
Dinitroaniline herbicides
Masako Ueji
National Institute for Agro-Environmental Sciences, Tsukuba, Japan
1
Introduction
Benfluralin, ethalfluralin, oryzalin, pendimethalin, prodiamine, trifluralin, etc., are
dinitroaniline herbicides used for selective weed control in agronomic and horti-
cultural applications (Figure 1). These compounds are used as soil-incorporated
herbicides for pre-emergence control of annual grass and broad-leaved weed on
cropland, lawns, and nonagricultural land. Germinating plants absorb dinitroaniline
herbicides through the young foliage and roots from the soil layer where the ap-
plied herbicides are present 1–2 cm beneath the soil surface. The absorbed chemicals
transport to the meristem, which act to impair cell division.
Dinitroaniline herbicides, in general, are very lipophilic, hence they are insoluble
in water. They are stable under acidic or alkaline conditions. Dinitroaniline herbicides
are potentially dissipated in the environment via photodegration and volatilization.
Owing to its low water solubility and high octanol/water partition coefficients,
dinitroaniline herbicides adsorb and bind to soil macromolecules and show minimal
leaching potential. Dinitroanilines herbicides show good soil residue activities with
soil half-lives ranging from 30 days for benfluralin and oryzalin to 6–7 months for
trifluralin.
1
N -Dealkylation (aerobic conditions) and reduction of the nitro group to
an amino moiety (anaerobic conditions) have been reported as major soil degradation
pathways.
Dinitroaniline herbicides show minimal plant systematic translocation properties
with the majority of the absorbed residues in the root tissues. Metabolites identified
include traces of N -dealkylation, alkyl and aryl hydroxylation and nitro reduction
products. Low levels of dinitroaniline herbicide residues have been reported in raw
agricultural commodities according to Good Agricultural Practice.
The tolerance for pesticide residues (TPR) in Japan of pendimethalin is 0.2 mg kg
−1
for cereals such as rice grain, wheat and corn; 0.05–0.2 mg kg
−1
for beans such as
red bean, soybean and peanut; 0.05–0.1 mg kg
−1
for fruits such as peach, orange, ap-
ple, banana, papaya, strawberry and grape; 0.05–0.2 mg kg
−1
for vegetables such as
cabbage, tomato, eggplant, carrot, sugar beat and onion; 0.05 mg kg
−1
for nuts such
as almond, chestnut and walnut; and 0.05–0.2 mg kg
−1
for potatoes. TPRs for triflu-
ralin are 0.05–0.1 mg kg
−1
for cereals, 0.05–0.15 mg kg
−1
for beans, 0.05 mg kg
−1
Handbook of Residue Analytical Methods for Agrochemicals.
C
2003 John Wiley & Sons Ltd.
390
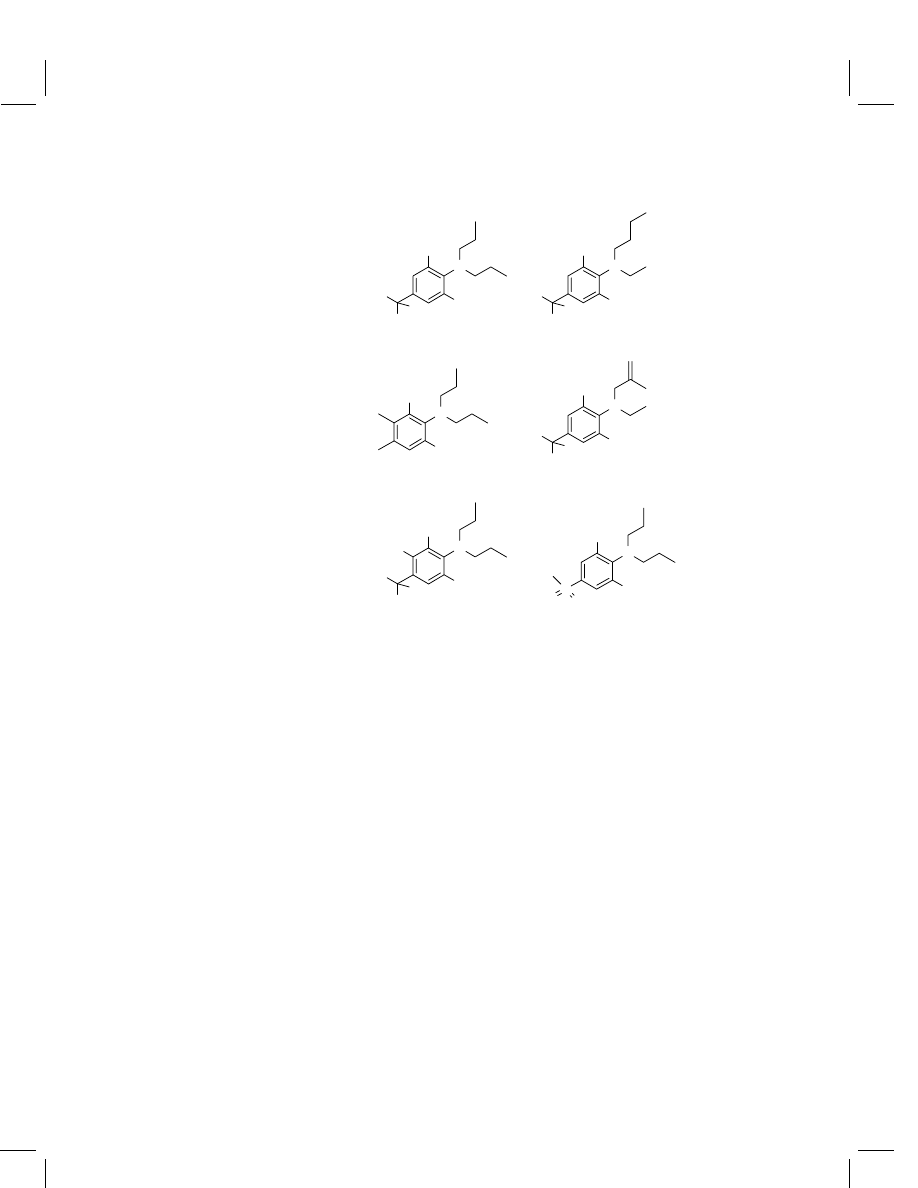
Compound class
N
F
F
F
F
NO
2
NO
2
Trifluralin
N
F
F
F
NO
2
NO
2
Benfluralin
N
NO
2
NO
2
Pendimethalin
N
F
F
NO
2
NO
2
Ethalfluralin
N
F
F
F
NO
2
NO
2
N
H
2
Prodiamine
N
S
NO
2
NO
2
O
O
H
2
N
Oryzalin
Figure 1
Structures of dinitroaniline herbicides
for fruits, 0.05–3 mg kg
−1
for vegetables, 2.0 mg kg
−1
for nuts, 0.05–0.15 mg kg
−1
for potatoes, and 15 mg kg
−1
for tea.
2
Residue analytical methods for dinitroaniline herbicides in crops, soil, and water
samples have been developed. The basic principle of the method consists of the fol-
lowing steps: extraction from the samples with acetone or other organic solvents,
purification using liquid–liquid partition and column chromatography, and quanti-
tative analysis by gas chromatography/nitrogen–phosphorus detection (GC/NPD) or
gas chromatography/electron capture detection (GC/ECD). Column chromatography
is used as the primary cleanup step to simplify the procedure and to enhance accuracy
and sensitivity for the residue method.
2
Analytical methodology for plant materials
2.1
Nature of the residues
Owing to the potential for low levels of residues (parent plus metabolites) in crop
tissues, the definition of dinitroaniline residues in crop samples is expressed as the
parent molecule only.

Dinitroaniline herbicides
391
2.2
Method principle
A homogenized sample of cereals, vegetables, fruits or potatoes (10–20 g) is
extracted with an organic solvent such as acetone and methanol. After filtra-
tion, the extract is concentrated to about 20 mL by rotary evaporation below
40
◦
C. The residue is transferred with 5% sodium chloride (NaCl) aqueous so-
lution and partitioned twice with n-hexane. The n-hexane extracts are dried with
anhydrous sodium sulfate and subjected to a Florisil column chromatographic
cleanup procedure. The eluate from the Florisil column is concentrated to dry-
ness and the residue is dissolved in an appropriate amount of acetone for analysis
by GC/NPD.
3
2.2.1
Extraction
(1) Vegetables and fruits
A 20-g sample of the minced vegetables or fruits is placed in a blender cup, 100 mL
of acetone are added and the mixture is shaken vigorously on a mechanical shaker
for 30 min. The homogenate is filtered under vacuum through a funnel fitted with a
filter paper, and the residue is shaken with 100 mL of acetone and then filtered again.
The filtrates are combined and concentrated to about 20 mL using a vacuum rotary
evaporator.
(2) Brown rice, wheat and bean
The cereal samples are milled with an ultracentrifuge mill and sieved through a 42-
mesh screen, then 10 g of the sieved sample are transferred into a 300-mL Erlenmeyer
flask and soaked in 30 mL of distilled water for 60 min. After 100 mL of acetone have
been added, the procedure described for vegetables and fruits is followed.
Residual pendimethalin in various crops was determined as follows.
4
A 10–20-g
amount of fruits or vegetables was extracted by blending twice with 200 mL of
methanol. Grasses and mint were extracted with 200 mL of methanol–water (1 : 1,
v/v). Nuts were extracted with 200 mL of n-hexane–2-propanol (3 : 1, v/v). For the
residue analysis of the dinitroaniline herbicides butralin, dinitramine, ethalfluralin,
pendimethalin, and trifluralin, a tomato sample (5 g) was extracted twice with 20 mL of
methanol in a Sorvall homogenizer and filtered through filter paper.
5
Benfluralin and
trifluralin residues in the sample (10 g) were extracted with 100 mL of acetonitrile–
water (99 : 1, v/v) in 250-mL screw-cap jars with Teflon liners rotated for 1 h on an
end-over-end shaker (40 rpm).
6
2.2.2
Cleanup
(1) Liquid–liquid partition
(a) NaCl solution–n-hexane partitioning
A 100-mL volume of 5% NaCl aqueous solution and 100 mL of n-hexane are added
to the concentrated extracts prepared in Section 2.2.1, and the mixture is shaken
vigorously for 5 min. The organic layer is collected, 50 mL of n-hexane are added to

392
Compound class
the aqueous layer and the mixture is shaken again. The n-hexane layers are collected,
dried with ca 20 g of anhydrous sodium sulfate, concentrated using a vacuum rotary
evaporator below 40
◦
C and dried under a gentle stream of nitrogen.
(b) Acetonitrile–n-hexane partitioning
In the case of plant samples having high oil contents (for example, rice grain, bean,
and corn), acetonitrile–n-hexane partitioning is used to remove the oily materials. The
concentrated residue obtained in Section (a) above is dissolved in 30 mL of n-hexane
and transferred into a separatory funnel, containing 30 mL of acetonitrile, and the
mixture is shaken vigorously. The acetonitrile layer is collected and another 30 mL of
acetonitrile are added and shaken with the n-hexane layer. The combined acetonitrile
phase is carefully evaporated to dryness.
(2) Column chromatography
(a) Solid-phase extraction (SPE) Florisil cartridge cleanup
An SPE Florisil cartridge is pre-washed with 5 mL of n-hexane–diethyl ether (49 : 1,
v/v) to remove any contaminants. To separate trifluralin, the concentrated residue
obtained in Section (1) (b) above in the flask is loaded in three portions of 5 mL of
the same solution on an SPE Florisil cartridge to the eluate containing trifluralin.
Pendimethalin is eluted with 30 mL of n-hexane–diethyl ether (19 : 1, v/v) after pre-
washing the SPE Florisil cartridge with 10 mL of n-hexane and discarding the eluate
from the cartridge three times with 5 mL of n-hexane. The eluate from the SPE Florisil
cartridge is evaporated to near dryness below 40
◦
C and the residue is made up to the
appropriate volume with acetone for gas chromatography (GC) analysis.
7
(b) SPE silica gel cartridge cleanup
Instead of an SPE Florisil cartridge, an SPE silica gel cartridge is also used. After
pre-washing the SPE silica gel cartridge with 5 mL of n-hexane, the concentrated
residue in the flask is dissolved in 8 mL of n-hexane, the solution is applied to the
cartridge, and then the eluate with n-hexane is discarded. Pendimethalin is eluted with
8 mL of n-hexane–diethyl ether (7 : 3, v/v).
(3) Gel permeation chromatography (GPC)
Cleanup of high oil content samples such as nuts, bean, corn, and rice grain was
accomplished with GPC, before applying column chromatography. Gelsomino et al.
8
analyzed the residues of 48 pesticides including dinitroaniline herbicides with GPC
followed by GC. The residues extracted with an organic solvent were dissolved in
3 mL of GPC mobile phase (ethyl acetate–cyclohexane, 1 : 1, v/v) and injected into
the GPC column. The purified organic fraction was collected, the collection volume of
which was determined from the calibration procedure with corn oil content according
to US Environmental Protection Agency (EPA) method No. 3640. For the determina-
tion of pendimethalin, the residual sample was transferred to the GPC column, and
the pendimethalin-containing eluate of 76–150 mL of cyclohexane–dichloromethane
(17 : 3, v/v) was collected and then evaporated to dryness for the next cleanup proce-
dure using column chromatography.
4

Dinitroaniline herbicides
393
2.2.3
Determination
(1) GC
To determine the residue levels of dinitroaniline herbicides, GC/NPD or GC/ECD
is used in general. An aliquot of GC-ready sample solution is injected into the gas
chromatograph under the conditions outlined below. Further confirmatory analysis is
carried out using gas chromatography/mass spectrometry (GC/MS) in the selected-ion
monitoring (SIM) mode.
(a) GC/NPD
Conditions: apparatus, Hewlett-Packard HP5890; column, DB-5 (30 m
× 0.53-mm
i.d.) with 1.5-µm film thickness; column temperature, 140
◦
C (1 min), increased at
10
◦
C min
−1
to 210
◦
C; inlet and detector temperature, 250 and 270
◦
C, respectively;
gas flow rates, He carrier gas 20 mL min
−1
, H
2
3.5 mL min
−1
, air 130 mL min
−1
; injec-
tion method, splitless mode; injection volume, 2 µL. The retention time of trifluralin
is 5.5 min.
7
Fewer interfering peaks are observed in GC/NPD than in GC/ECD.
(b) GC/ECD
Conditions: apparatus, Hewlett-Packard HP5890; column, DB-17HT (25 m
×
0.25-mm i.d.) with 0.15-µm film thickness; column temperature, 100
◦
C (1 min),
increased at 30
◦
C min
−1
to 180
◦
C and then 5
◦
C min
−1
to 210
◦
C; inlet and detector
temperature, 250 and 300
◦
C, respectively; gas flow rates, He carrier gas 1.5 mL min
−1
,
nitrogen make-up gas 60 mL min
−1
; injection method, splitless mode; injection vol-
ume, 2 µL. The retention time of pendimethalin is 8 min.
7
(c) Gas chromatography/ion trap detection (GC/ITD)
5, 9
Conditions: apparatus, Perkin-Elmer Model 8500; column, BP-1 (12 m
× 0.22-mm
i.d.) with 0.25-µm film thickness; column temperature, 85
◦
C (1 min), increased at
20
◦
C min
−1
to 180
◦
C (1 min) and then at 10
◦
C min
−1
to 250
◦
C; inlet and detector
temperature, 270 and 300
◦
C, respectively; gas flow rate, He carrier gas 10 psig
(1 psig
= 6895 Pa); injection method, splitless mode; injection volume, 2-µL; mass
range, m
/z 120–400; scan rate, 0.5 s per scan, 3-µscans; radiofrequency voltage,
1.1 MHz and 0–7.5 kV; automatic gain control, 78 µs–25 ms; solvent delay, 5 min.
The retention times of ethalfluralin, trifluralin, dinitramine, butralin and pendimethalin
are 6.6, 7.1, 8.1, 10.0 and 10.2 min, respectively.
(d) GC/MS
8
Conditions: apparatus, Hewlett-Packard HP5890 equipped with an HP5972 mass-
selective ion detector (quadruple); column, PTE-5 (30 m
× 0.25-mm i.d.) with
0.25-µm film thickness; column temperature, 50
◦
C (1 min), increased at 20
◦
C min
−1
to 150
◦
C (5 min) and then at 4
◦
C min
−1
to 280
◦
C (30 min); inlet and detector (GC/MS
transfer line) temperature, 250 and 280
◦
C, respectively; gas flow rate, He carrier gas
1 mL min
−1
; injection method, splitless mode; solvent delay, 3 min; electron ion-
ization voltage, 70 eV; scan rate, 1.5 scans s
−1
; scanned-mass range, m
/z 50–550.
The retention times of benfluralin, pendimethalin and trifluralin are 15.2, 25.1 and
15.1 min, respectively. The main ions of the benfluralin mass spectrum were at m
/z

394
Compound class
292, 264 and 335. Pendimethalin showed the most abundant ion at m
/z 252. Trifluralin
presented a fragmentation pattern with main ions at m
/z 306 and 263.
(2) High-performance liquid chromatography (HPLC)
HPLC has also been used to determine the residue levels of dinitroaniline herbi-
cides.
5
,10
Pendimethalin was quantified by HPLC under the following conditions:
apparatus, Spectroflow 400 solvent delivery system, Model 430 gradient former, and
Kratos Model 783 with UV absorbance detection at 239 nm; column, C
18
reversed-
phase (25 cm
× 3.0-mm i.d.); temperature, 40
◦
C; mobile phase, acetonitrile–water
(7 : 3, v/v); flow rate, 1 mL min
−1
.
2.2.4
Evaluation
Quantitation is performed by the calibration technique. A new calibration curve is
constructed with each dinitroaniline standard solution. The peak area is plotted against
the injected amount of standard. Each dinitroaniline in the sample is measured by using
the peak area for each standard. Before each set of measurements, the sensitivity and
stability of the GC and HPLC system is ascertained by injecting more than one
standard solution containing ca 0.05–2 mg L
−1
of each compound.
2.2.5
Recoveries, limit of detection (LOD) and limit of quantitation (LOQ)
The minimum detectable level is estimated with the dinitroaniline signal-to-noise
ratios (S/N). With fortification levels between 0.2 and 0.5 mg kg
−1
, the recovery of
trifluralin from plant matrices is 70–99% with the LOD/LOQ being 0.005 mg kg
−1
according to the analytical method of the Ministry of the Environment, Japan. In
multiresidue analysis by GC/NPD, the percent recoveries of pendimethalin from
each crop with a fortification level of 0.25 mg kg
−1
were brown rice 70, potato 70,
cabbage 80, lettuce 89, carrot 84, cucumber 64, shiitake 74, apple 76, strawberry 99,
and banana 99%. The LOD for each sample was 0.01 mg kg
−1
for pendimethalin.
11
In residue analysis by GC/ECD, recoveries of the majority of dinitroaniline her-
bicides from fortified samples of carrot, melon, and tomato at fortification levels
of 0.04–0.10 mg kg
−1
ranged from 79 to 92%. The LODs were benfluralin 0.001,
pendimethalin 0.002 and trifluralin 0.001 mg kg
−1
for the GC/ECD method.
8
The recoveries of five herbicides (ethalfluralin, trifluralin, dinitramine, butralin,
and pendimethalin) added to tomato in the range 0.1 to 1 mg kg
−1
were determined
using GC-ITD. The average recoveries ranged from 84 to 104%, and the detection
limit of these compounds was near 0.01 mg kg
−1
.
5
GC/MS in the SIM mode was carried out for confirmation of all positive and
ambiguous results obtained from GC analysis. GC/MS was effective as a multiresidue
screening method for crops; the mean recovery of trifluralin from green bean, cilantro,
apple, tomato, and green onion at a fortification level of 0.25 mg kg
−1
was 55% and
the LOD was 0.05 mg kg
−1
.
12
With the HPLC method, the recovery of pendimethalin from turf grass at a fortifi-
cation level of 0.25 mg kg
−1
was 97% and the LOD was 0.001 mg kg
−1
.
10

Dinitroaniline herbicides
395
2.2.6
Calculation of residues
The amount of dinitroaniline herbicide residue (R, mg kg
−1
) in the sample is calcu-
lated with the following equation:
R
= (W
i
/ V
i
)
/(V
f
/G)
where
G
= sample weight (g)
V
i
= injection volume into the gas chromatograph (µL) or high-performance liquid
chromatograph (µL)
V
f
= final sample volume (mL)
W
i
= amount of dinitroaniline herbicide for V
i
read from the calibration curve (ng)
3
Analytical methodology for soil
3.1
Nature of the residues
Dinitroaniline herbicides are generally stable in soil. Residue methods were developed
for both the parent molecule and selective soil degradates.
3.2
Method principle
Air-dried soil samples were screened through a 2-mm sieve, then the water content
in the soil was calculated after holding the soil samples for 5 h at 105
◦
C.
Residual dinitroaniline herbicides are generally extracted from 10–25 g of air-dried
soil samples using organic solvents such as ethyl acetate, acetonitrile, methylene chlo-
ride and acetone by sonication, mechanical shaking or Soxhlet extraction. If necessary,
the extract is then cleaned by a Florisil column or SPE. The extract is allowed to evap-
orate completely to dryness and the residue is dissolved in an appropriate volume of
the solvent for GC or HPLC analysis.
3.2.1
Extraction and cleanup
A 20-g sample of air-dried soil is extracted with 100 mL of ethyl acetate in a flask
shaker for 45 min. After shaking, the extract is decanted and separated. The soil is
re-extracted with 100 mL of ethyl acetate for 45 min. The combined soil extracts are
filtered through a Whatman No 1 filter paper and the filter cake is washed with an
additional 20 mL of ethyl acetate. The extracts are evaporated nearly to dryness, under
vacuum, using a rotary evaporator. The residue is dissolved in an appropriate volume
before GC analysis.
5
Garimella et al.
13
, investigated the effect on trifluralin recovery of different ex-
traction methods. A supercritical fluid extraction (SFE) procedure for the isolation of
the analytes from the matrices with a commercial SFE system (Dionex Model 703)

396
Compound class
was developed, and for analyte collection, C
18
traps (Dionex) were used. The collec-
tion tube was activated by passing methanol (1 mL) and acetone (1 mL), succes-
sively. The extraction of 3 g of soil sample was conducted at 20.3 mPa for 3 min and
then at 34.4 mPa for 17 min using highly purified CO
2
. The oven temperature was
maintained at 60
◦
C and the restrictor temperature at 125
◦
C. As a co-solvent, 15%
(v/v) acetone in CO
2
was used and the analyte was collected and transferred on to
the SPE tube. The SPE extract was used for GC or HPLC analysis. In the case of
liquid vortex extraction, 10 g of soil sample were vortexed three times for 2 min with
20 mL of acetone and equilibrated overnight. The samples were then vortexed four
times for 10 s and centrifuged at 870 g, and the supernatant was collected for GC or
HPLC analysis. Soxtec extraction was performed on an automated Soxhlet (Tecator
HT 1045 and HT2 1046 Soxtec). Samples of 5 g of soil in the extraction thimbles
were placed in the Soxtec apparatus with 75 mL of acetone in the extraction cup. The
temperature was set at 130
◦
C and the samples were boiled for 20 min followed by
15 min of rinsing. The acetone extract was concentrated for GC or HPLC analysis. By
comparing the extraction efficiencies with these extraction methods, SFE and liquid
vortex extraction of trifluralin from soil samples were determined to be preferable to
Soxtec extraction.
SPE purification was carried out continually after the SFE procedure. The SPE
tube was mounted on a vacuum manifold and preconditioned with 2 mL acetone and
2 mL of acetone–water (2 : 1, v/v) successively. The tube was connected with a 25-mL
reservoir into which the extract was transferred. After percolation, the tube was rinsed
with 10 mL of water–acetone (2 : 1, v/v) and the sorbent was dried under vacuum for
15 min. The residue was eluted with 5 mL of acetone into a volumetric flask. As well
as water–acetone (2 : 1, v/v), 2 mL of acetonitrile–water (1 : 1, v/v) were also used for
rinsing.
6
,13
3.2.2
Determination and evaluation
The determination of the residue levels by GC and HPLC and evaluation of the
residue levels were carried out by the procedures described for the plant material in
Sections 2.2.3 and 2.2.4, respectively.
3.2.3
Recoveries, limit of detection and limit of quantitation
For the determination of five herbicides (ethalfluralin, trifluralin, dinitramine, bu-
tralin, and pendimethalin) at fortification levels between 0.1 and 1 mg kg
−1
, soil was
extracted with ethyl acetate and the extract was purified on a Florisil column. The
residues were eluted with acetone and then analyzed by GC. The average recoveries
varied from 75 to 111% for GC/NPD and from 88 to 98% for GC/ITD with the LOD
being 0.01 mg kg
−1
for both GC/NPD and GC/ITD.
5
The recoveries of pendimethalin
at fortification levels ranging from 0.2 to 1 mg kg
−1
determined by GC/NPD were be-
tween 96 and 101% and the LOD was lower than 0.01 mg kg
−1
.
9
In the HPLC method for the simultaneous determination of dinitramine, ethalflu-
ralin, trifluralin, pendimethalin, and isopropalin, a Spherisorb ODS-2 column
(25 cm
× 4.6-mm i.d.) was used; the mobile phase was acetonitrile–water (11 : 9, v/v)
at a flow rate of 1.0 mL min
−1
with UV absorbance detection at 220 nm. The average
Dinitroaniline herbicides
397
recoveries for extraction with diethyl ether from soil were in the range 89–104% and
the LOD for these herbicides was 0.02 mg kg
−1
.
14
3.2.4
Calculation of residues
Calculation of residues in soil was carried out as described in Section 2.2.6.
3.3
Analytical method for soil metabolites
The fate of the dinitroaniline herbicides in soil is extremely complex and many
metabolites have been identified. Golab and Althaus
15
reported 28 metabolites iden-
tified in a degradation study of trifluralin in soil. Major degradation products of
dinitroaniline herbicides were formed by nitro reduction, N -dealkylation (mono-
dealkylated and completely dealkylated) and the ring formation of benzimidazole.
Analytical methods for fortified soils were developed for the simultaneous
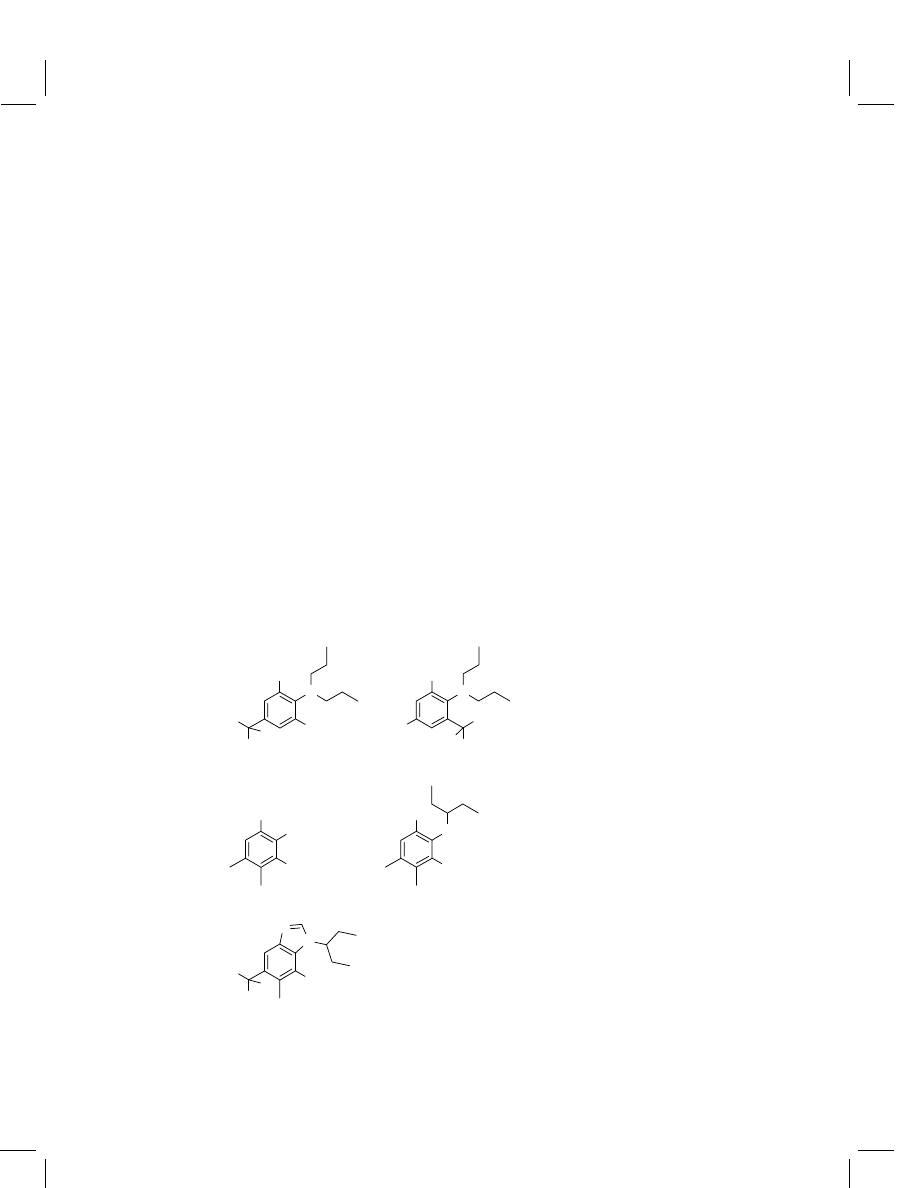
quantitation of the trifluralin metabolites, 2,6-dinitro-N -propyl-4-(trifluoromethyl)-
benzenamine (1) and 2,4-dinitro-N
,N-dipropyl-6-(trifluoromethyl)benzenamine
(2)
13
(Figure 2). The SFE method developed as described in Section 2.2.1 was
extended to the determination of these metabolites. From soil fortified with 0.5–
2.5 mg kg
−1
each of trifluralin, (1) and (2), the compounds were efficiently extracted
by this procedure. Trifluralin and its metabolites (1) and (2) are characterized by ab-
sorbance bands in both the ultraviolet (UV) and visible ranges for HPLC; however,
N
F
F
F
NO
2
NO
2
(1)
N
NO
2
F
F
F
O
2
N
(2)
NO
2
NH
(4)
NO
2
NH
2
NH
2
NO
2
(3)
F
F
F
N
N
NO
2
(5)
Figure 2
Structures of metabolites of trifluralin (1 and 2) and pendimethalin (3–5)

398
Compound class
in the research by Garimella et al.,
13
these compounds were monitored simultane-
ously in the visible range at 386 nm. This wavelength permitted the use of acetone,
which had a UV cutoff at 330 nm, as the mobile phase [acetone–water (4 : 1, v/v)].
The predicted degradation products of pendimethalin, via dealkylated pendi-
methalin (3,4-dimethyl-2,6-dinitroaniline) (3), partially reduced pendimethalin [N -
(1-ethylpropyl)-3,4-dimethyl-2-nitro-1,6-diaminobenzene] (4) and cyclized product
[N -(1-ethylpropyl)-5,6-dimethyl-7-nitrobenzimidazole] (5) (Figure 2) were deter-
mined. A 100-g sample of soil was extracted with 300 mL of methanol–concentrated
HCl (49 : 1, v/v) by shaking for 1 h. The extract was concentrated and partitioned
with n-hexane for pendimethalin and with n-hexane–ethyl acetate (1 : 1, v/v) for
pendimethalin degradation products and these compounds were detected by GC/ECD.
The GC conditions were as follows: column, megabore column packed with 3% OV-
17; inlet, column and detector temperatures, 235, 210 and 275
◦
C, respectively; gas
flow rate, nitrogen 20 mL min
−1
. With an injection volume of 3 µL, the retention
times were 3.5 min for pendimethalin, 1.8 min for (3), 3.0 min for (4) and 3.6 min
for (5). The recoveries of pendimethalin and degradation products with fortification
levels ranging from 0.2 to 1 mg kg
−1
determined by GC/ECD were more than 85%
for soil. A linear response was obtained between 0.1 and 5 ng.
16
4
Analytical methodology for water
4.1
Nature of the residues
Dinitroaniline herbicides have low soil mobility potential. Herbicide residues in the
treated field are usually incorporated into the upper layers of the soil mainly as
unextractable bound residue; therefore, the movement of dinitroaniline herbicides
from soil to the water compartment is minimal. Run-off is the principal route, which
could lead to the contamination of surface waters. Residue methods were developed
to measure the parent concentration in water samples.
4.2
Analytical method
Water (1000 mL) is transferred into a 2-L separatory funnel and extracted with two
portions of 50 mL of dichloromethane for 30 min with a mechanical shaker, and the
extracts are collected in a 200-mL Erlenmeyer flask. The combined extracts are filtered
through anhydrous sodium sulfate into a 300-mL round-bottom flask and evaporated
to dryness with a rotary evaporator under vacuum. The residue is dissolved in 1 mL
of n-hexane and an aliquot is analyzed by GC/NPD or GC/ITD under the conditions
described in Section 2.2.3.
9
Recoveries from water samples fortified with 0.0002
and 0.001 mg L
−1
of pendimethalin were in the range 94–110% by GC/NPD and
91–111% by GC/ITD. The detection limit was lower than 0.0001 mg L
−1
with both
methods.
Cabras et al.
14
reported an HPLC residue method for dinitroaniline herbicides.
A water sample was analyzed after purification and concentration on a Bond-Elut
C
18
cartridge (500-mg/2.8-mL). The cartridge was treated with 10 mL of methanol

Dinitroaniline herbicides
399
followed by 10 mL of water. A 100-mL water sample was added to the cartridge
using a reservoir and the cartridge was allowed to percolate slowly (1 mL min
−1
). The
reservoir was removed and the cartridge was washed with 5 mL of methanol–water
(1 : 1, v/v), followed by 5 mL of water. The cartridge was air-dried under vacuum for
2 min and then the dinitroaniline herbicides were eluted with 2 mL of diethyl ether. The
extract was evaporated completely to dryness and the residue was dissolved in 1 mL
of mobile phase, acetonitrile–water (3 : 1, v/v). The recoveries from water samples
fortified with 0.002 mg L
−1
of dinitramine, ethalfluralin, isopropalin, pendimethalin,
and trifluralin were 89–104%. The LOD was 0.0005 mg L
−1
in water for the five
herbicides.
Calculation of residues in water was carried out as described in Section 2.2.6 for
plant material.
References
1. T.R. Roberts, D.H. Hutson, P.W. Lee, P.H. Nicholls, and J.R. Plimmer, ‘Metabolic Pathways of
Agrochemicals,’ Royal Society of Chemistry, Cambridge (1998).
2. Ministry of Health, Labor and Welfare, Japan, ‘Specifications and Standards for Food, etc.,’
Ministry of Health, Labor and Welfare, Japan, Tokyo.
3. Ministry of the Environment, ‘Analytical Method for Trifluraline, Notification of Ministry of
the Environment, Japan,’ Ministry of the Environment, Tokyo (1976) (in Japanese).
4. J. Engebretson, G. Hall, M. Hengel, and T. Shibamoto, J. Agric. Food Chem., 49, 2198 (2001).
5. A.I. Garcia-Valcarcel, C. Sanchez-Brunete, L. Martinez, and J.I. Tadeo, J. Chromatogr., 719,
113 (1996).
6. A.A. Krause and H.D. Niemczyk, J. Environ. Sci. Health, B27, 39 (1992).
7. M. Ueji, H. Kobayashi, and K. Nakamura, ‘Analytical Methods for Pesticide Residues,’ Soft
Science, Tokyo, pp. 397, 508 (2001) (in Japanese).
8. A. Gelsomino, B. Petrovicova, S. Tiburtini, E. Magnaniand, and M. Felici, J. Chromatogr., 782,
105 (1997).
9. C. Sanchez-Brunete, L. Martine, and J.L. Tadeo, J. Agric. Food Chem., 42, 2210 (1994).
10. J.J. Gasper, J.R. Street, S.K. Harrison, and W.E. Pound, Weed Sci., 42, 586 (1994).
11. Y. Nakamura, Y. Tonogai, Y. Sekiguchi, Y. Tsumura, N. Nishida, K. Takakura, M. Isechi,
K. Yuasa, M. Nakamura, N. Kifune, K. Yamoto, S. Terasawa, T. Oshima, M. Miyata,
K. Kamakura, and Y. Ito, J. Agric. Food Chem., 42, 2508 (1994).
12. W. Liao, T. Joe, and W.G. Cusick, J. Assoc. Off. Anal. Chem., 74, 554 (1991).
13. U.I. Garimella, G.K. Stearman, and M.J.M. Wells, J. Agric. Food Chem., 48, 5874
(2000).
14. P. Cabras, M. Melis, L. Spanedda, and C. Tuberoso, J. Chromatogr., 585, 164 (1991).
15. T. Golab and W.A. Althaus, J. Agric. Food Chem., 27, 163 (1979).
16. G. Kulshrestha, S.B. Singh, S.P. Lal, and N.T. Yaduraju, Pestic. Manage. Sci., 56, 202 (2000).
Document Outline
- Front Matter
- Table of Contents
- Volume I
- Regulatory Guidance and Scientific Consideration for Residue Analytical Method Development and Validation
- Best Practices in the Generation and Analysis of Residues in Crop, Food and Feed
- Compound Class
- Anilides
- Chloroacetanilide Herbicides
- Dinitroaniline Herbicides
- Sulfonylurea Herbicides
- Triazine Herbicide Methodology
- Diphenyl Ethers
- Individual Compounds
- Volume II
- Index
Wyszukiwarka
Podobne podstrony:
Ćw 03c Izolacja limfocytów ze śledziony oraz określanie żywotności komórek
03c
03c KONTA WYNIKOWE I WYNIK FINANSOWY
03C Dziecko w sieci 3 10 2009
FIG-03C
Ćw 03c Izolacja limfocytów ze śledziony Obliczanie żywotności limfocytów
28 03c
FIG 03C
91942 abb
91942 08q
SB07 03C
91942 04m
91942 05d
91942 03d
91942 01e
więcej podobnych podstron