
Research Report
Eur Addict Res 2019;25:10–19
Intravenous Misuse of Methadone,
Buprenorphine and Buprenorphine-Naloxone
in Patients Under Opioid Maintenance Treatment:
A Cross-Sectional Multicentre Study
Fabio Lugoboni
a
Lorenzo Zamboni
a
Mauro Cibin
b
Stefano Tamburin
c
Gruppo InterSERT di Collaborazione Scientifica (GICS)
a
Department of Medicine, Addiction Medicine Unit, Verona University Hospital, Verona, Italy;
b
Department
of Psychiatry and Addictive Behaviours, Local Health Authority Serenissima, Venice, Italy;
c
Department of
Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy
Received: July 28, 2018
Accepted: December 5, 2018
Published online: January 9, 2019
Addicti
on
c
R
e
e
s ar h
Stefano Tamburin, MD, PhD
Department of Neurosciences
Biomedicine and Movement Sciences, University of Verona
Piazzale Scuro 10, IT–37134 Verona (Italy)
E-Mail stefano.tamburin
@
univr.it
© 2019 S. Karger AG, Basel
E-Mail karger@karger.com
www.karger.com/ear
DOI: 10.1159/000496112
Keywords
Benzodiazepine · Buprenorphine · Compliance · Concurrent
use · Misuse · Methadone · Mu agonist · Naloxone · Opioid ·
Opioid maintenance treatment · Overdose · Post-marketing
surveillance · Survey study
Abstract
Background: The act of intravenous misuse is common in
patients under opioid maintenance treatment (OMT), but in-
formation on associated factors is still limited. Objectives: To
explore factors associated with (a) intravenous OMT misuse,
(b) repeated misuse, (c) emergency room (ER) admission, (d)
misuse of different OMT types and (e) concurrent benzodi-
azepine misuse. Methods: We recruited 3,620 patients in 27
addiction units in Italy and collected data on the self-report-
ed rate of intravenous injection of methadone (MET), bu-
prenorphine (BUP), BUP-naloxone (NLX), OMT dosage and
type, experience of and reason for misuse, concurrent intra-
venous benzodiazepine misuse, pattern of misuse in relation
to admission to the addiction unit and ER admissions be-
cause of misuse. According to inclusion/exclusion criteria,
2,585 patients were included. Results: Intravenous misuse of
OMT substances was found in 28% of patients with no differ-
ence between OMT types and was associated with gender,
age, type of previous opioid abuse and intravenous benzo-
diazepine misuse. Repeated OMT misuse was reported by
20% (i.e., 71% of misusers) of patients and was associated
with positive OMT misuse experience and intravenous ben-
zodiazepine misuse. Admission to the ER because of misuse
complications was reported by 34% of patients, this out-
come being associated with gender, employment, type of
previous opioid abuse and intravenous benzodiazepine mis-
use. OMT dosage was lower than the recommended mainte-
nance dosage. Conclusions: We offered new information on
factors associated with intravenous OMT misuse, repeated
misuse and ER admission in Italian patients under OMT. Our
data indicate that BUP-NLX misuse is not different from that
of BUP or MET. Choosing the more expensive BUP-NLX over
MET will likely not lead to the expected reduction of the risk
of injection misuse of the OMT. Instead of prescribing new
and expensive OMT formulations, addiction unit physicians
and medical personnel should better focus on patient’s fea-
tures that are associated with a higher likelihood of misuse.
Care should be paid to concurrent benzodiazepine and OMT
misuse.
© 2019 S. Karger AG, Basel
Downloaded by:
Access provided by the University of Michigan Library
141.216.78.40 - 1/10/2019 11:34:42 AM

Misuse in Patients Under OMT
11
Eur Addict Res 2019;25:10–19
DOI: 10.1159/000496112
Introduction
Opioid maintenance treatment (OMT) with metha-
done (MET) or buprenorphine (BUP) increases retention
rate and reduces illicit opioid use, criminal behaviour and
the risk of HIV and viral hepatitis via needle sharing [1–3].
OMT plays a crucial role in the extinction of conditioned
addictive behaviour to opioids because it minimizes with-
drawal symptoms and attenuates the reinforcing effect of
street heroin, leading to its reduction or cessation [4].
Misuse and diversion, which include intravenous or
nasal administration, use of higher dosage, or illicit ac-
quisition, have been reported for OMT [5]. Patients
may prefer MET or BUP via nasal or injection route
because of the much shorter time to peak plasma con-
centration [6] that results in a higher reinforcing effect.
Injection of MET syrup and BUP tablets was reported
by several studies [1, 2, 7–15]. In addition to the nega-
tive effect on the treatment and rehabilitation of opioid
use, injection of MET and BUP raises the concern for
blood-borne infections, unwanted side effects or aller-
gic reactions to compounds that are present in MET
syrup and BUP tablets formulations (e.g., sorbitol or
silica), respiratory depression, local damage at the in-
jection site because larger-gauge needles are often used,
with venous thrombosis and pulmonary side effects [1,
2, 15–17].
Data on the prevalence of MET and BUP injection is
inconsistent across studies, with figures ranging from 5.0
to 35.8% for MET and from 9.1 to 46.5% for BUP [1, 9,
11, 12, 14, 18, 19].
Sublingual combination of BUP and low-dose nalox-
one (NLX) was marketed, assuming that NLX would an-
tagonize the euphoric properties of BUP, or precipitate
withdrawal symptoms in opioid-tolerant people when in-
jected intravenously, thus reducing the risk of diversion
and misuse [19, 20]. In contrast, when used sublingually,
BUP-NLX tablets skip hepatic first pass and offer good
plasma levels of BUP, with low NLX bioavailability result-
ing in low risk of severe and protracted withdrawal symp-
toms [21]. Despite these promising theoretical grounds,
post-marketing studies showed similar rates of injections
for MET and BUP-NLX, either as sublingual tablet or sol-
uble film formulations [22, 23].
According to previous reports, risk factors for OMT
misuse include younger age, risky behaviours associated
with opioid overdose, needle fixation (i.e., the act of in-
jecting becoming compulsive and rewarding), opioid and
benzodiazepine injection, polydrug abuse, self-treatment
of withdrawal symptoms, concurrent pain or psychiatric
symptoms, desire to obtain rapid onset of drug effect and
low OMT dosage [1, 7, 10, 11, 14, 18–20, 23].
Benzodiazepine is frequently co-prescribed with OMT
[24], despite the evidence that it is often misused [5, 25].
The combined use of benzodiazepine and opioid has been
associated with worse outcomes, higher risk of overdose
and admission to the emergency room (ER) and lower
adherence to OMT [26, 27]. Data on the concurrent in-
travenous misuse of benzodiazepine and single OMT ac-
tive principle is scanty [28].
In Italy, OMT is prescribed by public addiction units
that belong to the National Health Service. These addic-
tion units are general ones, in that they offer pharmaco-
logical treatment and rehabilitation to patients with dif-
ferent substance use disorders. Four OMT types may be
prescribed by addiction units in Italy, namely, MET low
concentration (0.1%), MET high concentration (0.5%),
BUP and BUP-NLX.
To offer new information on factors associated with
intravenous OMT injection, we studied a large sample
of patients under OMT recruited from a network of
addiction units in Italy, and collected data on the self-
reported rate of intravenous injection of MET, BUP
and BUP-NLX, OMT dosage and type, experience of
and reason for misuse, concurrent intravenous benzo-
diazepine misuse, pattern of misuse in relation to ad-
mission to the addiction unit, admissions to the ER
because of misuse, as well as demographic and clinical
variables. Among other substances of abuse, we fo-
cused on benzodiazepine because we were interested in
intravenous misuse. The aims of the study were (a) to
obtain reliable measures of intravenous injection of
different OMT types and benzodiazepine, and (b) to
explore factors associated with misuse, (c) repeated
misuse, and (d) admission to the ER because of misuse.
The findings of this study might help defining the
characteristics of the most vulnerable patients and pro-
vide useful information to better address and tailor
OMT.
Materials and Methods
From June to November 2015, 3,620 consecutive patients were
recruited from 27 Italian addiction units that belong to the Gruppo
InterSERT di Collaborazione Scientifica, a scientific collaborative
network dealing with substance use disorders and located in Italy.
The addiction units participating to the Gruppo InterSERT di Col-
laborazione Scientifica offer a wide coverage of the population of
Italian addicted patients. The procedures and treatments were
comparable across the addiction units and representative of stan-
dard care in Italy.
Downloaded by:
Access provided by the University of Michigan Library
141.216.78.40 - 1/10/2019 11:34:42 AM
Lugoboni et al.
Eur Addict Res 2019;25:10–19
12
DOI: 10.1159/000496112
The inclusion criteria were (a) age ≥18 years, (b) having been
on oral MET, BUP, or BUP-NLX treatment for at least 3 months;
(c) willing to participate to the study and to answer the question-
naire on misuse.
The exclusion criteria were (a) severe liver dysfunction, (b) se-
vere renal dysfunction, (c) other organ failure or severe medical
disorders, (d) psychosis, (e) dementia or cognitive impairment.
The study was conducted according to the Declaration of Hel-
sinki and approved by the Ethics Committee of the Verona Uni-
versity Hospital. All patients gave written informed consent for
participation in the study. No benefit was provided for participa-
tion in the study; it was voluntary and confidential.
For all the patients, demographic (gender: male, female; age
class: <
20, 20–29, 30–39, 40–49, ≥50; employment status: fully
employed, temporarily employed, unemployed; family status:
living with parents, single, married/engaged, homeless) and clin-
ical variables (type of previous opioid abuse: smoked, snorted,
injected; OMT type: MET 0.1%, MET 0.5%, BUP, BUP-NLX,
OMT dosage: mg) were recorded, based on self-report with an
anonymous questionnaire. Daily oral morphine milligram equiv-
alent dosage was calculated using standard dosage conversion
calculations [29, 30].
Patients were asked to fill an anonymous questionnaire on the
self-reported presence of intravenous injection (yes, no), rate of
repeated misuse (once, 2–20 times, >
20 times), main reason for
misuse (reward/euphoria, reduce withdrawal symptoms, enhance
drug effects), experience related to misuse (positive, negative) of
their current OMT (MET 0.1%, MET 0.5%, BUP, BUP-NLX), con-
current intravenous benzodiazepine misuse (never, once, 2–20
times, >
20 times), temporal pattern of misuse in relation to the ad-
diction unit access (before, after, both), and ER admissions be-
cause of misuse complications (yes, no).
No time frame was specified for OMT/benzodiazepine intrave-
nous misuse, except for the question on the temporal pattern of
misuse in relation to access to the admission unit.
A preliminary version of the questionnaire was administered
to a beta tester group of patients, who rated each question for eas-
iness to understand and answer to, and modified according to the
suggestions of the respondents.
Questionnaires were distributed to patients by the addiction
unit staff together with a covering letter explaining the aims of the
study and directions on the distribution and collection of data [31].
Patients were asked to complete the questionnaires and return
them in sealed envelopes to ensure they remained closed until
analysis. Patients were reassured that return/non-return would
not impact the treatment and were requested to answer honestly
to the questionnaire [31].
Statistical analysis was carried with the IBM SPSS version 20.0
statistical package. The Pearson’s χ
2
test with Yates’s correction
for continuity was used for categorical variables, while the un-
paired t test and the non-parametrical Mann-Whitney U test
were used for continuous ones. Logistic regression model analy-
sis was used to explore the association with misuse (dependent
variable: yes, no), repeated misuse (dependent variable: single
misuse, repeated misuse), and admission to the ER because of
misuse complications (dependent variable: yes, no), with the re-
sults expressed as ORs and 95% CIs. The goodness of fit of the
logistic regression model was assessed using the Hosmer and
Lemeshow test [32]. p < 0.05 (2-tailed) was taken as the signifi-
cance threshold for all the tests.
Results
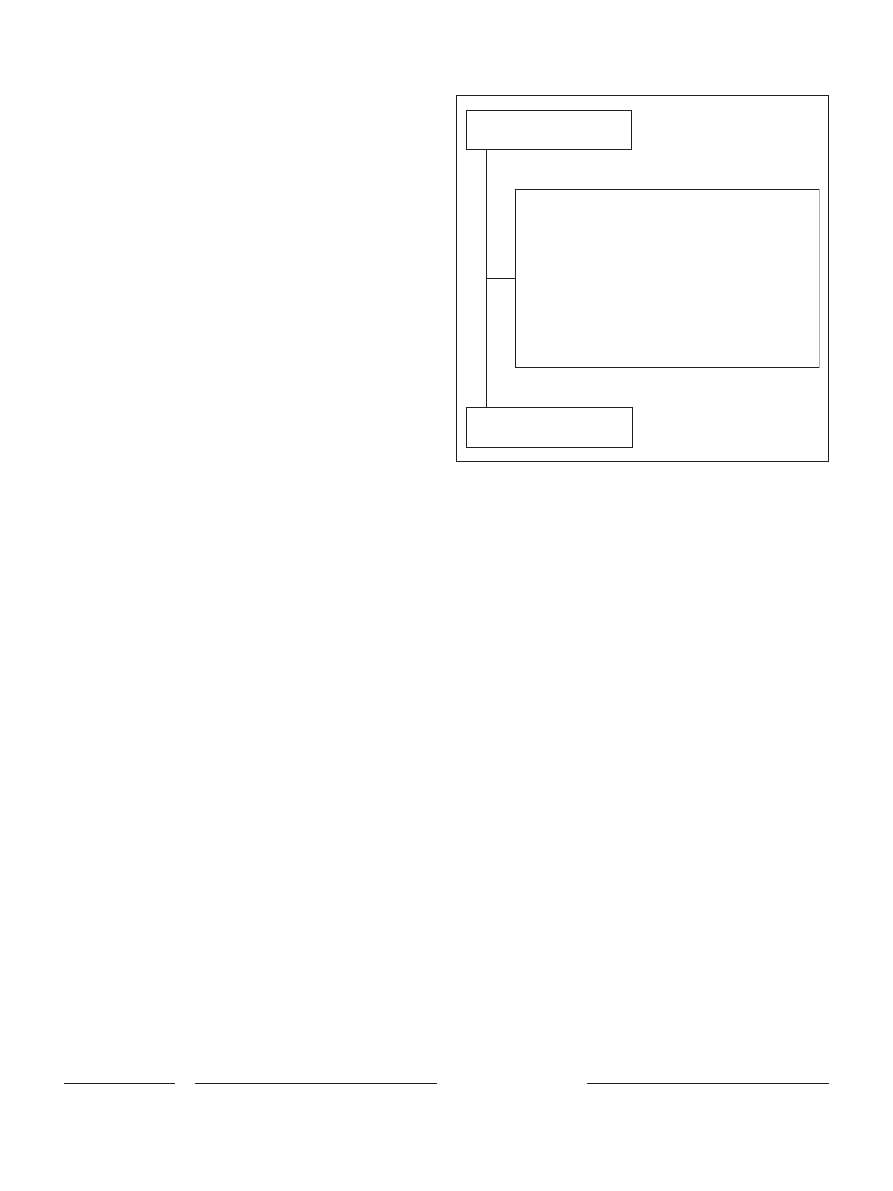
According to inclusion/exclusion criteria, 2,585
patients (2,079 males, 506 females; male/female ratio =
4.1) were included in the study (Fig. 1) and their data
analysed.
All the demographic characteristics of the patients
(age class, employment, family status) significantly dif-
fered according to gender (Table 1).
Among clinical variables, the type of previous opioid
abuse and OMT type significantly differed, while OMT
dosage was not significantly different according to gender
(Table 1). MET dosage was not significantly different
when comparing low concentration (MET 0.1%) to high
concentration (MET 0.5%) formulation, either in the
whole sample or according to gender (Table 1). BUP dos-
age was significantly higher when comparing BUP to
BUP-NLX formulation in the whole population (Mann-
Whitney U test: p = 0.006) and in males (p = 0.006), but
not in females (ns).
In the whole population, misuse of current OMT was
significantly more frequent for MET 0.5% (29%) than
MET 0.1% formulation (25%, p = 0.035), the rate of re-
peated misuse of current OMT was significantly higher
for BUP-NLX (once: 15%, 2–20 times: 35%, >
20 times:
50%) than BUP formulation (37, 26, and 37%; p = 0.008),
Assessed for eligibility
(n = 3,620)
Excluded (n = 1,035)
No urinalysis data (n = 14)
Opioid maintenance treatment <6 months (n = 105)
Unwilling or no time to participate in the study (n = 652)
Severe liver dysfunction (n = 41)
Severe renal dysfunction (n = 10)
Organ failure or severe medical disorders (n = 16)
Psychosis (n = 22)
Cognitive impairment (n = 18)
Incomplete questionnaire or missing data (n = 127)
More than 1 reason (n = 30)
Eligible and included
(n = 2,585)
Fig. 1.
Flow diagram of the study and reasons for patients’ exclusion.
Downloaded by:
Access provided by the University of Michigan Library
141.216.78.40 - 1/10/2019 11:34:42 AM
Misuse in Patients Under OMT
13
Eur Addict Res 2019;25:10–19
DOI: 10.1159/000496112
the temporal pattern of OMT misuse in relation to the
addiction unit access was significantly different across
OMT types (p = 0.032), and admission to the ER because
of misuse complications was significantly more frequent
for MET 0.5% (38%) than MET 0.1% formulation (30%,
p = 0.001; Table 2).
In men, the rate of repeated misuse of current OMT
was significantly higher for BUP-NLX (once: 15%, 2–20
times: 38%, >
20 times: 47%) than BUP formulation (34,
26, and 40%; p = 0.027), the main reason for current OMT
misuse was significantly different when comparing BUP
(reward/euphoria: 29%, reduce withdrawal symptoms:
58%, enhance drug effects: 13%) to BUP-NLX (31, 41,
and 28%; p = 0.042), the temporal pattern of OMT mis-
use in relation to the addiction unit access (overall: p =
0.018, MET 0.1 vs. MET 0.5%: p = 0.026; BUP vs. BUP-
NLX: p = 0.035) significantly differed, and admission to
the ER because of misuse complications was significant-
ly more frequent for MET 0.5% (38%) than MET 0.1%
formulation (29%; p < 0.001; online suppl. Table 1;
for all online suppl. material, see www.karger.com/
doi/10.1159/000496112).
In women, the misuse of current OMT was significant-
ly more frequent for MET 0.5% (29%) than MET 0.1%
formulation (18%; p = 0.019; online suppl. Table 2).
Distribution of the patients according to intravenous
OMT vs. benzodiazepine misuse indicated that the ma-
jority of them did not misuse any of the 2 drug classes
Table 1.
Demographic and clinical characteristics of the patients
Overall
†
(n = 2,585)
Males
†
(n = 2,079)
Females
†
(n = 506)
p value
‡
Age class, years, n (%)
<0.001
<20
33 (1)
15 (1)
18 (4)
20–29
577 (23)
445 (21)
132 (26)
30–39
756 (29)
604 (29)
152 (30)
40–49
879 (34)
722 (35)
157 (31)
≥50
340 (13)
293 (14)
47 (9)
Employment, n (%)
0.005
Fully employed
1,068 (41)
891 (43)
177 (35)
Temporarily employed
535 (21)
421 (20)
114 (23)
Unemployed
982 (38)
767 (37)
215 (42)
Family status, n (%)
<0.001
Living with parents
1,285 (50)
1,068 (51)
17 (43)
Single
606 (23)
517 (25)
89 (18)
Married/engaged
611 (24)
421 (20)
190 (37)
Homeless
83 (3)
73 (4)
10 (2)
Type of previous opioid abuse, n (%)
0.003
Smoked
484 (19)
364 (17)
120 (24)
Snorted
488 (19)
407 (20)
81 (16)
Injected
1,613 (62)
1,308 (63)
305 (60)
OMT type, n (%)
0.014
MET 0.1%
590 (23)
462 (22)
128 (25)
MET 0.5%
1,356 (53)
1,075 (52)
281 (56)
BUP
400 (15)
341 (16)
59 (12)
BUP-NLX
239 (9)
201 (10)
38 (7)
OMT dosage, mg
MET 0.1%
51.0±52.7
51.6±54.4
48.6±46.0
ns
MET 0.5%
50.1±45.0
49.8±41.7
51.2±55.8
ns
BUP
10.6±11.8
*
10.6±12.1
*
10.3±9.9
ns
BUP-NLX
8.1±9.8
8.0±10.4
8.4±5.9
ns
†
Percentage of column.
‡
p value for comparison between males and females (Pearson’s χ
2
test for categorical variables, unpaired t test or Mann-Whitney U
test for continuous ones).
* Significant BUP versus BUP-NLX comparison (Mann-Whitney U test).
ns, non significant; BUP, buprenorphine; MET, methadone; NLX, naloxone; OMT, opioid maintenance treatment.
Downloaded by:
Access provided by the University of Michigan Library
141.216.78.40 - 1/10/2019 11:34:42 AM

Lugoboni et al.
Eur Addict Res 2019;25:10–19
14
DOI: 10.1159/000496112
(58%), while the remaining population was divided into
3 groups of similar size, that is, those misusing OMT
(13%), benzodiazepine (15%) and both (14%; p < 0.0001;
Table 3).
The multivariate logistic regression model showed
that gender, age class, type of previous opioid abuse,
and intravenous benzodiazepine misuse were signifi-
cantly associated with OMT misuse (Table 4), while
the other covariates (employment, family status, OMT
type, daily oral morphine milligram equivalent dos-
age, pattern of benzodiazepine misuse) were not sig-
nificant.
According to the multivariate logistic regression mod-
el, the experience of OMT misuse, and intravenous ben-
zodiazepine misuse were the only variables significantly
associated with repeated OMT misuse (Table 4), while the
other covariates (gender, age class, employment, family
status, type of previous opioid abuse, OMT type, daily
oral morphine milligram equivalent dosage, main reason
for OMT misuse, temporal pattern of benzodiazepine
misuse) were not significant.
The multivariate logistic regression model showed
gender, employment, type of previous opioid abuse and
intravenous benzodiazepine misuse to be significantly as-
Table 2.
Characteristics of intravenous misuse in the whole population (n = 2,585)
OMT type, n (%)
p value
MET 0.1%
†
MET 0.5%
†
BUP
†
BUP-NLX
†
Overall
‡
MET
§
BUP
¶
Misuse of current OMT (n = 2,585)
ns
0.035
ns
Yes (n = 718, 28%)
146 (25)
399 (29)
101 (25)
72 (30)
No (n = 1,867, 72%)
444 (75)
957 (71)
299 (75)
167 (70)
Repeated misuse rate for current OMT (n = 718)
0.035
ns
0.008
Once (n = 206, 29%)
49 (33)
109 (27)
37 (37)
11 (15)
2–20 Times (n = 182, 25%)
33 (23)
98 (25)
26 (26)
25 (35)
>20 Times (n = 330, 46%)
64 (44)
192 (48)
38 (37)
36 (50)
Main reason for current OMT misuse (n = 718)
ns
ns
ns
Reward/euphoria (n = 204, 28%)
38 (26)
115 (29)
28 (28)
23 (32)
Reduce withdrawal symptoms (n = 380, 53%)
85 (58)
206 (52)
58 (57)
31 (43)
Enhance drug effect (n = 134, 19%)
23 (16)
78 (19)
15 (15)
18 (25)
Experience of current OMT misuse (n = 718)
ns
ns
ns
Positive (n = 320, 45%)
70 (48)
174 (44)
41 (42)
35 (49)
Negative (n = 398, 55%)
76 (52)
225 (56)
60 (58)
37 (51)
Temporal pattern of OMT misuse (n = 718)
0.032
ns
ns
Before access to the AU (n = 208, 29%)
55 (37)
109 (27)
27 (27)
17 (24)
After access to the AU (n = 321, 45%)
58 (40)
174 (44)
56 (55)
33 (46)
Both (n = 189, 26%)
33 (23)
116 (29)
18 (18)
22 (30)
Concurrent BZD misuse (n = 2,585)
ns
ns
ns
Never (n = 1,831, 71%)
418 (71)
936 (69)
299 (75)
178 (74)
Once (n = 238, 9%)
54 (9)
121 (9)
40 (10)
23 (10)
2–20 times (n = 236, 9%)
60 (10)
133 (10)
23 (6)
20 (8)
>20 times (n = 280, 11%)
58 (10)
166 (12)
38 (9)
18 (8)
Temporal pattern of BZD misuse (n = 754)
Before access to the AU (n = 374, 50%)
90 (52)
201 (48)
54 (53)
29 (48)
ns
ns
ns
After access to the AU (n = 177, 23%)
43 (25)
96 (23)
22 (22)
16 (26)
Both (n = 203, 27%)
39 (23)
123 (29)
25 (25)
16 (26)
ER admission because of misuse (n = 2,585)
0.001
0.001
ns
Yes (n = 887, 34%)
178 (30)
513 (38)
120 (30)
76 (32)
No (n = 1,698, 66%)
412 (70)
843 (62)
280 (70)
163 (68)
†
Percentage of column.
‡
p value for the comparison between the 4 OMT types (Pearson’s χ
2
test).
§
p value for the comparison between the 2 MET formulations (MET 0.1 versus MET 0.5%, Pearson’s χ
2
test).
¶
p value for the comparison between the 2 BUP formulations (BUP vs. BUP-NLX, Pearson’s χ
2
test).
ns, non significant; AU, addiction unit; BZD, benzodiazepine; BUP, buprenorphine; ER, emergency room; MET, methadone; NLX, nalox-
one; OMT, opioid maintenance treatment.
Downloaded by:
Access provided by the University of Michigan Library
141.216.78.40 - 1/10/2019 11:34:42 AM
Misuse in Patients Under OMT
15
Eur Addict Res 2019;25:10–19
DOI: 10.1159/000496112
sociated with admission to the ER because of misuse com-
plications (Table 4), while the other covariates (age class,
family status, OMT type, daily oral morphine milligram
equivalent dosage, rate of repeated OMT misuse, main
reason for OMT misuse, experience of OMT misuse, tem-
poral pattern of OMT and benzodiazepine misuse) were
not significant.
Discussion
The present multicentre study, which is to the best of
our knowledge, one of the largest one on OMT misuse,
yielded the following main findings: (a) OMT misuse was
found in 28% of patients with no difference between
OMT types, and was associated with gender, age, type of
previous opioid abuse and intravenous benzodiazepine
misuse; (b) repeated OMT misuse was reported by 20%
(i.e., 71% of misusers) of patients, and was associated with
positive OMT misuse experience and intravenous benzo-
diazepine misuse; (c) 34% of patients reported admission
to the ER because of misuse complications, this outcome
being associated with gender, employment, type of previ-
ous opioid abuse and intravenous benzodiazepine mis-
use.
Baseline demographic (age, employment, family sta-
tus) and clinical variables (type of previous opioid
abuse, OMT type) significantly differed according to
gender, indicating that male and female cohorts did not
overlap. In keeping with previous reports [28, 31, 33],
men were largely overrepresented in our sample, and
this gender unbalance might have biased the findings
for the whole population and their generalization to
women. Univariate analyses for OMT types were per-
formed in the whole sample and separately according
to gender, and it suggested some differences between
men and women. They included (a) OMT misuse was
significantly more frequent for MET 0.5% vs. MET
0.1% in women; (b) repeated OMT misuse rate was sig-
nificantly higher for BUP-NLX vs. BUP in men; (c) ad-
mission to the ER because of misuse complications was
significantly higher for MET 0.5% vs. MET 0.1% in
men; (d) the main reason for misuse significantly dif-
fered when comparing BUP-NLX vs. BUP in men.
However, these findings should be interpreted with
caution because of the univariate model, and the differ-
ent statistical power for male and female populations,
the former being more than 4 times larger than the lat-
ter. Multivariate analyses documented gender-related
differences, in that female sex appeared to be signifi-
cantly and inversely associated with OMT misuse (OR
0.74) and admission to the ER because of misuse com-
plications (OR 0.59).
The main finding of this study was that, in keeping
with previous reports [1, 9, 11, 14, 18, 19, 31, 34], nearly
one third of patients under OMT reported misuse, this
outcome not being influenced by OMT type or dosage in
the multivariate model. In accordance with previous
studies [35, 36], age class was significantly and inversely
associated with misuse, suggesting that younger patients
under OMT should be more strictly monitored. We
could not document any significant association between
other demographic variables, such as family and employ-
ment status, and misuse, thus not confirming previous
reports [11, 31, 34].
Our data contradict the notion that BUP-NLX may re-
duce diversion and misuse [19, 21] but are in keeping with
post-marketing reports of non-significant difference be-
tween BUP-NLX and BUP or MET injection rate in OMT
patients [22, 23], and experimental evidence that BUP and
BUP-NLX are similarly reinforcing in recently detoxified
heroin abusers [37]. Opioid pharmacology may explain this
apparently paradoxical finding. BUP has a higher binding
affinity for the mu opioid receptor and a longer effect than
Table 3.
Distribution of the patients according to intravenous OMT and BZD misuse
Overall
†
(n = 2,585)
Males
†
(n = 2,079)
Females
†
(n = 506)
No misuse, n (%)
1,489 (58)
1,187 (57)
302 (60)
Misuse of OMT only, n (%)
342 (13)
282 (14)
60 (12)
Misuse of BZD only, n (%)
378 (15)
299 (14)
79 (15)
Concurrent OMT and BZD misuse, n (%)
376 (14)
311 (15)
65 (13)
p
value (Pearson’s χ
2
test, 2 × 2 table)
<0.0001
<0.0001
<0.0001
†
Percentage of column.
BZD, benzodiazepine; OMT, opioid maintenance treatment.
Downloaded by:
Access provided by the University of Michigan Library
141.216.78.40 - 1/10/2019 11:34:42 AM

Lugoboni et al.
Eur Addict Res 2019;25:10–19
16
DOI: 10.1159/000496112
NLX [38]. NLX dose in the BUP-NLX formulation might
not be sufficient to antagonize BUP effect on the mu recep-
tor [28] and/or its antagonist effect might be too short. This
view is supported by an experimental study showing that
intranasal administration of BUP-NLX causes modest but
transient unpleasant effect related to opioid withdrawal,
followed by delayed agonist effect of BUP [38].
One fifth of our patients, and nearly 3 quarters of mis-
users, reported repeated OMT misuse, which was signifi-
cantly associated with positive misuse experience in the
Table 4.
Results of the multivariate logistic regression model analysis
Significant covariates
OR (95% CI)
p value
Dependent variable 1: OMT misuse
Gender
Male
1
Female
0.74 (0.57–0.96)
0.021
Age, years
<30
1
30–39
0.44 (0.34–0.58)
<0.001
40–49
0.26 (0.20–0.35)
<0.001
≥50
0.17 (0.12–0.24)
<0.001
Type of previous opioid abuse
Smoked
1
Snorted
1.54 (1.02–2.32)
0.04
Injected
4.95 (3.52–6.96)
<0.001
Intravenous BZD misuse
Never
1
Once
3.32 (2.45–4.52)
<0.001
2–20 times
3.56 (2.61–4.84)
<0.001
>20 times
4.03 (3.02–5.38)
<0.001
Dependent variable 2: repeated OMT misuse
Experience of OMT misuse
Negative
1
Positive
3.13 (2.17–4.52)
<0.001
Intravenous BZD misuse
Never
1
Once
1.75 (1.06–2.89)
0.028
2–20 times
1.81 (1.10–2.98)
0.02
>20 times
2.02 (1.25–3.28)
0.004
Dependent variable 3: admission to the ER because
of misuse complications
Gender
Male
1
Female
0.59 (0.36–0.96)
0.035
Employment
Fully employed
1
Temporarily employed
1.74 (1.05–2.86)
0.031
Unemployed
2.49 (1.63–3.82)
<0.001
Type of previous opioid abuse
Smoked
1
Snorted
ns
Injected
3.89 (1.78–8.47)
0.001
Intravenous BZD misuse
Never
1
Once
ns
2–20 times
2.71 (1.61–4.57)
<0.001
>20 times
2.97 (1.80–4.87)
<0.001
Here only covariates that turned out to be significant in the multivariate logistic regression model analysis are reported.
ns, non significant; BZD, benzodiazepine; ER, emergency room; OMT, opioid maintenance treatment.
Downloaded by:
Access provided by the University of Michigan Library
141.216.78.40 - 1/10/2019 11:34:42 AM

Misuse in Patients Under OMT
17
Eur Addict Res 2019;25:10–19
DOI: 10.1159/000496112
multivariate analysis. The finding that positive effect of
misuse was similar between MET, BUP and BUP-NLX
might explain why OMT type did not influence repeated
misuse in the multivariate model, and is in keeping with a
previous report of misuse experience influencing repeat-
ed misuse [33]. Male patients injected BUP-NLX more
frequently than BUP to enhance drug effects than to re-
duce withdrawal symptoms, suggesting that BUP-NLX
might reduce misuse in patients who are more sensitive to
the negative effect of withdrawal, while it may worsen this
phenomenon in those who prefer a long-lasting drug ef-
fect. Future studies should explore whether these differ-
ences across patients, or their personality profiles, can
help predicting misuse to different OMT formulations.
Another variable that may have influenced misuse is
the ease of injecting, which is higher for BUP and BUP-
NLX than MET, the latter being more viscous and requir-
ing larger gauge needles [39].
One third of our patients reported admission to the ER
during their lifetime because of misuse complications.
This number is higher than that of patients reporting mis-
use to current OMT because the answer encompassed ad-
missions due to any OMT, either current or previous,
and/or other drugs, including benzodiazepine. Reasons
for ER admission were based on self-report and could not
be cross-checked with ER/hospital databases because the
questionnaires were anonymous. Employment was sig-
nificantly associated to ER admissions, in that temporar-
ily employed and unemployed patients had ORs of 1.74
and 2.49, respectively, in comparison to fully employed
ones. This finding underscores the importance of social
factors and the role of psychosocial support to improve
misuse outcomes [31].
In accordance with previous reports [1], previous opi-
oid injection was associated with a higher likelihood of
misuse (OR 4.95) and ER admission (OR 3.89) in com-
parison to smoking and snorting. Injection provides fast-
er drug delivery and onset compared to the oral route,
and once people start injecting opioids, they often engage
in risky injection practices [40]. This finding suggests
more caution when delivering OMT to patients who pre-
viously injected opioids.
Concurrent intravenous benzodiazepine misuse was
reported by 29% of patients, half of whom misused also
OMT. Benzodiazepine misuse and its frequency were
associated with OMT misuse, repeated misuse and ER
admission, suggesting that it represents the variable
more significantly correlated to OMT misuse outcomes
in our study. This finding is in keeping with previous
studies and suggest considering overall drug misuse in-
stead of focusing on OMT misuse only [31], and paying
attention to concurrent anxiety disorders or sleep dis-
orders that may require benzodiazepine prescription
[24, 41].
The main strengths of this study are the large sample size
and the multicentre design that allowed sampling a large
population of Italian patients under OMT. Another
strength is the high return rate (78%), in that only 18% of
patients refused to participate to the study, and 4% of the
questionnaires were incomplete and thus not analysed.
These figures are higher than those from a similar report on
Finnish OMT patients, where the return rate was 60% [31].
The main limitation of the present study is that OMT
dosage was below the recommended maintenance dose
range. The average daily dose of MET, either high or low
concentration, was around 50 mg, while international ap-
plied guidelines, such as the NICE guidance, suggest the
usual maintenance dose range of 60–120 mg daily [42].
Similarly, the average BUP daily dose was 11 mg and that
of BUP-NLX was 8 mg, both of them below the usual
maintenance daily dose range of 12–24 mg [42]. The low
OMT dosage might be the main reason for intravenous
misuse in our patients and a potential bias in the interpre-
tation of the data, since previous studies reported an as-
sociation between sub-optimal OMT doses and higher
misuse to reduce withdrawal symptoms [11, 20, 31, 34].
However, the daily oral morphine milligram equivalent
dosage was not significant in any of the 3 multivariate
analyses, possibly arguing against the importance of this
factor.
We may speculate that the low OMT dosage in our
sample might be related to the finding that 45% of the pa-
tients started misuse after accessing the addiction unit. In
Italy, outpatients receive OMT directly and with no cost
from the National Health Service addiction units, while
other sources, such as prescription from general practi-
tioners and private addiction specialists, are nearly ab-
sent. The large number of addiction units involved in this
study is representative of standard care in Italy, and thus
we recommend that caution to OMT under-dosage be
exercised in order to avoid intravenous misuse. Male pa-
tients experienced misuse more frequently for MET 0.5%
(45%) than MET 0.1% (39%) and for BUP (53%) than
BUP-NLX (43%). Since OMT misuse is known to be di-
rectly related to drug availability [43], this finding under-
scores the importance of strict monitoring of misuse in
the addiction units, especially for high concentration
MET and BUP.
Another limitation is that data was self-reported by pa-
tients, who, despite being assured of the confidentiality of
Downloaded by:
Access provided by the University of Michigan Library
141.216.78.40 - 1/10/2019 11:34:42 AM

Lugoboni et al.
Eur Addict Res 2019;25:10–19
18
DOI: 10.1159/000496112
the survey, might have under-reported some sensitive
pieces of information because of shame or fear of negative
judgement [31]. However, studies based on self-report
are considered sufficiently reliable and valid in the field
of addiction medicine [44], and there was no other way
to collect information on misuse that is usually not re-
corded in the patient’s clinical files. Other limitations are
that respondents might have been the more motivated
and compliant patients, and the absence of data on psy-
chiatric comorbidity.
In conclusion, we offered new information on OMT
misuse in a large group of Italian patients. Our data indi-
cate that BUP-NLX misuse is not different from that of
BUP or MET. These results may be helpful for better tai-
loring OMT to reduce misuse and its complications. For
example, particular care should be paid to men and pa-
tients who previously injected opioids or with intrave-
nous benzodiazepine misuse, in that they are at higher
risk of misuse and worse outcome with OMT. They also
indicate that choosing the more expansive BUP-NLX
over MET will likely not lead to the expected reduction of
the risk of injection misuse of the OMT. Addiction unit
physicians and medical personnel should better focus on
patient’s features that are associated with higher likeli-
hood of misuse.
Acknowledgements
None.
Ethics Statement
The study was conducted according to the Declaration of Hel-
sinki and approved by the Ethics Committee of the Verona Uni-
versity Hospital. All patients gave written informed consent for
participation to the study. No benefit was provided for participa-
tion in the study that was voluntary and confidential.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
None.
Authors Contributions
F.L.: designed the study, gathered the data, developed the data-
base, interpreted the data, drafted and revised the manuscript. L.Z.:
designed the study, gathered the data, developed the database, in-
terpreted the data and revised the manuscript. M.C.: designed the
study, gathered the data, interpreted the data and revised the man-
uscript. S.T.: designed the study, developed the database, conduct-
ed the statistical analysis, interpreted the data, drafted and revised
the manuscript. All authors read and approved of the final version
of the manuscript.
Appendix
Members of the Gruppo InterSERT di Collaborazione Scientifica
(GICS) in alphabetical order: L. Andreoli, V. Balestra, O. Betti, C.
Biasin, C. Bossi, A. Bottazzo, A. Bove, R. Bressan, B. Buson, E. Cac-
camo, V. Calderan, S. Cancian, F. Cantachin, D. Cantiero, G. Can-
zian, D. Cargnelutti, L. Carraro, D. Casalboni, R. Casari, G. Certa, P.
Civitelli, M. Codogno, T. Cozzi, D. Danieli, L. De Cecco, A. Dei Ros-
si, E. Dell’Antonio, R. Del Zotto, M. Faccini, M. Fadelli, E. Favero, A.
Fiore, B. Fona, A. Franceschini, E. Gaiga, M. Gardiolo, N. Gentile, G.
Gerra, N. Ghezzo, M. Giacomin, L. Giannessi, G. Giuli, G. Guescini,
B. Hanife, S. Laus, G. Mantovani, A. Manzoni, S. Marescatto, M.
Mazzo, D. Meneghello, C. Meneguzzi, D. E. Milan, D. Mussi, M.
Monfredini, E. Nardi, F. Nardozi, A. Natoli, M. Pagnin, P. Pagnin, A.
Pani, V. Pavani, P. Pellachin, F. Peroni, V. Peroni, T. Pezzotti, M. C.
Pieri, L. Povellato, D. Prosa, B. Pupulin, G. Raschi, C. Resentera, M.
Residori, P. Righetti, M. Ripoli, P. Riscica, V. Rizzetto, M. Rotini, A.
Rovea, R. Sabbioni, D. Saccon, E. Santo, E. Savoini, M. Scarzella, P.
Simonetto, C. Smacchia, M. Stellato, C. Stimolo, L. Suardi, M. Trev-
isan, G. Urzino, A. Vaiana, A. Valent, M. Vidal, A. Zamai, A. Zanchet-
tin, V. Zavan, G. Zecchinato, M. Zerman, G. Zinfollino.
References
1 Humeniuk R, Ali R, McGregor C, Darke S:
Prevalence and correlates of intravenous
methadone syrup administration in Adelaide,
Australia. Addiction 2003;
98:
413–418.
2 Nordmann S, Frauger E, Pauly V, Orléans V,
Pradel V, Mallaret M, et al: Misuse of bu-
prenorphine maintenance treatment since in-
troduction of its generic forms: OPPIDUM
survey. Pharmacoepidemiol Drug Saf 2012;
21:
184–190.
3 Mattick RP, Breen C, Kimber J, Davoli M: Bu-
prenorphine maintenance versus placebo or
methadone maintenance for opioid depen-
dence. Cochrane Database Syst Rev
2014:CD002207.
4 Bell J: Pharmacological maintenance treat-
ments of opiate addiction. Br J Clin Pharma-
col 2014;
77:
253–263.
5 Casati A, Sedefov R, Pfeiffer-Gerschel T: Mis-
use of medicines in the European Union: a
systematic review of the literature. Eur Addict
Res 2012;
18:
228–245.
6 Dale O, Hoffer C, Sheffels P, Kharasch ED:
Disposition of nasal, intravenous, and oral
methadone in healthy volunteers. Clin Phar-
macol Ther 2002;
72:
536–545.
7 Robinson GM, Kemp R, Lee C, Cranston D:
Patients in methadone maintenance treatment
who inject methadone syrup: a preliminary
study. Drug Alcohol Rev 2000;
19:
447–450.
Downloaded by:
Access provided by the University of Michigan Library
141.216.78.40 - 1/10/2019 11:34:42 AM

Misuse in Patients Under OMT
19
Eur Addict Res 2019;25:10–19
DOI: 10.1159/000496112
8 Darke S: Self-report among injecting drug us-
ers: a review. Drug Alcohol Depend 1998;
51:
253–263.
9 Chevalley AE, Besson J, Croquette-Krokar M,
Davidson C, Dubois JA, Uehlinger C, et al:
Prevalence of methadone injection in three
Swiss cities. Presse Med 2005;
34:
776–780.
10 Rosenblum A, Parrino M, Schnoll SH, Fong
C, Maxwell C, Cleland CM, et al: Prescription
opioid abuse among enrollees into metha-
done maintenance treatment. Drug Alcohol
Depend 2007;
90:
64–71.
11 Roux P, Villes V, Bry D, Spire B, Feroni I,
Marcellin F, et al: Buprenorphine sniffing as a
response to inadequate care in substituted pa-
tients: results from the Subazur survey in
south-eastern France. Addict Behav 2008;
33:
1625–1629.
12 Winstock AR, Lea T, Sheridan J: Prevalence of
diversion and injection of methadone and bu-
prenorphine among clients receiving opioid
treatment at community pharmacies in New
South Wales, Australia. Int J Drug Policy
2008;
19:
450–458.
13 Maremmani I, Pacini M, Pani PP, Popovic D,
Romano A, Maremmani AG, et al: Use of
street methadone in Italian heroin addicts
presenting for opioid agonist treatment. J Ad-
dict Dis 2009;
28:
382–388.
14 Judson G, Bird R, O’Connor P, Bevin T, Loan
R, Schroder M, et al: Drug injecting in pa-
tients in New Zealand methadone mainte-
nance treatment programs: an anonymous
survey. Drug Alcohol Rev 2010;
29:
41–46.
15 Bouquié R, Wainstein L, Pilet P, Mussini JM,
Deslandes G, Clouet J, et al: Crushed and in-
jected buprenorphine tablets: characteristics
of princeps and generic solutions. PLoS One
2014;
9:e113991.
16 Quaglio G, Talamini G, Lechi A, Venturini L,
Lugoboni F, Mezzelani P; et al: Study of 2708
heroin-related deaths in north-eastern Italy
1985–98 to establish the main causes of death.
Addiction 2001;
96:
1127–1137.
17 Weimer MB, Korthuis PT, Behonick GS,
Wunsch MJ: The source of methadone in
overdose deaths in Western Virginia in 2004.
J Addict Med 2011;
5:
188–202.
18 Moratti E, Kashanpour H, Lombardelli T,
Maisto M: Intravenous misuse of buprenor-
phine: characteristics and extent among
patients undergoing drug maintenance
therapy. Clin Drug Investig 2010;
30(suppl
1):
3–11.
19 Cicero TJ, Ellis MS, Surratt HL, Kurtz SP: Fac-
tors contributing to the rise of buprenorphine
misuse: 2008–2013. Drug Alcohol Depend
2014;
142:
98–104.
20 Comer SD, Sullivan MA, Vosburg SK, Ma-
nubay J, Amass L, Cooper ZD, et al: Abuse
liability of intravenous buprenorphine/nalox-
one and buprenorphine alone in buprenor-
phine-maintained intravenous heroin abus-
ers. Addiction 2010;
105:
709–718.
21 Soyka M: New developments in the manage-
ment of opioid dependence: focus on sublin-
gual buprenorphine-naloxone. Subst Abuse
Rehabil 2015;
6:
1–14.
22 Bruce RD, Govindasamy S, Sylla L, Kamarul-
zaman A, Altice F: Lack of reduction in bu-
prenorphine injection after introduction of
co-formulated buprenorphine/naloxone to
the Malaysian market. Am J Drug Alcohol
Abuse 2009;
35:
68–72.
23 Larance B, Lintzeris N, Ali R, Dietze P, Mat-
tick R, Jenkinson R, et al: The diversion and
injection of a buprenorphine-naloxone solu-
ble film formulation. Drug Alcohol Depend
2014;
136:
21–27.
24 Chen KW, Berger CC, Forde DP, D’Adamo C,
Weintraub E, Gandhi D: Benzodiazepine use
and misuse among patients in a methadone
program. BMC Psychiatry 2011;
11:
90.
25 Pauly V, Pradel V, Pourcel L, Nordmann S,
Frauger E, Lapeyre-Mestre M, et al: Estimated
magnitude of diversion and abuse of opioids
relative to benzodiazepines in France. Drug
Alcohol Depend 2012;
126:
13–20.
26 Herbert A, Gilbert R, Cottrell D, Li L: Causes
of death up to 10 years after admissions to
hospitals for self-inflicted, drug-related or al-
cohol-related, or violent injury during adoles-
cence: a retrospective, nationwide, cohort
study. Lancet 2017;
390:
577–587.
27 Sun EC, Dixit A, Humphreys K, Darnall BD,
Baker LC, Mackey S: Association between
concurrent use of prescription opioids and
benzodiazepines and overdose: retrospective
analysis. BMJ 2017;
356:j760.
28 Vicknasingam B, Mazlan M, Schottenfeld RS,
Chawarski MC: Injection of buprenorphine
and buprenorphine/naloxone tablets in Ma-
laysia. Drug Alcohol Depend 2010;
111:
44–
49.
29 Nielsen S, Degenhardt L, Hoban B, Gisev N:
Comparing Opioids: A Guide to Estimating
Oral Morphine Equivalents (OME) in Re-
search. Technical Report No. 329. Sydney,
National Drug and Alcohol Research Centre,
University of New South Wales, 2014.
30 Lugoboni F, Zamboni L, Federico A, Tambu-
rin S; Gruppo InterSERT di Collaborazione
Scientifica (GICS): Erectile dysfunction and
quality of life in men receiving methadone or
buprenorphine maintenance treatment. A
cross-sectional multicentre study. PLoS One
2017;
12:e0188994.
31 Launonen E, Wallace I, Kotovirta E, Alho H,
Simojoki K: Factors associated with non-ad-
herence and misuse of opioid maintenance
treatment medications and intoxicating drugs
among Finnish maintenance treatment pa-
tients. Drug Alcohol Depend 2016;
162:
227–
235.
32 Hosmer DW, Lemeshow S: Applied Logistic
Regression (ed 2). New York, Wiley, 2000.
33 Duffy P, Baldwin H: The nature of methadone
diversion in England: a Merseyside case
study. Harm Reduct J 2012;
9:
3.
34 Vidal-Trecan G, Varescon I, Nabet N, Bois-
sonnas A: Intravenous use of prescribed sub-
lingual buprenorphine tablets by drug users
receiving maintenance therapy in France.
Drug Alcohol Depend 2003;
69:
175–181.
35 West NA, Severtson SG, Green JL, Dart RC:
Trends in abuse and misuse of prescription
opioids among older adults. Drug Alcohol
Depend 2015;
149:
117–121.
36 Krause D, Plörer D, Koller G, Martin G, Win-
ter C, Adam R, et al: High concomitant mis-
use of fentanyl in subjects on opioid mainte-
nance treatment. Subst Use Misuse 2017;
52:
639–645.
37 Comer SD, Collins ED: Self-administration of
intravenous buprenorphine and the bu-
prenorphine/naloxone combination by re-
cently detoxified heroin abusers. J Pharmacol
Exp Ther 2002;
303:
695–703.
38 Walsh SL, Nuzzo PA, Babalonis S, Casselton
V, Lofwall MR: Intranasal buprenorphine
alone and in combination with naloxone:
abuse liability and reinforcing efficacy in
physically dependent opioid abusers. Drug
Alcohol Depend 2016;
162:
190–198.
39 Guichard A, Lert F, Calderon C, Gaigi H, Ma-
guet O, Soletti J, et al: Illicit drug use and in-
jection practices among drug users on metha-
done and buprenorphine maintenance treat-
ment in France. Addiction 2003;
98:
1585–1597.
40 Jones CM: Trends and key correlates of pre-
scription opioid injection misuse in the Unit-
ed States. Addict Behav 2018;
78:
145–152.
41 Bouvier BA, Waye KM, Elston B, Hadland SE,
Green TC, Marshall BDL: Prevalence and cor-
relates of benzodiazepine use and misuse
among young adults who use prescription
opioids non-medically. Drug Alcohol De-
pend 2018;
183:
73–77.
42 NICE: Methadone and buprenorphine for the
management of opioid dependence. https://
w w w . n i c e . o r g . u k / g u i d a n c e / T A 1 1 4 /
chapter/1-Guidance (accessed on November
4, 2018).
43 Degenhardt L, Larance BK, Bell JR, Winstock
AR, Lintzeris N, Ali RL, et al: Injection of
medications used in opioid substitution treat-
ment in Australia after the introduction of a
mixed partial agonist-antagonist formula-
tion. Med J Aust 2009;
131:
161–165.
44 Darke S, Topp L, Ross J: The injection of
methadone and benzodiazepines among Syd-
ney injecting drug users 1996–2000: 5-year
monitoring of trends from the illicit drug re-
porting system. Drug Alcohol Rev 2002;
21:
27–32.
Downloaded by:
Access provided by the University of Michigan Library
141.216.78.40 - 1/10/2019 11:34:42 AM
Document Outline
- TabellenTitel
- TabellenFussnote
- TabellenTitel
- StartZeile
- Zwischenlinie
- TabellenFussnote
- TabellenTitel
- TabellenFussnote
- TabellenTitel
- TabellenFussnote
Wyszukiwarka
Podobne podstrony:
10 Metody otrzymywania zwierzat transgenicznychid 10950 ppt
10 dźwigniaid 10541 ppt
wyklad 10 MNE
Kosci, kregoslup 28[1][1][1] 10 06 dla studentow
10 budowa i rozwój OUN
10 Hist BNid 10866 ppt
POKREWIEŃSTWO I INBRED 22 4 10
Prezentacja JMichalska PSP w obliczu zagrozen cywilizacyjn 10 2007
Mat 10 Ceramika
BLS 10
10 0 Reprezentacja Binarna
10 4id 10454 ppt
10 Reprezentacja liczb w systemie komputerowymid 11082 ppt
więcej podobnych podstron