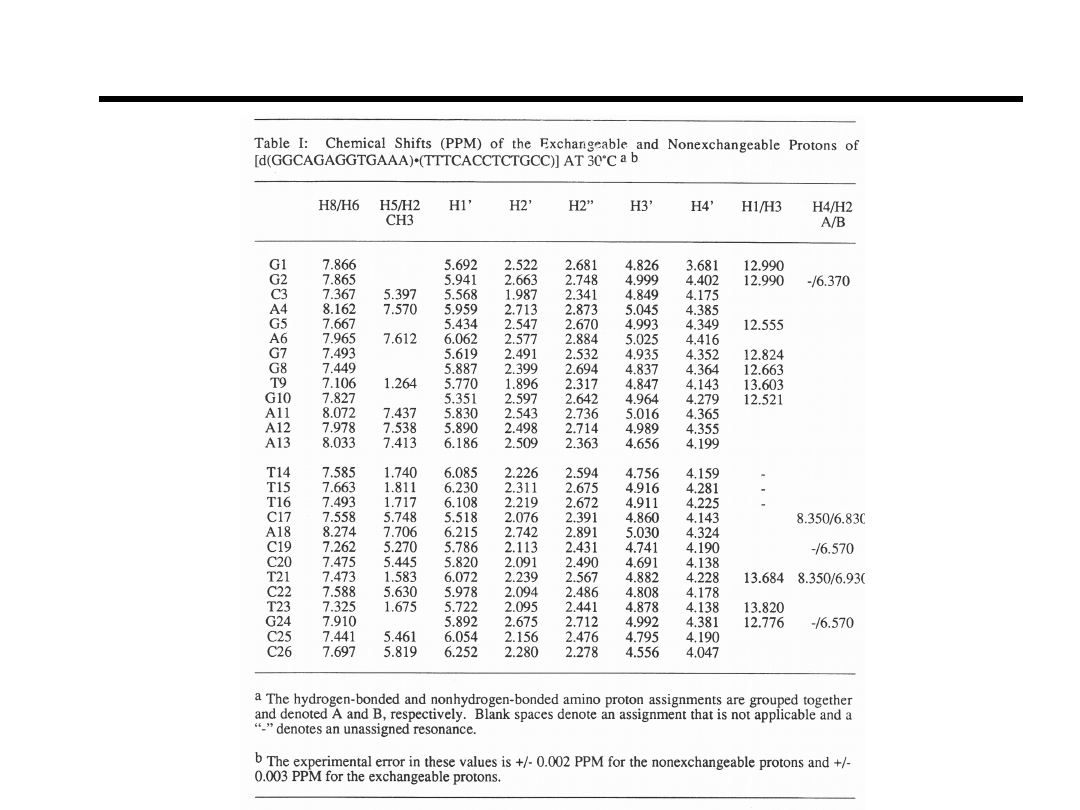
Proton Chemical Shifts of DR1
~97% of all protons
are assigned
Bishop et.al., Bioch
(1994).
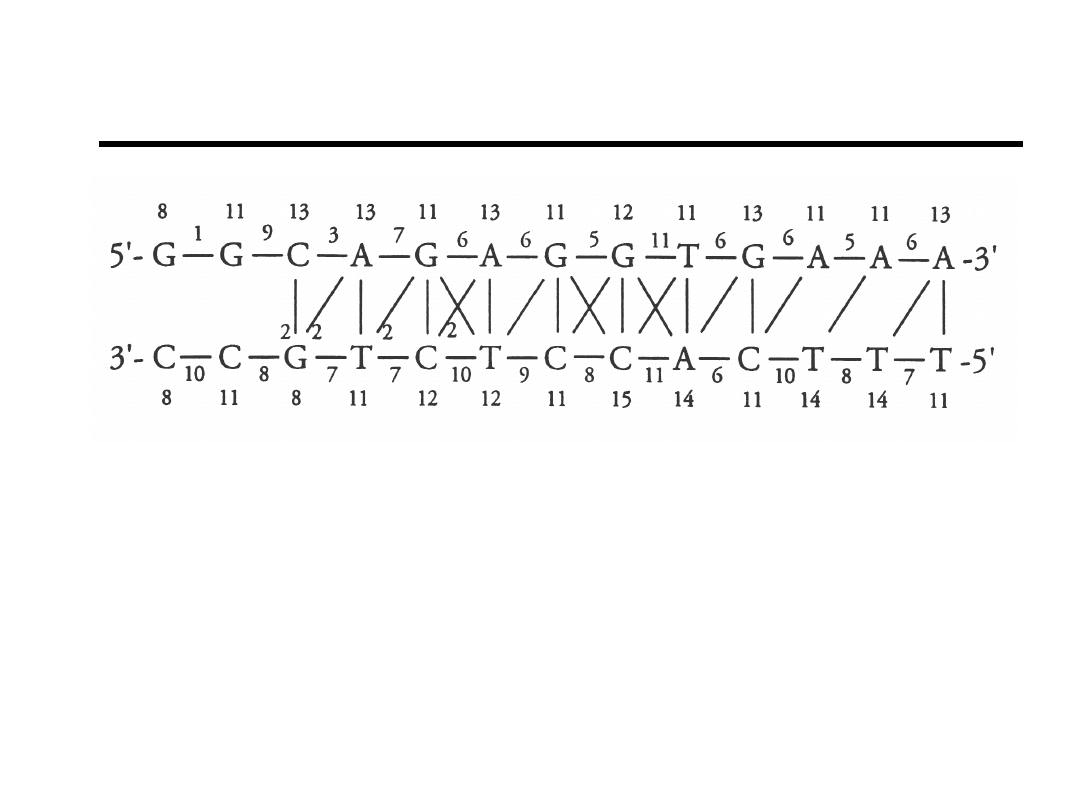
Distribution of Distance Constraints
502 NOE derived distance constraints.
Bishop et.al., Bioch
(1994).
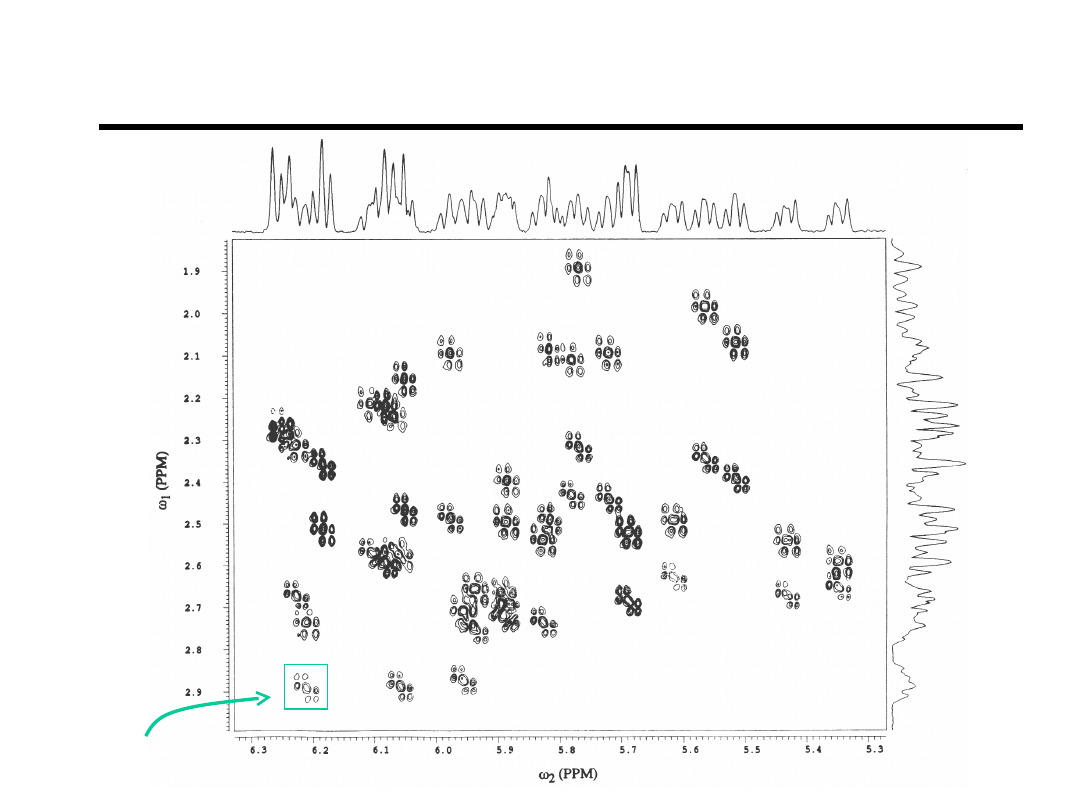
E.COSY H1’-to-H2’H2”
A18,H1’-A18,H2”
Bishop et.al., Bioch
(1994).
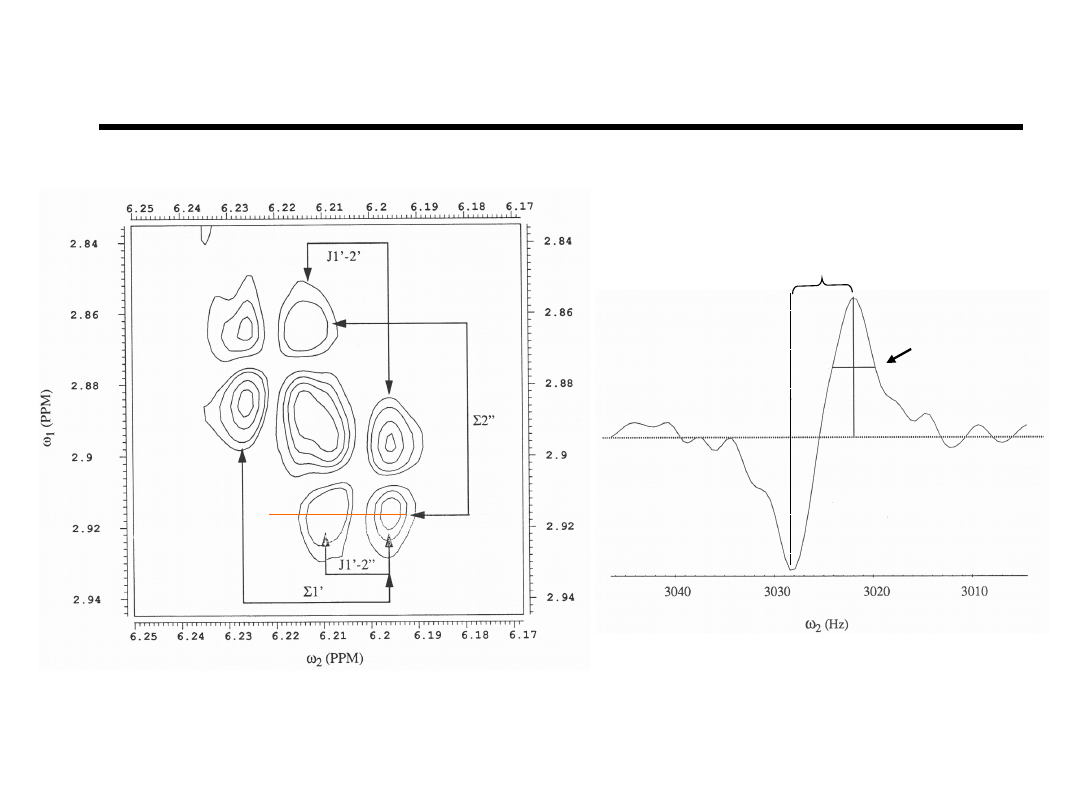
E.COSY A18,H1’-to-H2’H2”
5.9 Hz = J
1’-2”
Linewidth
~4.9 Hz
Bishop et.al., Bioch
(1994).
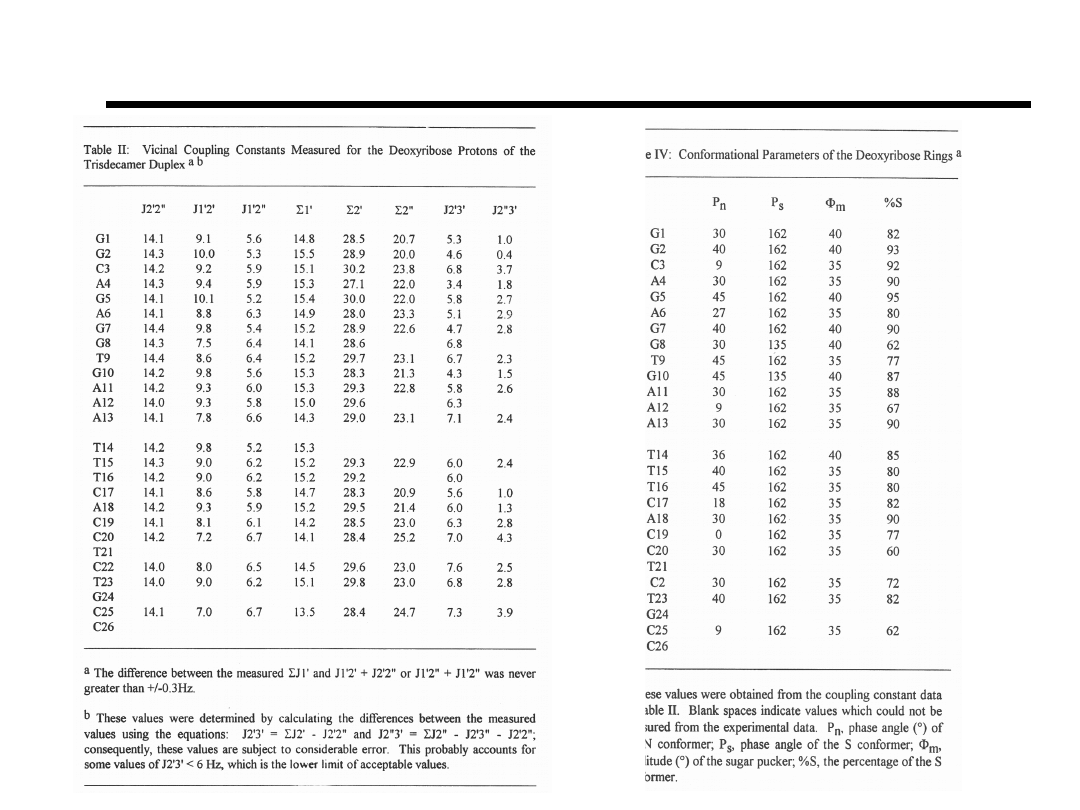
Coupling Constants and Conformations for Sugars
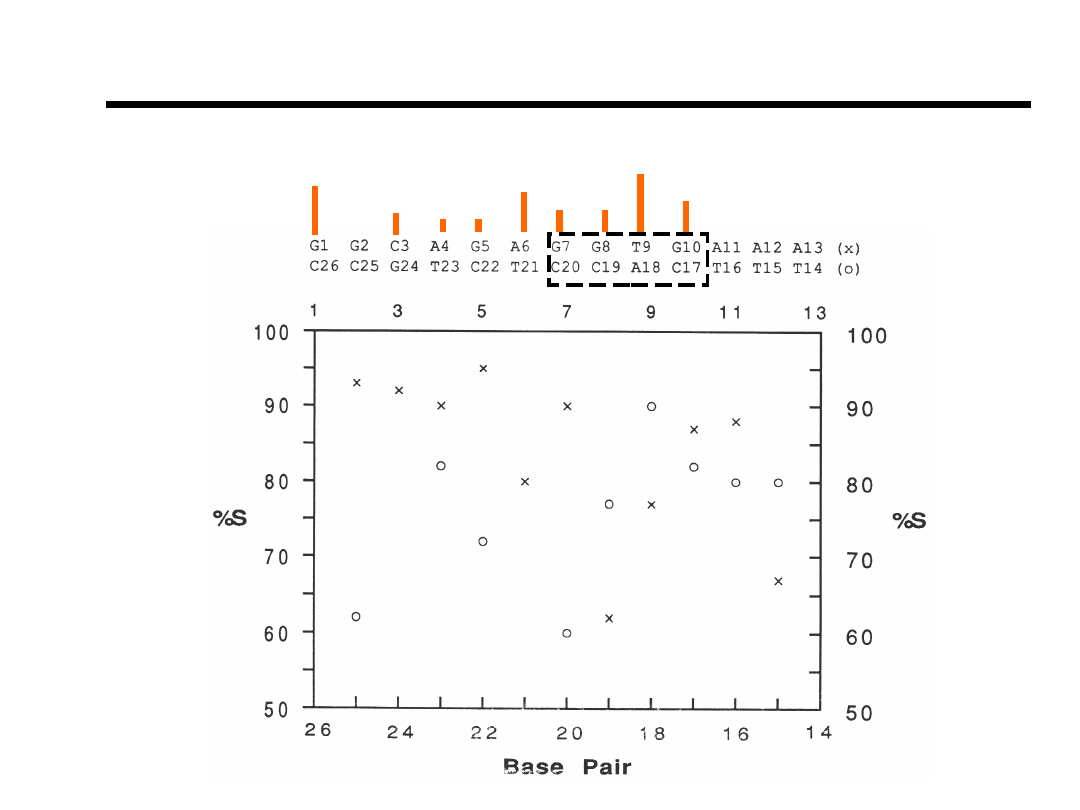
%S versus Base-pair
?
? ?
?
Relative imino
proton exchange rate.
Bishop et.al., Bioch
(1994).
V
constraint
V
total
= V
bondlength
+ V
bondangles
, V
dihedral
+ V
electrostatics
+ V
NOE
+ V
jcoupling
V
NOE
=
Structure Determination
k
2
(r-r
l
)
2 when r<rl
0
when r
l
< r <r
u
k
3
(r-r
u
)
2 when r
u
< r
4k
2
(r-r
u
)
2 when r>r
u
all NOEs
r
u
r
l
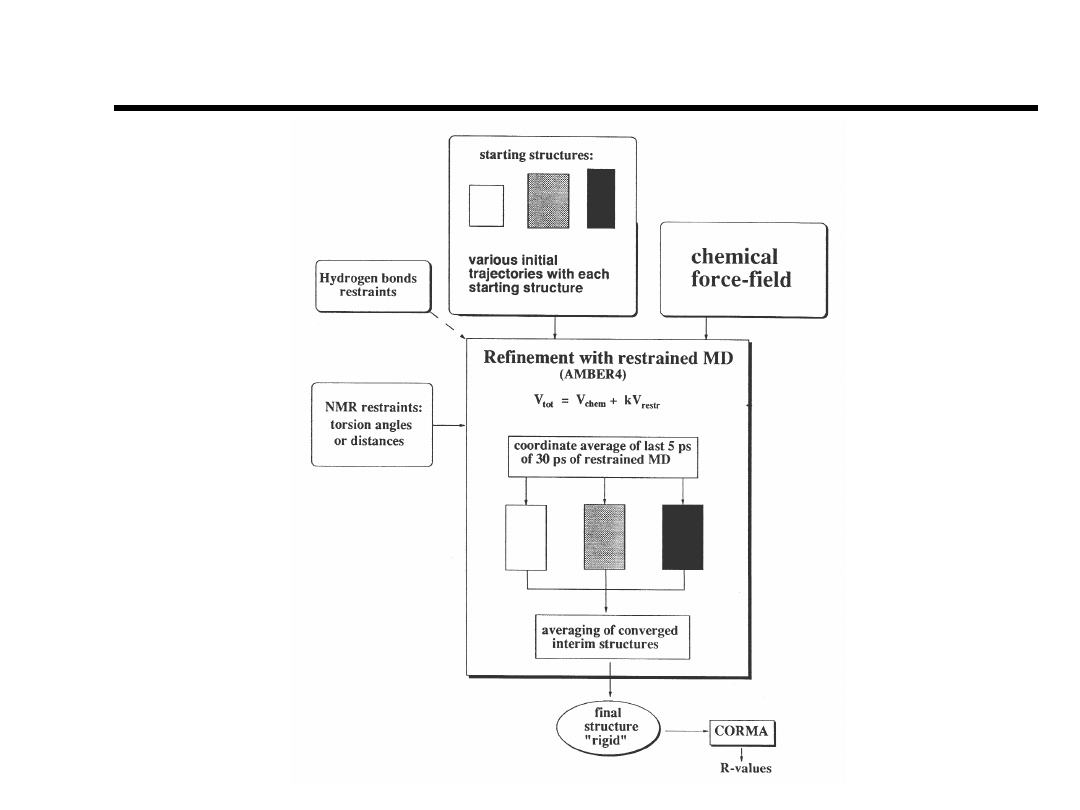
Structure Determination
NATO ASI Vol H87
“NMR of Biol. Macr.”
James et al., (1994).
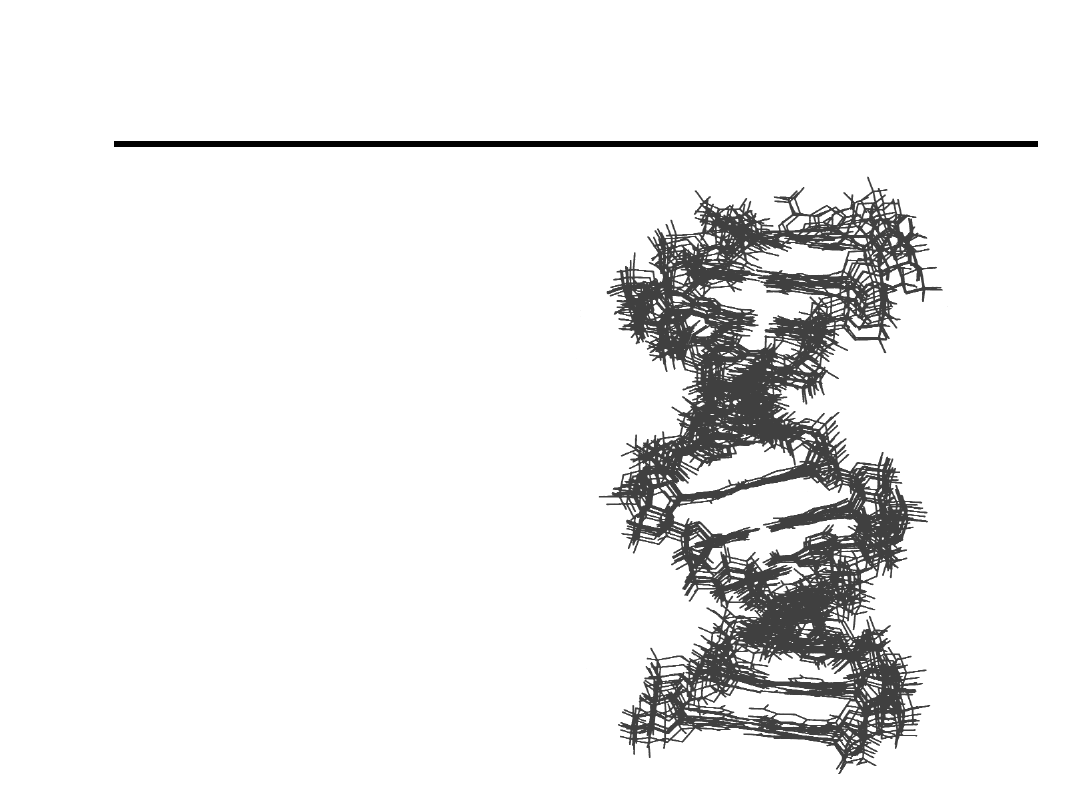
Structure Determination
NATO ASI Vol H87
“NMR of Biol. Macr.”
James et al., (1994).
10 rMD structures
of [d(AGCTTGCCTTGAG)-
[CTCAAGGCAAGCT)]
RMSD = 0.9Å
267 distance restraints
130 torsion angle restraints
Document Outline
- Slide 1
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
- Slide 19
- Slide 20
- Slide 21
- Slide 22
- Slide 23
- Slide 24
- Slide 25
Wyszukiwarka
Podobne podstrony:
Automated NMR structure calculation
196 Capital structure Intro lecture 1id 18514 ppt
197 Capital structure lecture Gdansk 2006 Lecture 2id 18521 ppt
lecture5 6 data structure 2
lecture5 6 data structure
196 Capital structure Intro lecture 1id 18514 ppt
Morphology and characterization of 3D micro porous structured
Eurocode 6 Part 3 1996 2006 Design of Masonry Structures Simplified Calculation Methods for Maso
Structures sp11
4 Plant Structure, Growth and Development, before ppt
Homework Data Structures
Lesley Jeffries Discovering language The structure of modern English
Eurocode 5 EN 1995 1 1 Design Of Timber Structures Part 1 1 General Rules
CS Structured Cabling
[38]QUERCETIN AND ITS DERIVATIVES CHEMICAL STRUCTURE AND BIOACTIVITY – A REVIEW
Butterworth Finite element analysis of Structural Steelwork Beam to Column Bolted Connections (2)
Syntheses, structural and antimicrobial studies of a new N allylamide
słowka gramatyka Turkish Sentence Structure
więcej podobnych podstron