Neuroscience Research 36 (2000) 175 – 182
Update article
Protein – protein interactions in neurotransmitter release
Sumiko Mochida *
Department of Physiology, Tokyo Medical Uni
6ersity,
1
-
1
Shinjuku-
6
-chome, Shinjuku-ku, Tokyo
160
-
8402
, Japan
Received 1 November 1999; accepted 10 December 1999
Abstract
The arrival of a nerve impulse at a nerve terminal leads to the opening of voltage-gated Ca
2 +
channels and a rapid influx of
Ca
2 +
. The increase in Ca
2 +
concentration at the active zone from the basal level of 100 – 200 mM triggers the fusion of docked
synaptic vesicles, resulting in neurotransmitter release. A large number of proteins have been identified at nerve terminals and a
cascade of protein – protein interactions has been suggested to be involved in the cycling of synaptic vesicle states. Functional
studies in last half decade on synaptic-terminal proteins, including Ca
2 +
channels, have revealed that the SNARE core complex,
consisting of synaptobrevin VAMP, a synaptic vesicle-associated protein, syntaxin and SNAP-25, synaptic membrane-associated
proteins, acts as the membrane fusion machinery and that proteins interacting with the SNARE complex play essential roles in
synaptic vesicle exocytosis by regulating assembly and disassembly of the SNARE complex. © 2000 Elsevier Science Ireland Ltd.
All rights reserved.
Keywords
:
Neurotransmitter release; Synaptic vesicle; Exocytosis; Snare proteins; Synaptic terminal proteins; Ca
2 +
channels
www.elsevier.com/locate/neures
1. Introduction
Exocytosis in neurons requires proteins known as
SNAREs A set of three synaptic membrane proteins,
the synaptic vesicle protein synaptobrevin (also known
as VAMP, vesicle-associated membrane protein) and
the plasma membrane proteins syntaxin and SNAP-25
(synaptosome-associated protein of 25 kDa), were orig-
inally identified as membrane receptors for NSF (N-
ethylmaleimide-sensitive factor) and SNAPs (soluble
NSF attachment proteins) and were therefore, desig-
nated as SNAREs(SNAP receptors) (So¨llner et al.,
1993a). This finding directly linked these proteins to
exocytosis, as NSF and SNAPs are soluble proteins
known to be essential for many intracellular vesicle
fusion reactions. A second line of evidence linking these
proteins to exocytosis came from the discovery that
tetanus and bolutinum neurotoxins, a group of eight
related paralytic neurotoxins produced by Clostridia,
block neuronal exocytosis by selectively proteolyzing
the individual SNARE proteins (Niemann et al., 1994;
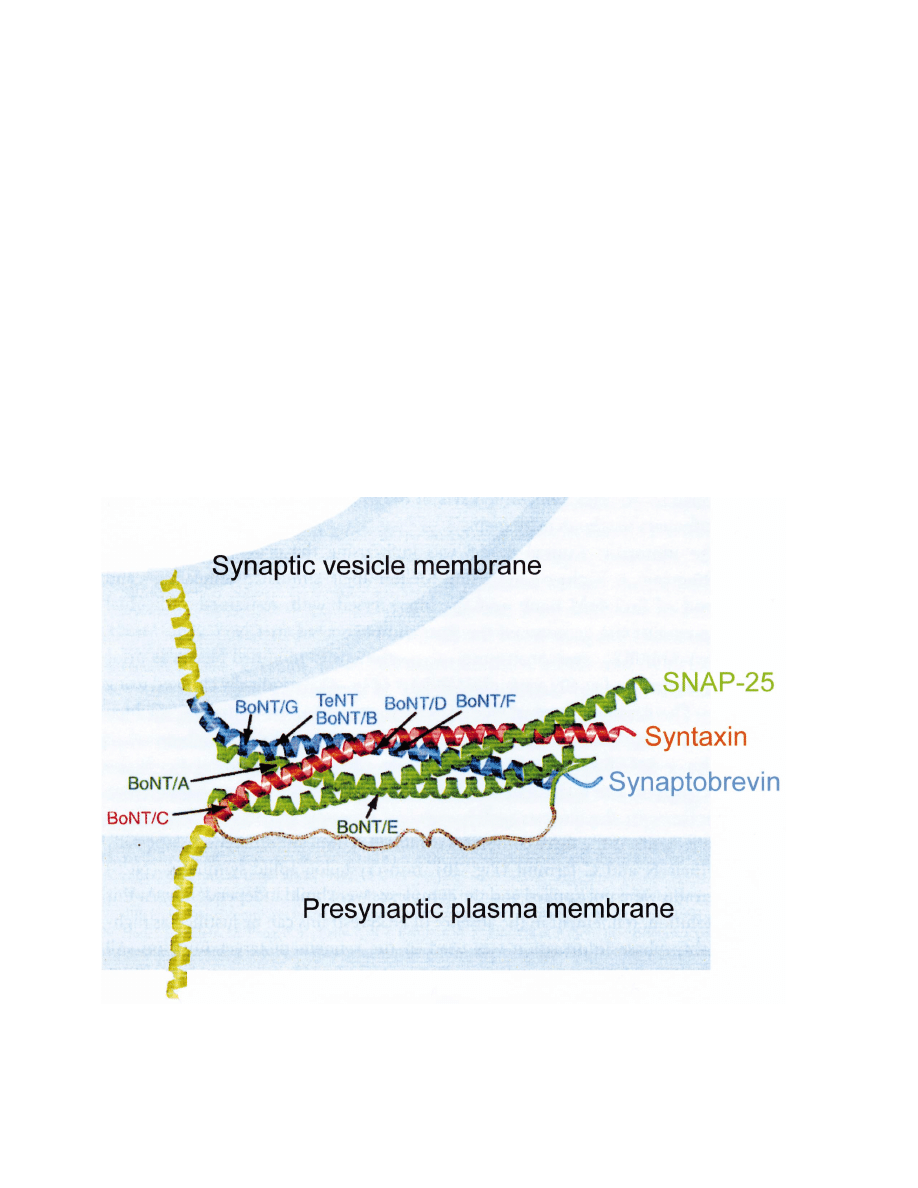
Monteccuco and Schiavo, 1995; Fig. 1). Furthermore,
proteins related to syntaxin, SNAP-25 and VAMP are
essential for a variety of other membrane transport
reactions. This has been best characterized in yeast,
where proteins homologous to the SNAREs have been
shown to be important in trafficking throughout the
secretory pathway (Bennett and Scheller, 1993; Ferro-
Novick and Jahn, 1994). These lines of evidence consid-
ered together implicate the SNAREs in neuronal
exocytosis. SNARE-associated proteins, including small
GTP-binding proteins and Ca
2 +
-binding proteins, have
been identified in nerve terminals and their regulatory
roles in exocytosis have been discussed in a number of
excellent reviews (Su¨dhof, 1995; Augustine et al., 1996;
Bean and Scheller, 1997; Hanson et al., 1997). This
article will focus on recent findings concerning synaptic
protein – protein interactions implicated in nerve im-
pulse-evoked exocytosis.
* Tel.: + 81-33-516140 ext. 248; fax: + 81-35-3790658.
E-mail address
:
mochida@tokyo-med.ac.jp (S. Mochida)
0168-0102/00/$ - see front matter © 2000 Elsevier Science Ireland Ltd. All rights reserved.
PII: S 0 1 6 8 - 0 1 0 2 ( 9 9 ) 0 0 1 2 8 - 5
S. Mochida
/
Neuroscience Research
36 (2000) 175 – 182
176
2. SNARE complexes
SNAREs assemble with a 1:1:1 stoichiometry into
stable ternary complexes that are disassembled by NSF,
an ATPase, working together with
a-SNAP (So¨llner et
al., 1993b; Hayashi et al., 1995). Are SNAREs involved
in vesicle docking or fusion? SNAREs represented by
synaptobrevin are vesicle-membrane, or ‘v-SNAREs’,
whereas SNAREs represented by syntaxin and SNAP-
25 are target-membrane, or ‘t-SNAREs’ (So¨llner et al.,
1993a; Rothman, 1994). As v and t-SNAREs are pre-
dominantly located on the vesicles and target mem-
branes, respectively, it has been proposed that the
formation of SNARE complexes may play a critical
role in establishing and stabilizing membrane docking
(So¨llner et al., 1993a; Rothman, 1994). However, func-
tional experiments using clostridial neurotoxins have
shown that disruption of the v-SNARE, synaptobrevin
(Hunt et al., 1994; Sweeney et al., 1995), or that of the
t-SNARE, SNAP-25 (Banerjee et al., 1996), does not
affect the docking or priming of synaptic vesicles. Ge-
netic deletion of the t-SNARE, syntaxin or the v-
SNARE in Drosophila had profound effects on the
function of secretory pathways, with complete loss of
synaptic transmission (Broadie et al., 1995; Schulze et
al., 1995; Sweeney et al., 1995; Deitcher et al., 1998).
These results indicate that SNAREs do not play an
essential role in docking.
Recent in vitro experiments revealed that SNARE
complexes are the minimal machinery required for
membrane fusion (Weber et al., 1998). Weber et al.
(1998) demonstrated that recombinant synaptobrevin
and the syntaxin/SNAP-25 complex, reconstituted into
separate lipid vesicles assemble into trans SNARE com-
plexes, designated as ‘SNAREpins’, linking two mem-
branes. This leads to spontaneous lipid mixing,
considered to be an index of fusion between the docked
membranes at physiological temperature. SNARE com-
plexes are formed by coiled-coil interactions of the
a-helices of syntaxin, SNAP-25 and synaptobrevin
(Chapman et al., 1995a) immediately before fusion
(Sutton et al., 1998). Electrostatic calculations show a
pronounced charge distribution of the synaptic fusion
complex, the cumulative electrostatic potential may
Fig. 1. SNAREs. SNAREs, consisting of three synaptic membrane proteins, namely synaptobrevin/VAMP, a synaptic vesicle protein and syntaxin
and SNAP-25 which are plasma membrane proteins, form the four
a-helix bundles of the synaptic fusion complex (Sutton et al., 1998). SNAREs
are targets of clostridial neurotoxins that block neurotransmitter release. Synaptobrevin is cleaved by the botulinum neurotoxins (BoNT) B, D,
G and F and tetanus toxin (TeNT). Syntaxin is cleaved by BoNT C1. SNAP-25 is cleaved by BoNTA and E.
S. Mochida
/
Neuroscience Research
36 (2000) 175 – 182
177
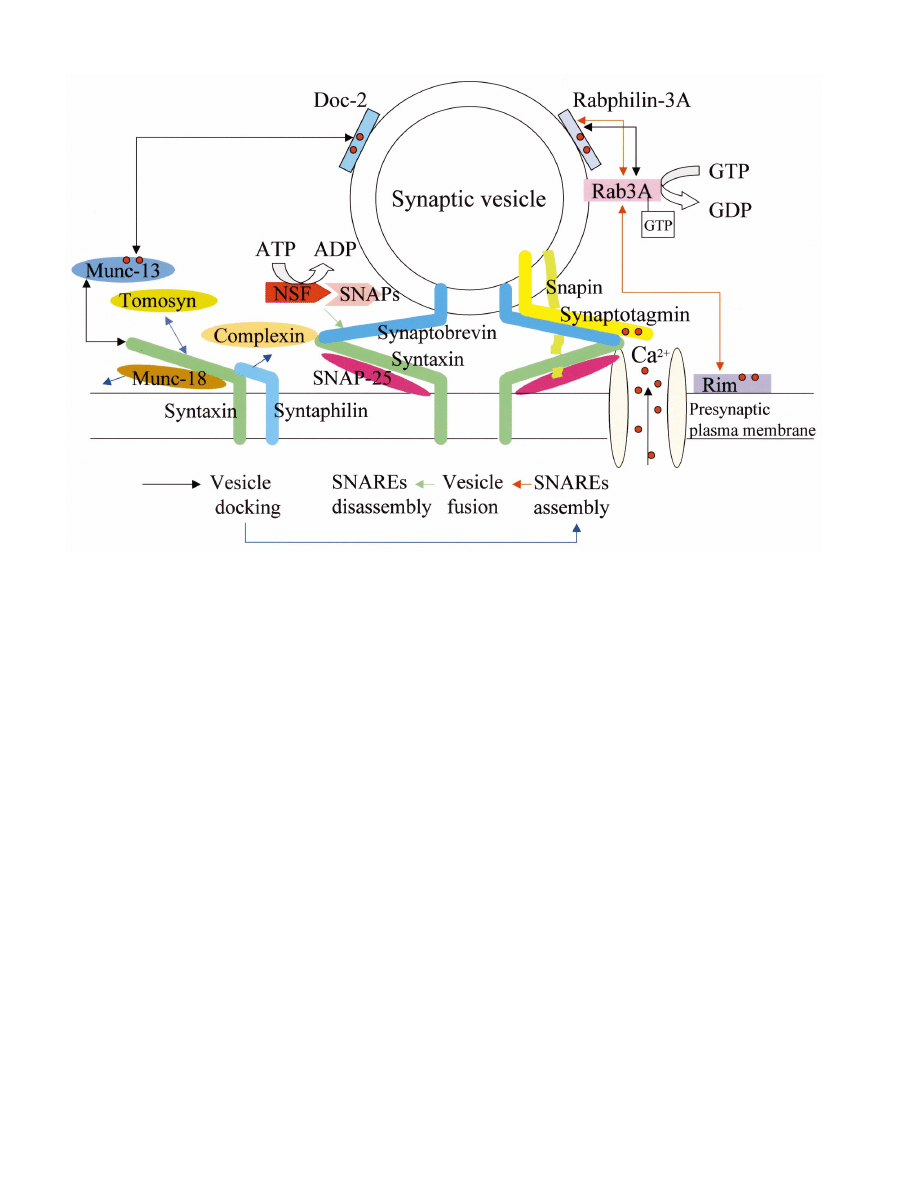
Fig. 2. Hypothetical model of exocytosis regulated by the SNAREs and SNARE-interacting proteins. The simplest model for exocytosis is that
SNARE assembly, triggered by the binding of Ca
2 +
to synaptotagmin, induces the fusion of synaptic vesicles docked at the active zone close to
Ca
2 +
channels. Most of the SNARE-interacting proteins shown in this figure regulate SNARE assembly and disassembly. Arrows indicate
protein – protein interactions related to the cascade of synaptic vesicle states shown below. Each color of arrows corresponds to the color of arrows
in the diagram of the synaptic vesicle cascade. More complicated models have, however, been suggested based on a number of studies on synaptic
proteins. Ca
2 +
(red circles) may act as a signal for Ca
2 +
-binding proteins to promote progression of synaptic vesicles to the next stage. Moreover,
the SNARE-interacting proteins, such as snapin, complexin and rab3A, could modulate SNARE complex formation and may thus regulate the
strength of synaptic transmission underlying synaptic plasticity.
promote membrane fusion by affecting by membrane
surface (Sutton et al., 1998). It remains to be deter-
mined how the SNARE complexes are formed and
whether the free energy released by the assembly of the
synaptic fusion complex is sufficient to induce lipid
mixing. Chen et al. (1999) suggested that rapid signal-
ing in neurons is achieved by organizing the SNARE
complexes for very rapid Ca
2 +
-triggered assembly. A
model for Ca
2 +
-triggered synaptic vesicle fusion pro-
poses that synaptotagmin, a Ca
2 +
-binding protein (see
Ca
2 +
-binding proteins), acts as an electrostatic switch,
promoting a structural rearrangement in the fusion
machinery (Shao et al., 1997).
According to the above mechanism of fusion, disas-
sembly of SNARE complexes by an ATPase, NSF,
together with
a-SNAP may occur after the fusion of
synaptic vesicles. In support of this, Littleton et al.
(1998), using syntaxin and NSF mutants of Drosophila,
found evidence that NSF disassembles SNAREs resid-
ing in the presynaptic membrane after the fusion. On
the other hand, Xu et al. (1998) suggested that there is
an equilibrium between the assembly and disassembly
of SNAREs in the absence of exocytosis, based on the
finding of a blockade of exocytosis of chromaffin cells
by botulinum toxins, which are known only to act on
SNARE proteins in the disassembled state. Accord-
ingly, it remains to be clarified when and how SNARE-
protein disassembly occurs.
3. Proteins interacting with SNAREs
A multitude of proteins control the SNAREs (Fig.
2). Complexin binds to SNARE complexes and regu-
lates the functions of SNAREs in competition with
a-SNAP (McMahon et al., 1995). The inhibitory roles
of complexin II on neurotransmitter release, have been
suggested by functional studies in Aplysia buccal gan-
glia (Ono et al., 1998). Injection of recombinant com-
plexin II and
a-SNAP into presynaptic neurons, caused

S. Mochida
/
Neuroscience Research
36 (2000) 175 – 182
178
depression and facilitation of neurotransmitter release,
respectively. The effect of complexin II was reversed by
a subsequent injection of recombinant
a-SNAP and
vice versa. Enhancement of synaptic transmission by
recombinant SNAPs at giant synapses of the squid has
also been reported (DeBello et al., 1995). A recent
study in complexin II-deficient mice reported that ordi-
nary synaptic transmission and short-term plasticity are
normal but long-term potentiation (LTP) in hippocam-
pus is impaired (Takahashi et al., 1999). Complexin
appears to be a multi-functional modulator of neuro-
transmitter release, regulating the formation of the
SNARE complex.
Snapin is a protein exclusively located on synaptic
vesicle membranes that associates with the SNARE
complex through direct interaction with SNAP-25,
modulating
sequential
interactions
between
the
SNAREs and synaptotagmin, a Ca
2 +
sensor (see cal-
cium-binding proteins). Recombinant snapin injected
into presynaptic neurons reversibly inhibited neuro-
transmitter release at synapses between rat superior
cervical ganglion neurons (SCGNs) in culture (Ilardi et
al., 1999). SNARE complex formation was also regu-
lated by cytoplasmic syntaxin-interacting proteins, such
as Munc-18 and tomosyn. In vitro binding studies
showed that Munc-18 interacts with syntaxin prevent-
ing its binding to SNAP-25 or synaptobrevin and
thereby precluding formation of the SNARE complex
(Pevsner, et al., 1994), while tomosyn promotes
SNARE complex assembly (Fujita et al., 1998a). Func-
tional studies on squid giant synapses, demonstrated
that peptides corresponding to a partial sequence of
munc-18, inhibited exocytosis, indicating that the inter-
action of munc-18 with syntaxin is essential for the
fusion of docked vesicles (Dresbach et al., 1998).
Syntaphilin, a plasma membrane-associated protein,
competes with SNAP-25 for binding to syntaxin and
inhibits the SNARE complex formation by binding to
syntaxin at nerve terminals. Transient overexpression of
syntaphilin in cultured hippocampal neurons, signifi-
cantly reduces neurotransmitter release. Furthermore,
introduction of the syntaphilin coiled-coil domain into
presynaptic neurons of the SCGNs synapse inhibits
synaptic transmission. Syntaphilin may function as a
molecular clamp that controls the availability of free
syntaxin for the assembly of the SNARE complex and
thereby regulates synaptic vesicle exocytosis (Lao et al.,
in press). Septin CDCrel-1 a GTPase associated with
synaptic vesicles, also binds to syntaxin via the SNARE
interaction domain and inhibits exocytosis by prevent-
ing vesicle docking (Beites et al., 1999).
3
.
1
. GTP-binding proteins and associated proteins
A GTP-binding protein, rab3, which is a vesicle-asso-
ciated protein has a GTPase motif and GTP/GDP
binding domains (Touchot et al., 1987; Matsui et al.,
1988). The rab3 homologue Ypt1p is known to regulate
the formation of SNARE complexes in yeast via tran-
sient interactions (Lian et al., 1994; Søgaard, et al.,
1994; Lupashin and Waters, 1997). At nerve terminals,
rab3A and its binding proteins, rabphilin3A (Shirataki
et al., 1993) and Rim (Wang et al., 1997) are involved
in exocytosis via hydrolysis of GTP (Bean and Scheller,
1997). Using hippocampal neurons from rab3A-mutant
mice, Geppert et al. (1994a, 1997) demonstrated a
function for rab3A in limiting exocytosis. rab3A has
also been shown to be essential for generation of LTP
at mossy fiber synapses in the hippocampal CA3 region
(Castillo et al., 1997). Rabphgilin-3A a synaptic vesicle
protein, is proposed to act as a rab3A effector protein
by binding to rab3A in a GTP-dependent manner.
Injection of recombinant rabphilin-3A protein into
squid giant synapses inhibited exocytosis (Burns et al.,
1998); however, studies on rabphilin-knockout mice
revealed that this protein is not required for rab3A to
regulate neurotransmitter release (Schu¨lter et al., 1999).
Rim is a protein associated with the plasma membrane
at the active zone and binds to rab3A complexed with
GTP, suggesting that Rim serves as a regulator (a
promoter) of synaptic vesicle fusion by inducing the
formation of a GTP-dependent complex between
synaptic vesicles and plasma membranes (Wang et al.,
1997). However, until now, no functional studies of
Rim have been performed in neurons.
4. Ca
2 +
channels
The SNARE complex interacts with N-type and P/Q-
type Ca
2 +
channels that provide Ca
2 +
for triggering
exocytosis in the peripheral and central nervous system
(Sheng et al., 1994a,b; Rettig et al., 1996). Disruption
of Ca
2 +
channel interactions with SNAREs by a pep-
tide sequence of the syntaxin-binding site of N-type
Ca
2 +
channels altered the Ca
2 +
-dependence of neuro-
transmitter release at neuromuscular junctions of Xeno-
pus (Rettig et al., 1997). This interaction was found to
be essential for synchronous neurotransmitter release.
The peptide sequence of the syntaxin-binding site of
N-type Ca
2 +
channels inhibited synchronous transmit-
ter release, while it increased the asynchronous trans-
mitter release that follows a train of action potentials at
synapses formed by SCGNs (Mochida et al., 1996). In
addition to mediating Ca
2 +
entry, N-type Ca
2 +
chan-
nels may have direct effects on the transmitter release
process via interaction with SNARE proteins. Introduc-
tion of the peptide sequence of the syntaxin-binding site
of N-type Ca
2 +
channels decreased the voltage-depen-
dent enhancement of Ca
2 +
-independent transmitter re-
lease, suggesting that the N-type Ca
2 +
channel serves
as a voltage sensor that enhances the docking and/or

S. Mochida
/
Neuroscience Research
36 (2000) 175 – 182
179
exocytosis of synaptic vesicles via its interaction with
SNARE proteins (Mochida et al., 1998b). The interac-
tion appears to tether SNARE complexes to Ca
2 +
channels, thereby localizing the fusion machinery near
the site of Ca
2 +
influx and potentiating synaptic trans-
mission. However, studies in which syntaxin was ex-
pressed in Xenopus oocytes (Bezprozvanny et al., 1995;
Wiser et al., 1996) and in which mutant syntaxin lack-
ing the Ca
2 +
channel binding site was expressed in
Drosophila (Wu et al., 1999), suggest that syntaxin also
functions to inhibit Ca
2 +
channels.
5. Calcium-binding proteins
Calcium-binding proteins containing two C2 do-
mains, homologous to the Ca
2 +
-binding regulatory
region of PKC, are considered to act as Ca
2 +
sensors
in nerve terminals. Synaptotagmin a synaptic vesicle
protein which binds to Ca
2 +
and phospholipids via its
C2 domains, has been best characterized as a Ca
2 +
sensor in exocytosis (Brose et al., 1992; Chapman et al.,
1995b). Twelve synaptotagmin isoforms have been
identified. Synaptotagmin I (and II) interacts directly
with syntaxin. This interaction is regulated by Ca
2 +
but requires more than 200
mM for half-maximal bind-
ing (Li et al., 1994). This approximates the Ca
2 +
requirement for synaptic vesicle exocytosis and suggests
a mechanism whereby Ca
2 +
triggers exocytosis by reg-
ulating synaptotagmin I (and II) interaction with syn-
taxin (Li et al., 1994) and other SNAREs (Schiavo et
al., 1997). This idea was supported not only by bio-
chemical evidence that synaptotagmin undergoes a
Ca
2 +
-dependent conformational change (Brose et al.,
1992; Shao et al., 1997), but also by the following
functional evidence. Ca
2 +
-dependent neurotransmitter
release was severely impaired in synaptotagmin I-
knockout mice (Geppert et al., 1994b) and at synapses
following injection of C2 domain peptides (Bommert et
al., 1993) or antibodies against the C2A domain
(Mikoshiba et al., 1995; Mochida et al., 1997). A recent
study demonstrated that Ca
2 +
triggers the penetration
of synaptotagmin I into membranes and simultaneously
enhances the binding of synaptotagmin I to the
SNARE complex, supporting the molecular model in
which synaptotagmin triggers exocytosis via its interac-
tions with membranes and SNARE complexes (Davis
et al., 1999). In addition, synaptic efficacy may be
modulated by changes in the ratio of synaptotagmin
isomers at the synaptic vesicles (Littleton et al., 1999).
Synaptotagmin IV forms hetero-oligomers with synap-
totagmin I, resulting in the formation of synaptotagmin
clusters that cannot effectively penetrate into the mem-
brane, thereby changing the Ca
2 +
sensitivity of vesicle
fusion and decreasing evoked neurotransmission.
Other Ca
2 +
-binding proteins containing C2 domains,
such as rabphilin-3A, Munc-13 and Doc2, are thought
to participate in vesicle trafficking or translocation to a
readily releasable pool prior to docking/fusion at the
active zone (Burns et al., 1998; Mochida et al., 1998a).
Rabphlin-3A and doc2 are synaptic vesicle-associated
proteins (Shirataki et al., 1993; Orita et al., 1995) and
Munc-13 is a membrane-associated protein (Brose et
al., 1995). Doc2 interacts with Munc-13 (Orita et al.,
1997) which, in turn, interacts directly with syntaxin in
a different state (Betz et al., 1997). These protein –
protein interactions may regulate the progression of
synaptic vesicles to the docked and primed states (Su¨d-
hof, 1995) in Ca
2 +
-dependent manner. Rabphilin-3A
interacts with rab3A, which interacts with rim in a
GTP-dependent manner (see GTP-binding proteins).
Rim is also a Ca
2 +
-binding protein containing C2
domains (Wang et al., 1997).
5
.
1
. Protein phosphorylation
There are several lines of evidence suggesting that the
proteins involved in exocytosis are targets for modula-
tion by second messenger systems. Exocytosis from
chromaffin cells is enhanced by protein kinase C (PKC)
via an increase in the size of the readily releasable pool
of secretory granules (Gillis et al., 1996). This could be
attributable to phosphorylation of SNAREs or/and
SNARE-interacting proteins. Munc-13, which interacts
with the Munc-18-syntaxin complex, has phorbol ester-
and diacylglycerol-binding domains (Maruyama and
Brenner, 1991; Brose et al., 1995). Overexpression of
Munc-13 at the neuromuscular junctions of Xenopus
increased the facilitatory actions of phorbol ester on
transmitter release, suggesting that this protein is a
target for the diacylglycerol second messenger pathway
(Betz et al., 1998). Nitric oxide, which stimulates Ca
2 +
-
independent transmitter release from synaptosomes, en-
hances the formation of the SNARE complex and
inhibits the binding of Munc-18 to syntaxin (Meffert et
al., 1996). PKC or cyclin-dependent kinase 5 phospho-
rylates Munc-18 (Fujita et al., 1998b; Fletcher et al.,
1999). Phosphorylation of Munc-18 increases the level
of v-SNARE interaction with syntaxin and the secre-
tory response. These lines of evidence indicate that
phosphorylation of SNAREs or synaptic terminal
proteins that interact with SNAREs modulate the effi-
ciency of synaptic transmission. Phosphorylation of
SNAP-25 decreased the amount of syntaxin co-im-
munoprecipitated with SNAP-25 (Shimazaki et al.,
1996), suggesting that SNARE complex formation is
inhibited by phosphorylation of SNAP-25 Following
phosphorylation by PKA, the binding of
a-SNAP to
the SNARE complex is 10 times weaker than that of
the dephosphorylated form (Hirling and Scheller, 1996).
These data suggest that phosphorylation of SNAREs
S. Mochida
/
Neuroscience Research
36 (2000) 175 – 182
180
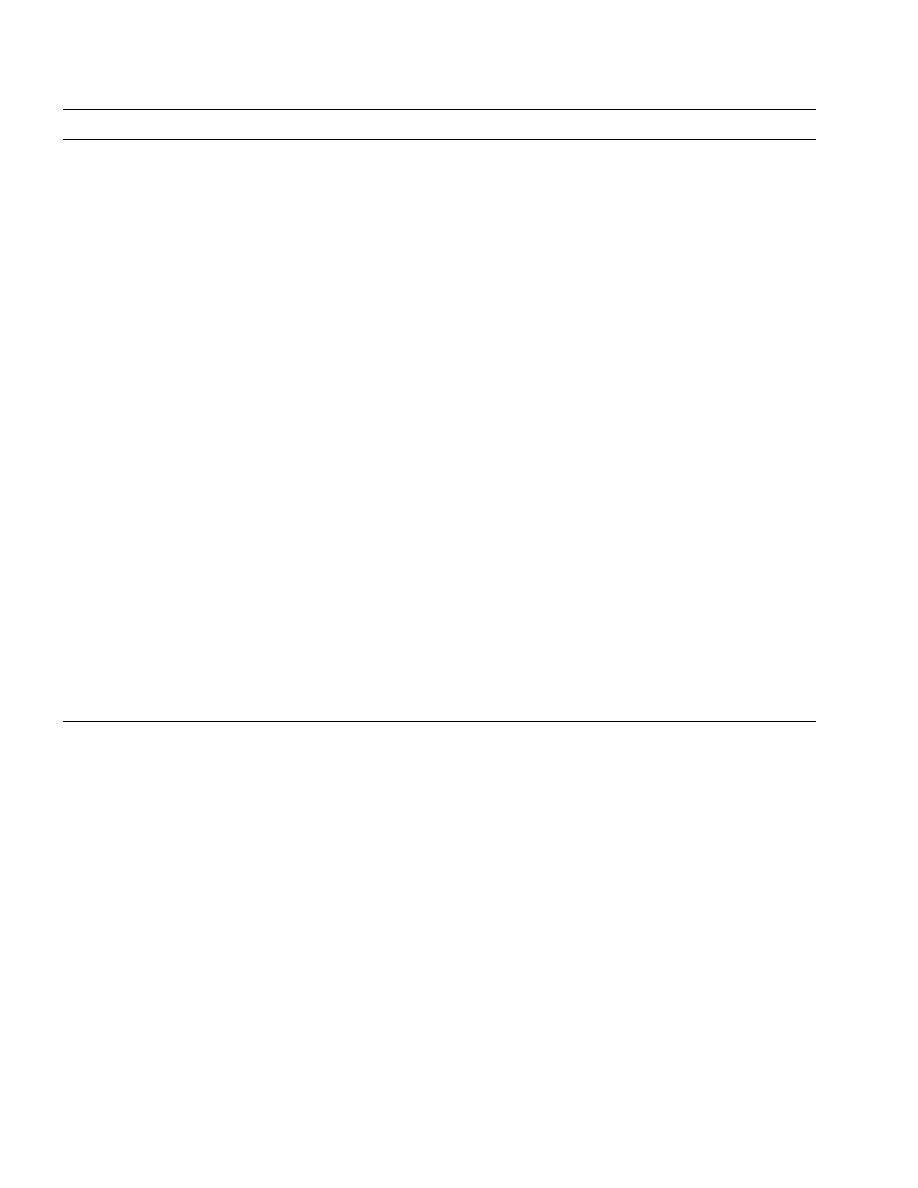
Table 1
SNARES and SNARE-associated proteins implicated in exocytosis
a
Proteins
Classification
Localization
Speculated functions in neurotransmitter release
Synaptobrevin/
SNARES (SNARE core complex)
Synaptic vesicles
Fusion machinery
VAMP
Syntaxin
Plasma membranes
Plasma membranes
SNAP-25
NSF
SNARES disassembly
SNARE core complex-interacting
Cytoplasm
proteins
(an ATPase)
Cytoplasm
a-SNAP
SNAREs disassembly
Cytoplasm
Modulation of SNAREs assembly
Complexin
Synaptic vesicles
Snapin
Modulation of SNAREs-synaptotagmin interaction
N-(and P/Q-
Plasma membranes
(1) Synchronous neurotransmitter release
type) Ca
2+
channels
(2) Transmission of voltage signal to SNARE complexes
Cytoplasm and plasma
Tomosyn
Syntaxin-interacting proteins
Stimulation of SNAREs assembly
membranes
Munc-18
Cytoplasmic face of
(1) Inhibition of SNAREs assembly
(2) Essential for synaptic vesicle fusion
plasma membranes
Plasma membranes
Inhibition of SNAREs assembly
Syntaphilin
Cytoplasmic face of
Munc-13
(1) Enhancement of SNAREs assembly by diacylglycelol
(2) Promotion of synaptic vesicle trafficking by interac-
plasma membranes
tion with Doc2
Rab3A
GTP-binding proteins and associ-
Synaptic vesicles
(1) Limiting fusion machinery
ated proteins
(2) Generation of LTP
(a GTPase)
(3) Recruitment of synaptic vesicles to the active zone
Rabphilin-3A
Synaptic vesicles
(1) Modulation of synaptic vesicle fusion
(2) Modulation of synaptic vesicle trafficking
Active zone
Promotion of synaptic vesicle fusion
Rim
Synaptotagmin
Trigger of synaptic vesicle fusion
Ca
2+
-binding proteins containing
Synaptic vesicles
two C2 domains
I
Synaptic vesicles
Promotion of synaptic vesicle trafficking by interaction
Doc 2
with Munc-13
Cytoplasmic face of
Munc-13
(1) Enhancement of snares assembly by diacylglycelol
(2) Promotion of synaptic vesicle trafficking by interac-
plasma membranes
tion with Doc2
Rabphilin-3A
Synaptic vesicles
(1) Modulation of synaptic vesicle fusion
(2) Modulation of synaptic vesicle trafficking
Active zone
Rim
Promotion of synaptic vesicle fusion
a
Although several isoforms of proteins have been detected, the best-characterized protein is listed on the table.
and related proteins may also be responsible for synap-
tic depression.
6. Conclusions
The hypothetical functions of SNAREs and SNARE-
interacting proteins in synaptic vesicle exocytosis are
summarized in Fig. 2 and Table 1. The protein – protein
interactions appear to be very complicated. However,
SNAREs are probably essential components for synap-
tic vesicle fusion machinery and most of the other
proteins described in this article regulate the assembly
or disassembly of SNAREs. Moreover, the SNARE-in-
teracting proteins may regulate the efficiency and
strength of synaptic transmission underlying synaptic
plasticity and memory by modulating the SNARE com-
plex formation. Ca
2 +
-binding proteins could act as key
proteins that induce the progression of synaptic vesicles
to the next stage along the maturation pathway.
Acknowledgements
Fig. 1 is printed by permission from Nature, 395,
347 – 353, 1998 (Copyright: Macmillan Magazines),

S. Mochida
/
Neuroscience Research
36 (2000) 175 – 182
181
with kind agreement of Dr Reinhard Jahn (Department
of Neurobiology, Max – Planck-Institute for Biophysical
Chemistry, Go¨ttingen, Germany). I thank Dr Michael
J. Seager (Neurobiologie des Canaux Ioniques, IN-
SERM, Marseille, France) for critical reading of the
manuscript.
References
Augustine, G.J., Burns, M.E., DeBello, W.M., Pettit, D.L.,
Schweizer, F.E., 1996. Exocytosis: proteins and perturbations.
Annu. Rev. Pharmacol. Toxicol. 36, 659 – 701.
Banerjee, A., Kowalchyk, J.A., DasGupta, B.R., Martin, T.F.J.,
1996. SNAP-25 is required for a late postdocking step in Ca
2 +
-
dependent exocytosis. J. Biol. Chem. 271, 20227 – 20230.
Bean, A.J., Scheller, R.H., 1997. Better late than never: a role for
rabs late in exocytosis. Neuron 19, 751 – 754.
Beites, C.L., Xie, H., Bowser, R., Trimble, W.S., 1999. The septin
CDCrel-1 binds syntaxin and inhibits exocytosis. Nat. Neurosci.
2, 434 – 439.
Bennett, M.K., Scheller, R.H., 1993. The molecular machinery for
secretion is conserved from yeast to neurons. Proc. Natl. Acad.
Sci. USA 90, 2559 – 2563.
Betz, A., Okamoto, M., Benseler, F., Brose, N., 1997. Direct interac-
tion of the rat unc-13 homologue Munc13-1 with the N-terminal
of syntaxin. J. Biol. Chem. 272, 2520 – 2526.
Betz, A., Ashley, U., Rickmann, M., et al., 1998. Munc13-1 is a
presynaptic phorbol ester receptor that enhances neurotransmitter
release. Neuron 21, 123 – 136.
Bezprozvanny, I., Scheller, R.H., Tsien, R.W., 1995. Functional
impact of syntaxin on gating of N-type and Q-type calcium
channels. Nature 378, 623 – 626.
Bommert, K., Charton, M.P., DeBello, W.M., et al., 1993. Inhibition
of neurotransmitter release by C2-domain peptides implicates
synaptotagmin in exocytosis. Nature 363, 163 – 165.
Broadie, K., Prokop, A., Bellen, H.J., et al., 1995. Syntaxin and
VAMP function downstream of vesicle docking in Drosophila.
Neuron 15, 663 – 673.
Brose, N., Petrenko, A.G., Su¨dhof, T.C., Jahn, R., 1992. Synaptotag-
min: a calcium sensor on the synaptic vesicle surface. Science 256,
1021 – 1025.
Brose, N., Hofmann, K., Hata, Y., Su¨dhof, T.C., 1995. Mammalian
homologues of Caenorhabditis elegans unc-13 gene define novel
family of C2-domain proteins. J. Biol. Chem. 270, 25273 – 25280.
Burns, E.M., Sasaki, T., Takai, Y., Augustine, G.J., 1998. Rabphilin-
3A: a multifunctional regulator of synaptic vesicle traffic. J. Gen.
Physiol. 111, 243 – 255.
Castillo, P.E., Janz, R., Su¨dhof, T.C., et al., 1997. Rab3A is essential
for mossy fibre long-term potentiation in the hippocampus. Na-
ture 388, 590 – 593.
Chapman, E.R., An, S., Barton, N., Jahn, R., 1995a. SNAP-25, a
t-SNARE which binds to both syntaxin and VAMP via domains
that may form cioled coils. J. Biol. Chem. 269, 27427 – 27432.
Chapman, E.R., Hanson, P.I., An, S., Jahn, R., 1995b. Ca
2 +
regu-
lates the interaction between synaptotagmin and syntaxin 1. J.
Biol. Chem. 270, 23667 – 23671.
Chen, Y.A., Scales, S.J., Patel, S.M., Doung, Y.-C., Scheller, R.H.,
1999. SNARE complex formation is triggered by Ca
2 +
and drives
membrane fusion. Cell 97, 165 – 174.
Davis, A.F., Bai, J., Fasshauer, D., et al., 1999. Kinetics of synapto-
tagmin responses to Ca
2 +
and assembly with core snare complex
onto membranes. Neuron 24, 363 – 376.
DeBello, W.M., O’Conner, V., Dresbach, T., et al., 1995. SNAP-me-
diated protein – protein interactions essential for neurotransmitter
release. Nature 373, 626 – 630.
Deitcher, D.L., Ueda, A., Stewart, B.A., et al., 1998. Distinct require-
ments for evoked and spontaneous release of neurotransmitter are
revealed by mutations in the Drosophila gene neuronal-VAMP. J.
Neurosci. 18, 2028 – 2039.
Dresbach, T., Burns, M.E., O’Conner, V., et al., 1998. A neuronal
Sec1 homolog regulates neurotransmitter release at the squid
giant synapse. J. Neurosci. 18, 2923 – 2932.
Ferro-Novick, S., Jahn, R., 1994. Vesicle fusion from yeast to man.
Nature 370, 191 – 193.
Fletcher, A.I., Shuang, R., Giovannucci, D.R., et al., 1999. Regula-
tion of exocytosis by cyclin-dependent kinase 5 via phosphoryla-
toin of munc-18. J. Biol. Chem. 274, 4027 – 4035.
Fujita, Y., Shirataki, H., Sakisaka, T., et al., 1998a. Tomosyn: a
syntaxin-1-binding protein that forms a novel complex in the
neurotransmitter process. Neuron 20, 905 – 915.
Fujita, Y., Sasaki, T., Fukui, K., et al., 1998b. Phosphorylation of
munc-18/n – Sec1/rbSec1 by protein kinase C. J. Biol. Chem. 271,
7265 – 7268.
Geppert, M., Bolshakov, V.Y., Siegelbaum, S.A., et al., 1994a. Role
of the rab3A in neurotransmitter release. Nature 369, 493 – 497.
Geppert, M., Goda, Y., Hammer, R.E., et al., 1994b. Synaptotagmin
I: a major Ca
2 +
sensor for transmitter release at a central
synapse. Cell 79, 717 – 727.
Geppert, M., Goda, Y., Stevens, C.F., Su¨dhof, T.C., 1997. The small
GTP-binding protein rab3A regulates a late step in synaptic
vesicle fusion. Nature 387, 810 – 814.
Gillis, K.D., Mo¨bner, R., Neher, E., 1996. Protein kinase C enhances
exocytosis from chromaffin cells by increasing the size of the
readily releasable pool of secretory granules. Neuron 16, 1209 –
1220.
Hanson, P.I., Heuser, J.E., Jahn, R., 1997. Neurotransmitter release
— 4 years of SNARE complexes. Curr. Opin. Neurobiol. 7,
310 – 315.
Hayashi, T., Yamasaki, S., Nauenburg, S., Binz, T., Niemann, H.,
1995. Disassembly of the reconstituted synaptic vesicle membrane
fusion complex in vitro. EMBO J. 14, 2317 – 2325.
Hirling, H., Scheller, R.H., 1996. Phosphorylation of synaptic vesicle
proteins: modulation of the
aSNAP interaction with the core
complex. Proc. Natl. Acad. Sci. USA 93, 11945 – 11949.
Hunt, J.M., Bommert, K., Charlton, M.P., et al., 1994. A post-dock-
ing role for VAMP in synaptic vesicle fusion. Neuron 12, 1269 –
1279.
Ilardi, J.M., Mochida, S., Sheng, Z.-H., 1999. Snapin: a SNARE-as-
sociated protein implicated in synaptic transmission. Nat. Neu-
rosci. 2, 119 – 124.
Lao, G., Scheuss, V., Gerwin, C.M., Su, Q., Mochida, S., Rettig, J.,
Sheng, Z.-H., 2000. Syntaphilin: a syntaxin-1 clamp that controls
SNARE assembly. Neuron (In press).
Li, C., Ullrich, B., Zhang, J.Z., Anderson, R.G.W., Brose, N.,
Su¨dhof, T.C., 1994. Ca
2 +
-dependent and -independent activities
of neural and non neural synaptotagmins. Nature 375, 594 – 599.
Lian, J.P., Stone, S., Jiang, Y., Lyons, P., Ferro-Novik, S., 1994.
Ypt1p implicated in v-SNARE activation. Nature 372, 698 – 701.
Littleton, J.T., Chapman, E.R., Kreber, R., et al., 1998. Temperature-
sensitive paralytic mutations demonstrate that synaptic exocytosis
requires SNARE complex assembly and disassembly. Neuron 21,
401 – 413.
Littleton, J.T., Serano, T.L., Rubin, G.M., Ganetzky, B., Chapman,
E.R., 1999. Synaptic function modulated by changes in the ratio
of synaptotagmin I and IV. Nature 400, 757 – 760.
Lupashin, V.V., Waters, M.G., 1997. t-SNARE activation through
transient interaction with rab-like guanosine triphosphate. Science
276, 1255 – 1258.
Maruyama, I.N., Brenner, S., 1991. A phorbol ester/diacylglycerol-
binding protein encoded by the unc-13 gene of Caenorhabditis
elegans. Proc. Natl. Acad. Sci. USA 88, 5729 – 5733.

S. Mochida
/
Neuroscience Research
36 (2000) 175 – 182
182
Matsui, Y., Kikuchi, A., Kondo, J., et al., 1988. Nucleotide and
deduced amino acid sequences of a GTP-binding protein family
with molecular weights of 25000 from bovine brain. J. Biol.
Chem. 263, 11071 – 11074.
McMahon, H.T., Missler, M., Li, C., Su¨dhof, T.C., 1995. Complex-
ins: cytosolic proteins that regulate SNAP receptor function. Cell
83, 111 – 119.
Meffert, M.K., Calakos, N.C., Scheller, R.H., Schulman, H., 1996.
Nitric oxide modulates synaptic vesicle docking/fusion reactions.
Neuron 16, 1229 – 1236.
Mikoshiba, K., Fukuda, M., Moreira, J.E., et al., 1995. Role of the
C2A domain of synaptotagmin in transmitter release as deter-
mined by specific antibody injection into the squid giant synapse
terminal. Proc. Natl. Acad. Sci. USA 92, 10703 – 10707.
Mochida, S., Sheng, Z.-H., Baker, C., Kobayashi, H., Catterall,
W.A., 1996. Inhibition of neurotransmission by peptides contain-
ing the synaptic protein interaction site of N-type Ca
2 +
channels.
Neuron 17, 781 – 788.
Mochida, S., Fukuda, M., Niinobe, M., Kobayashi, H., Mikoshiba,
K., 1997. Role of synaptotagmin C2 domains in neurotransmitter
secretion and inositol high-polyphosphate binding at mammalian
cholinergic synapses. Neuroscience 77, 937 – 943.
Mochida, S., Orita, S., Sakaguchi, G., Sasaki, T., Takai, Y., 1998a.
Role of the Doc2a-Munc-13-1 interaction in the neurotransmitter
release process. Proc. Natl. Acad. Sci. USA 95, 11418 – 11422.
Mochida, S., Yokoyama, C.T., Kim, D., Itoh, K., Catterall, W.A.,
1998b. Evidence for a voltage-dependent enhancement of neuro-
transmitter release mediated via the synaptic protein interaction
site of N-type Ca
2 +
channels. Proc. Natl. Acad. Sci. USA 95,
14523 – 14528.
Monteccuco, C., Schiavo, G., 1995. Structure and function of tetanus
and botulinum neurotoxins. Quart. Rev. Biophys. 28, 423 – 472.
Niemann, H., Blasi, J., Jahn, R., 1994. Clostridial neurotoxins: new
tools for dissecting exocytosis. Trens. Cell. Biol. 4, 179 – 185.
Ono, S., Baux, G., Sekiguchi, M., et al., 1998. Regulatory roles of
complexins in neurotransmitter release from mature presynaptic
nerve terminals. Eur. J. Neurosci. 10, 2143 – 2152.
Orita, S., Sasaki, T., Komura, R., et al., 1995. Molecular cloning of
an isoform of Doc2 having two C2-like domains. Biochem. Bio-
phys. Res. Commun. 206, 439 – 448.
Orita, S., Naito, A., Sakaguchi, G., Sasaki, T., Takai, Y., 1997.
Physical and functional interactions in Ca
2 +
-dependent exocyto-
sis machinery. J. Biol. Chem. 272, 16081 – 16084.
Pevsner, J., Hsu, S.-C., Braun, J.E.A., et al., 1994. Specificity and
regulation of a synaptic vesicle docking complex. Neuron 13,
353 – 361.
Rettig, J., Sheng, Z.-H., Kim, D.K., et al., 1996. Isoform-specific
interaction of the
a1A subunits of brain Ca
2 +
channels with
presynaptic proteins syntaxin and SNAP-25. Proc. Natl. Acad.
Sci. USA 93, 7363 – 7368.
Rettig, J., Heinermann, C., Ashery, U., et al., 1997. Alteration of
Ca
2 +
dependence of neurotransmitter release by disruption of
Ca
2 +
channel/syntaxin interaction. J. Neurosci. 17, 6647 – 6656.
Rothman, J.E., 1994. Mechanisms of intracellular protein transport.
Nature 372, 55 – 63.
Schiavo, G., Stenbeck, G., Rothman, J.E., So¨llner, T.H., 1997.
Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the
plasma membrane t-SNARE, SNAP-25, can explain docked vesi-
cles at neurotoxin-treated synapses. Proc. Natl. Acad. Sci. USA
94, 997 – 1001.
Schu¨lter, O.M., Schnell, E., Verhage, M., et al., 1999. Rabphilin
knock out mice reveal that rabphilin is not required for rab3
function in regulating neurotransmitter release. J. Neurosci. 19,
5834 – 5846.
Schulze, K.L., Broadie, K., Perin, M.S., Bellen, H.J., 1995. Genetic
and
electrophysiological
studies
of
Drosophila
syntaxin-1A
demonstrate its role in nonneuronal secretion and neurotransmis-
sion. Cell 80, 311 – 320.
Shao, X., Li, C., Fernandez, I., et al., 1997. Synaptotagmin-syntaxin
interaction: the C2 domain as a Ca
2 +
-dependent electrostatic
switch. Neuron 18, 133 – 142.
Sheng, Z.-H., Rettig, J., Takahashi, M., Catterall, W.A., 1994a.
Identification of a syntaxin-binding site on N-type calcium chan-
nels. Neuron 13, 1303 – 1313.
Sheng, Z.-H., Rettig, J., Cook, T., Catterall, W.A., 1994b. Calcium-
dependent interaction of N-type calcium channels with the synap-
tic core-complex. Nature 379, 451 – 454.
Shimazaki, Y., Nishiki, T., Omori, A., et al., 1996. Phosphorylation
of 25-kDa synaptosome-associated protein. J. Biol. Chem. 271,
14548 – 14553.
Shirataki, H., Kaibuchi, K., Sakoda, T., et al., 1993. Rabphilin-3A, a
putative target protein for smg p25/rab3A p25 small GTP-binding
protein related to synaptotagmin. Mol. Cell. Biol. 13, 2061 – 2068.
Søgaard, M., Tani, K., Ye, R.R., et al., 1994. A rab protein is
required for the assembly of SNARE complexes in the docking of
transport vesicles. Cell 78, 937 – 948.
So¨llner, T., Whiteheat, S.W., Brunner, M., et al., 1993a. SNAP
receptors implicated in vesicle targeting and fusion. Nature 362,
318 – 324.
So¨llner, T., Bennett, M.K., Whiteheat, S.W., Scheller, R.H., Roth-
man, J.E., 1993b. A protein assembly-disassembly pathway in
vitro that may correspond to sequential steps of synaptic vesicle
docking, activation and fusion. Cell 75, 409 – 418.
Su¨dhof, T.C., 1995. The synaptic vesicle cycle: a cascade of protein –
protein interactions. Nature 375, 645 – 653.
Sutton, R.B., Fasshauer, D., Jahn, R., Brunger, A.T., 1998. Crystal
structure of a SNARE complex involved in synaptic exocytosis at
2.4 A
, resolution. Nature 395, 347–353.
Sweeney, S.T., Broadie, K., Keane, J., Niemann, H., O’Kane, C.J.,
1995. Targeted expression of tetanus toxin light chain in
Drosophila specifically eliminates synaptic transmission and
causes behavioral defects. Neuron 14, 341 – 351.
Takahashi, S., Ujihara, H., Huang, G.-Z., et al., 1999. Reduced
hippocampal LTP in mice lacking a presynaptic protein: com-
plexin II. Eur. J. Neurosci. 11, 2359 – 2366.
Touchot, N., Chardin, P., Tavitian, A., 1987. Four additional mem-
bers of the ras gene superfamily isolated by an oligonucleotide
strategy: molecular cloning of YPT-related cDNAs from a rat
brain library. Proc. Natl. Acad. Sci. USA 84, 8210 – 8214.
Wang, Y., Okamoto, M., Schmitz, F., Hoffmann, K., Su¨dhof, T.C.,
1997. Rim is a putative Rab3 effector in regulating synaptic-vesi-
cle fusion. Nature 388, 593 – 598.
Weber, T., Zemelman, B.V., McNew, J.A., et al., 1998. SNAREpins:
minimal machinery for membrane fusion. Cell 92, 759 – 772.
Wiser, O., Bennett, M.K., Atlas, D., 1996. Functional interaction of
syntaxin and SNAP-25 with voltage-sensitive L-and N-type Ca
2 +
channels. EMBO J. 15, 4100 – 4110.
Wu, M.N., Fergestad, T., Lloyed, T.E., et al., 1999. Syntaxin 1A
interacts with multiple exocytic proteins to regulate neurotrans-
mitter release in vivo. Neuron 23, 593 – 605.
Xu, T., Binz, T., Niemann, H., Neher, E., 1998. Multiple kinetic
components of exocytosis distinguished by neurotoxin sensitivity.
Natu. Neurosci. 1, 192 – 200.
.
Wyszukiwarka
Podobne podstrony:
egzocytoza 5fantastic pl
2011Wykład1 chemii ogólnej 5fantastic pl
fizjologia 5fantastic pl
POPRAWA WSZYSTKICH KOLOKWIËW. 5fantastic.pl , Ćwiczenia
Osocze a mocz. 5fantastic.pl , Ćwiczenia
Zjazd5s1 v2. 5fantastic.pl , Ćwiczenia
wykład 1. 5fantastic.pl , Ćwiczenia
podstawy produkcji roślinnej. 5fantastic.pl , Ćwiczenia(2)
Dobrostan owiec. 5fantastic.pl , Wykłady(1)
kolos 2. 5fantastic.pl , Ćwiczenia(1)
MODU 0 MODU Y SZCZEG Y. 5fantastic.pl , Wykłady(1)
sprawozdanie. 5fantastic.pl , Ćwiczenia
Chwasty1. 5fantastic.pl , Ćwiczenia
więcej podobnych podstron