
Carboxylic Acid Derivatives and Nitriles
Carboxylic Acid Derivatives:
There are really only four things to worry about under this heading; acid chlorides,
anhydrides, esters and amides. We’ll start with the halides:
Acid Chlorides
Acid chlorides (or bromides) are easily prepared from carboxylic acids, and are extremely
reactive. However, they are very easy to prepare, by two common methods:
1) Thionyl Chloride (SOCl
2
):
O
R
OH
SOCl
2
O
R
Cl
+ SO
2
+ HCl
O
O
OH
OEt
SOCl
2
O
O
Cl
OEt
2) Oxalyl chloride (a much nicer reaction!):
O
R
OH
O
R
Cl
+ CO
2
+ CO + HCl
O
Cl
O
Cl
(Oxalyl Chloride)
Cl
Cl
OH
O
HO
O
Cl-CO-CO-Cl
DMF / CH
2
Cl
2
Cl
Cl
Cl
O
Cl
O
Why make acid chlorides? They are the fastest way to get to any other carboxylic acid
derivative, or to a number of other carbonyl compounds:
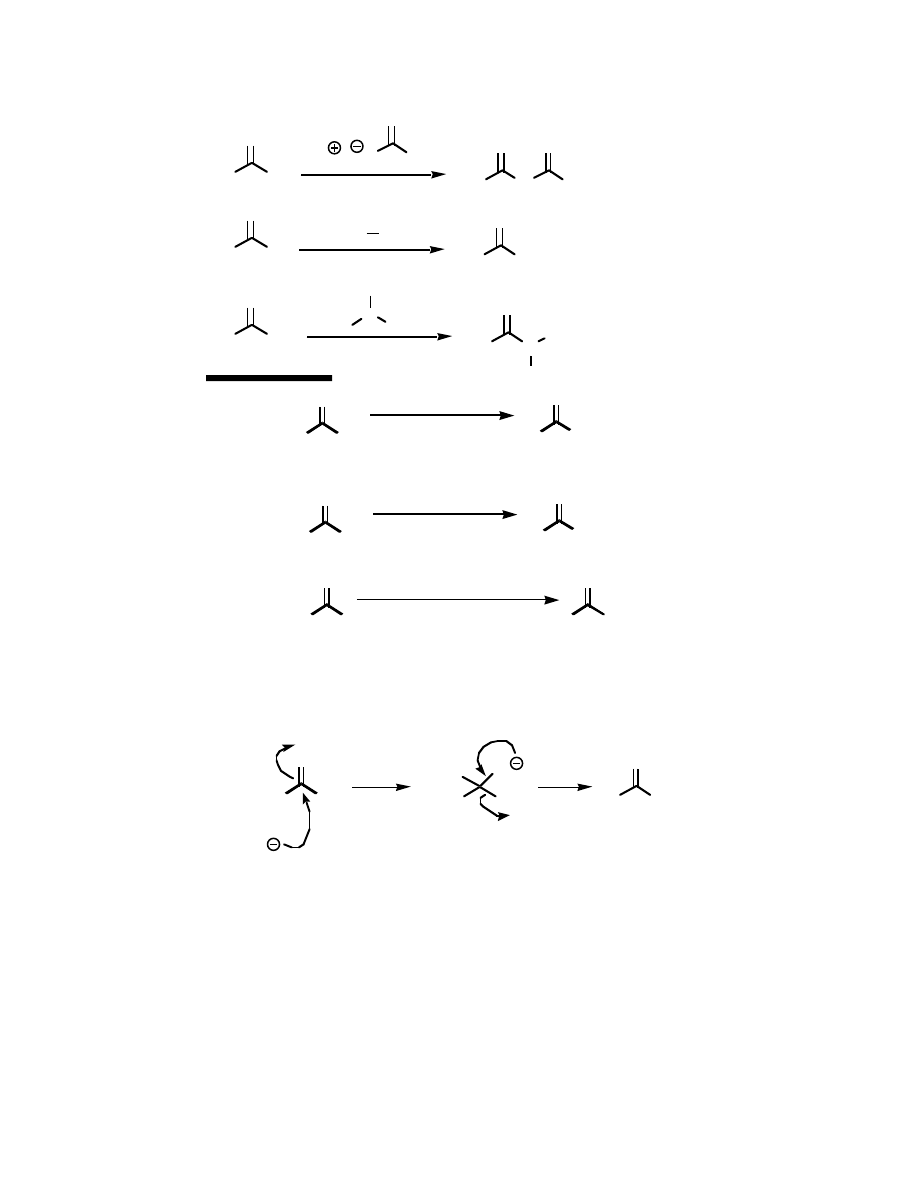
O
R
Cl
O
R'
O
Na
O
R
O
R'
O
(anhydride)
O
R
Cl
HO R'
O
R
OR'
(ester)
O
R
Cl
O
R
N
(amide)
H
N
R'
R''
R''
R'
O
R
Cl
LiAlH(Ot-Bu)
3
O
R
H
O
R
Cl
O
R
R'
R'
2
CuLi
O
R
Cl
O
R
Ar
AlCl
3
Aromatic Compound (Ar)
The mechanism for all of the above reactions (except the last [Friedel-Crafts]) is pretty
much the same – a nucleophile adds to the electrophilic carbonyl group, creating a tetrahedral
intermediate. The electrons on oxygen then pop down, expelling the good leaving group (Cl
-
).
This type of reaction is frequently called an addition-elimination reaction:
O
R
Nu
O
R
Cl
Nu
R
Cl
Nu
O
Acid Anhydrides:
Symmetrical anhydrides are typically made by mixing an acid under dehydrating
conditions. For the most part, this is a difficult reaction to perform, and since the reactivity of
anhydrides is so similar to that of acid chlorides, anhydrides are not commonly used in synthesis.
There are two exceptions to this statement. First, acetic anhydride is fairly easy to prepare in
bulk from dirt-cheap acetic acid, and so it is often used in place of acetyl chloride (MeCOCl).
Molecules which have two carboxylic acid functions in proximity often form cyclic anhydrides -
these are usually fairly easy to prepare. A couple of examples are maleic anhydride and phthalic
anhydride:
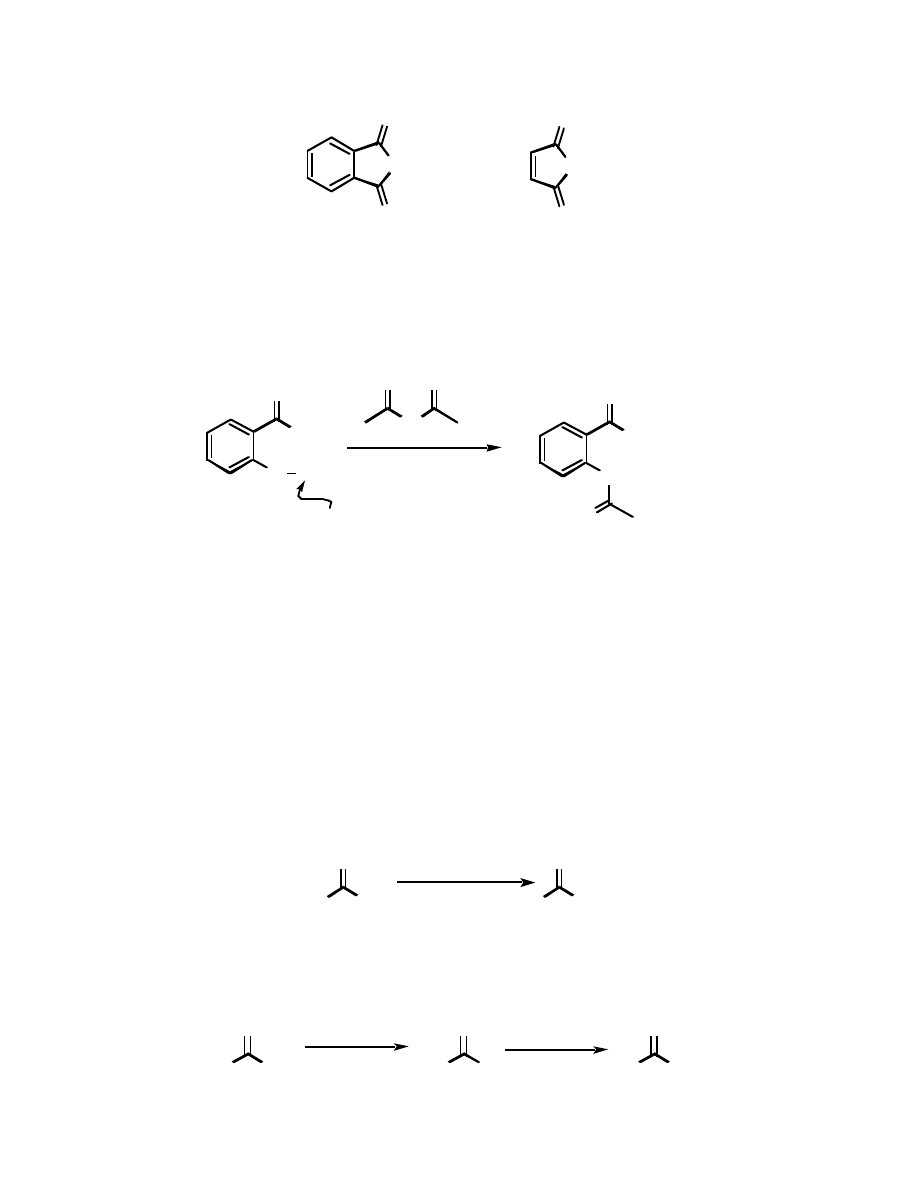
O
O
O
O
O
O
Phthalic anhydride
Maleic anhydride
Again, the only really commonly used anhydride is acetic anhydride. This reagent can be
used to acetylate functional groups such as alcohols and amines. Acetylation can modify both
the chemistry and biological activity of a compound. In the case of aspirin, for example,
acetylation of the relatively acidic phenol alcohol of salicylic acid leads to a compound that
doesn’t dissolve your stomach lining so easily...
O
OH
O
Salicylic acid
H
Acidic proton
O
O
O
O
OH
O
O
Acetyl salicylic acid, Aspirin
(acidic proton gone!!)
So, remember...anhydrides react just about like acid chlorides.
Esters
Preparation of Esters:
This is probably the most common type of organic compounds. They are found in most
biological systems (along with amides), are responsible for many smells (such as the smell of
apples), and are a good way to mask an acidic proton (i.e. a form of protecting group for
carboxylic acids!).
There are two common ways to prepare esters. The easiest by far is the Fischer
esterification. For this reaction, a carboxylic acid is dissolved in an alcohol (such as methanol),
along with a few drops of HCl. Because this reaction requires such a huge excess of the alcohol,
you can imagine that this method only works for relatively cheap and readily available alcohols
(methanol, ethanol, etc.):
R
OH
O
R'OH as solvent
HCl (catalyst)
R
OR'
O
Another way to make esters is to turn the acid into the acid chloride, and then add the
alcohol along with a small amount of base (to pick up the HCl generated by the reaction). This
reaction sequence requires only one equivalent of the alcohol, and thus is valuable in cases where
the alcohol is difficult to prepare or expensive:
R
OH
O
R
Cl
O
R
OR'
O
R'OH
Et
3
N
SOCl
2
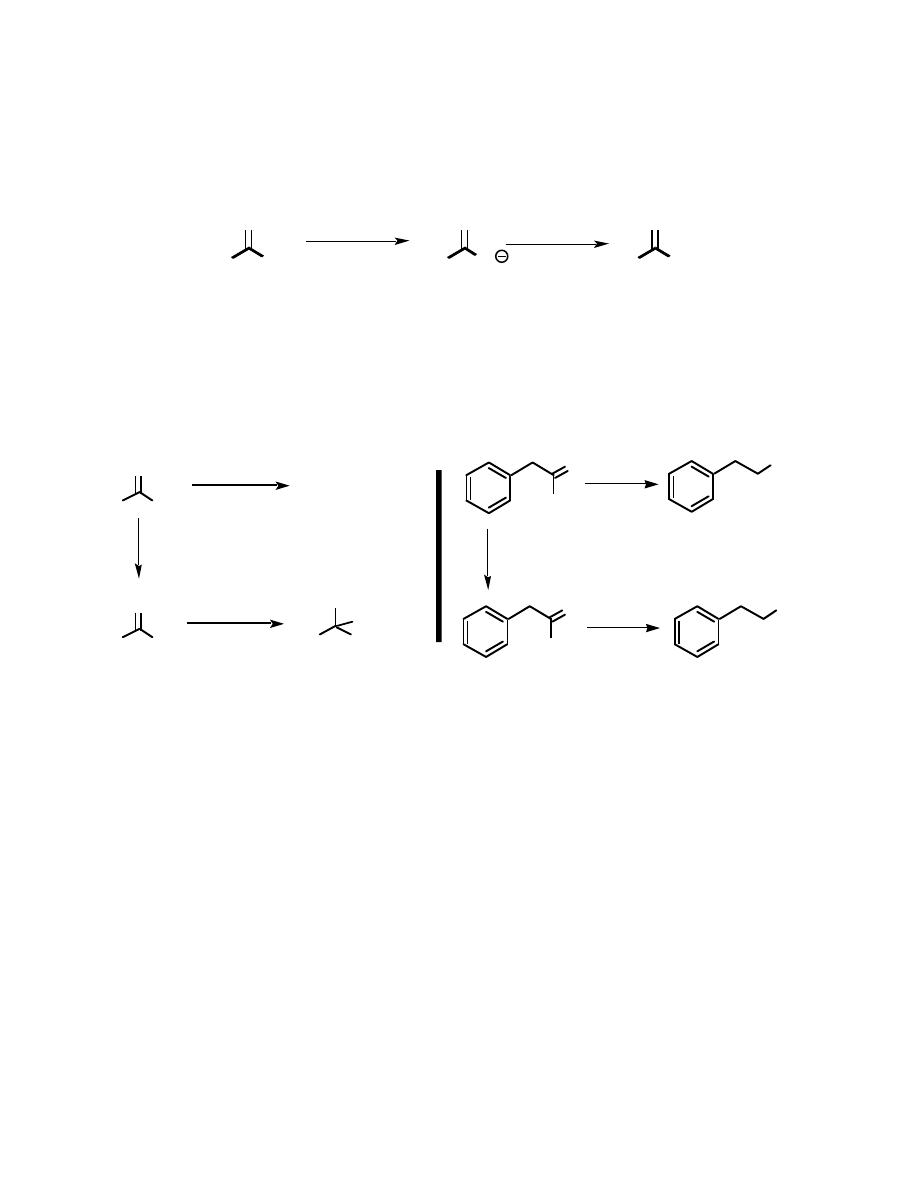
Certain esters can also be prepared by mixing the sodium or potassium salt of the acid
with an alkyl halide. This reaction is of very limited scope (it only works with primary alkyl
halides, but it does work particularly well with allylic and benzylic compounds), but it has some
real synthetic potential, as will be discussed later.
R
OH
O
R
O
O
R
OR'
O
R'Br
DMF or
DMSO
KOH
Uses of Esters:
One of the most useful aspects of esters is that they mask the acidic proton of the
carboxylic acid. As you have seen, most reactions of organo-metallics (RM compounds) will
not work at all with carboxylic acids. However, if we convert the acid to an ester, these
reactions will take place. Furthermore, reductions frequently occur in higher yield when
performed on an ester rather than an acid. Finally, esters are very common in nature, and thus
are often the final goal of synthesis.
R
OH
O
MeMgBr
R
OMe
O
X
MeOH/H
2
SO
4
MeMgBr
R
CH
3
OH
CH
3
O
OH
O
OEt
EtOH/H
2
SO
4
LiAlH
4
LiAlH
4
OH
OH
42% yield
96% yield
Reactions of Esters:
You have already seen many of the reactions possible with esters - reduction to primary
alcohols and addition of RM type reagents to form tertiary alcohols. Another common reaction
is called saponification, and turns the ester back into the respective acid and alcohol. While this
reaction can be done under either acidic or basic conditions, it is usually done with base. The
mechanism goes something like this:
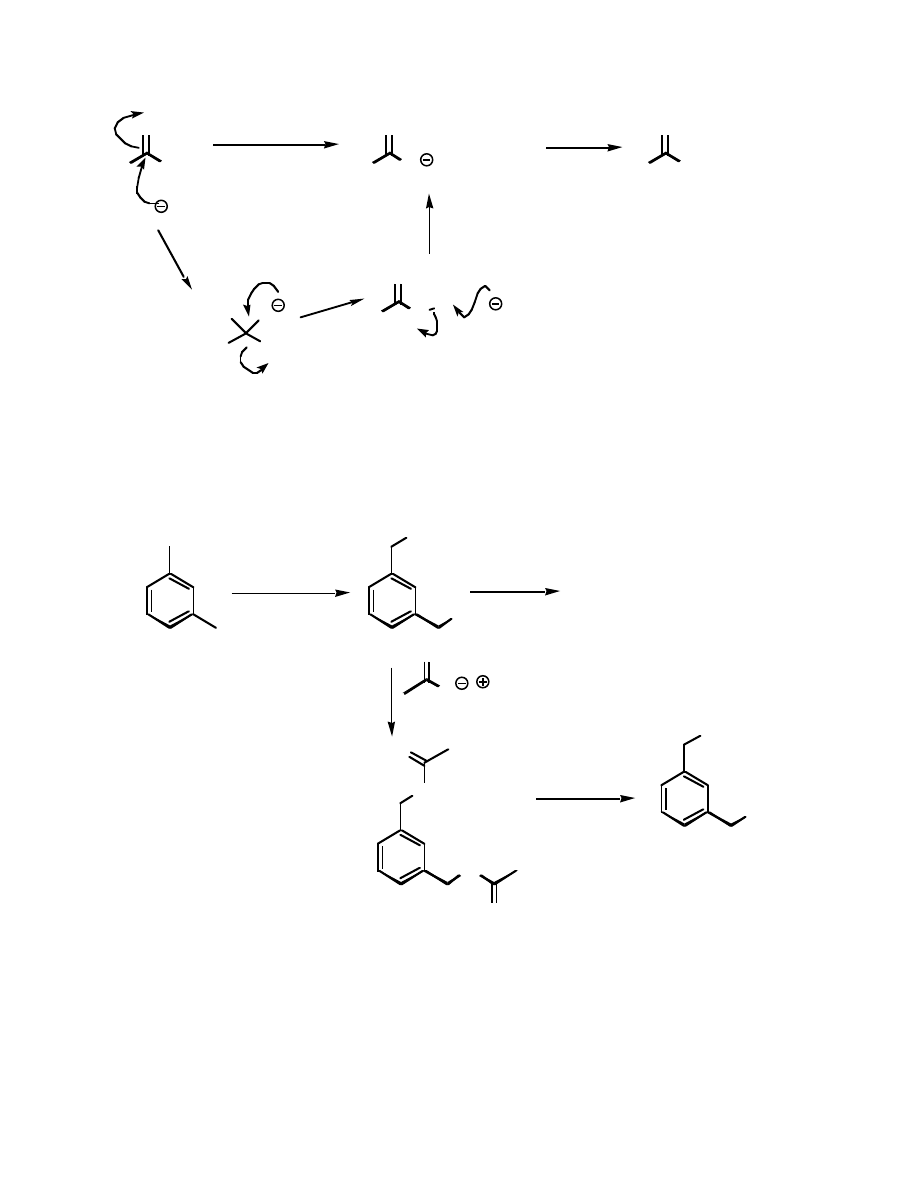
R
OR'
O
KOH / H
2
O
R
O
O
+ HOR'
OH
R
OR'
HO
O
H
3
O
+
R
OH
O
+ HOR'
R
O
O
H
OR'
+
The deprotonation of the acid by the alkoxide is what makes this reaction essentially
irreversible.
The combination of esterification / saponification is an excellent method for the
formation of allylic and benzylic alcohols. You should recall that allylic or benzylic bromides
are easy to prepare. The resulting primary halides are generally resistant to substitution by
simple hydroxide, so typically the esterification (with potassium acetate) and saponification steps
are used to produce the alcohol in excellent yields:
NBS / light
(or Br
2
/ light)
Br
Br
KOH
NO REACTION!
O
O
K
(in DMF, 40°C)
O
O
O
O
96% yield
KOH / H
2
O
100°C
OH
OH
100% yield
“Ester-Transposition” - this term refers to swapping one “OR” portion of an ester for
another, as shown in the schemes below (this reaction can take place under either acidic or basic
conditions):

OMe
O
EtOH / H
+
OEt
O
OMe
O
NaOEt
OEt
O
(large excess)
These reactions are typically run in a LARGE excess of the alcohol / alcoholate to be
“swapped” – this is required because as the reaction proceeds, large amounts of the initial
alcohol are produced. This whole concept will likely be more clear when I explain it during
lecture. While not overly important now, this concept will be important when we discuss
malonic ester chemistry in a few weeks.
Amides
Preparation
Amides are a very common class of nitrogen-containing carbonyl compound. They are
generally prepared by the reaction of an acid chloride with ammonia, or a primary or secondary
amine (why not tertiary amines - think about this!):
O
R
Cl
R
N
R'
H
O
R
N
R
R'
+
O
Cl
Me
N
H
H
O
N
Me
H
+
R
N
R'
H
H
Cl
Me
N
H
H
H
Cl
Amides are the least reactive (and thus, most stable) of the carboxylic acid derivatives.
Because of the strong donation of π-electrons from the amine fragment, the amide carbonyl
group is particularly unreactive:
N
O
Me
N
O
Me
Reactions:
Like esters, amides can be hydrolyzed back to the initial carboxylic acid and amine.
However, this reaction is not often performed – generally, the amide is the goal of syntheis.
Your text adequately outlines the mechanism for both the basic and acidic hydrolysis of amides -
you should be familiar with this.

A more common reaction with amides is their reduction. Unlike the reduction of acids
and esters, however, the lithium aluminum hydride reduction of an amide yields an amine – thus
making this a synthetically useful reaction:
N
H
N
H
Me
O
N
N
H
N
H
Me
N
LiAlH
4
Nitriles
Preparation
Primary alkyl nitriles are generally prepared by the reaction of potassium or sodium
cyanide with a primary alkyl halide. Secondary and tertiary nitriles cannot be formed by this
route, and are thus made by the dehydration of an amide (usually with SOCl
2
, but occasionally
also with P
2
O
5
):
O
R
NH
2
SOCl
2
heat
C
R
N
R
Br
KCN
R
C
N
Reactions
As you have already seen that nitriles can be hydrolyzed to amides and carboxylic acids
quite readily. Your text provides a good description of the mechanism.
R
_
CN
H
3
O
+
H
2
O
120°
R
_
COOH
R
_
CN
HO
-
H
2
O
120°
R
_
CONH
2
210°
HO
-
R
_
COOH
Otherwise, nitriles behave quite similarly to the carbonyl compounds. The nitrile carbon
is electrophilic, and the nitrogen can easily stabilize a negative charge. The nitrile nitrogen is
also easily protonated.
reduction:
Like amides, nitriles are reduced in LiAlH
4
to primary amines. The reaction is quite
clean, usually affording the amine in >80% yield. However, because nitriles are often made
from amides, it is often easier to convert the amide to the amine, rather than go through the
nitrile. The only real exception to this is in the preparation of amino acids, which we shall
discuss in the weeks to come.
By using a weaker reducing agent, it is possible to stop reduction after the addition of
only one hydride, leaving an imine. As you recall, imines are usually prepared by reacting an
amine with a ketone or aldehyde - it is not surprising, then, that this imine can be hydrolyzed to
an aldehyde. Again remember that there are usually easier ways to make aldehydes:
R
C
N
1) LiAlH
4
2) H
3
O
+
R
NH
2
1) DIBAL-H (-78°C)
2) H
2
O
R
O
H
And yes, you can also add Grignard reagents to nitriles. In this case, you also end up
with an imine which is in this case hydrolyzed to a ketone. And again remember, there are
other ways to make the same ketones (i.e. you only have to remember one route...)
Br
C N
O
OH
C
N
Et
H
O
Cl
O
Et
KCN
1) Mg then CO
2
SOCl
2
1) EtMgBr
Et
2
CuLi
H
3
O
+
/ H
2
O
2) H
3
O
+
2) H
3
O
+
Wyszukiwarka
Podobne podstrony:
triazenes carboxylic acid alkylation
Polypeptide Synthesis, Ring Opening Polymerization of alfa Amino Acid N Carboxyanhydrides
Polypeptide Synthesis, Ring Opening Polymerization of alfa Amino Acid N Carboxyanhydrides
derivation flow equation prof J Kleppe
hydrobromic acid eros rh031
peracetic acid eros rp034
p toluenesulfonic acid eros rt134
[38]QUERCETIN AND ITS DERIVATIVES CHEMICAL STRUCTURE AND BIOACTIVITY – A REVIEW
glyoxylic acid eros rg009
Acrylic (and Methacrylic) Acid Polymers
formic acid eros rf025
hypophosphorous acid eros rh075
CALC1 L 4 Derivatives
8 acid mine drainage 1
Derivational Morphology
extraction and analysis of indole derivatives from fungal biomass Journal of Basic Microbiology 34 (
więcej podobnych podstron