
170
Ann. N.Y. Acad. Sci. 1014: 170–178 (2004). © 2004 New York Academy of Sciences.
doi: 10.1196/annals.1294.018
Principles of Exocytosis and Membrane Fusion
REINHARD JAHN
Department of Neurobiology, Max-Planck-Institute for Biophysical Chemistry,
Göttingen, Germany
A
BSTRACT
: Exocytosis is a ubiquitous process occurring in every eukaryotic
cell including processes as diverse as membrane expansion during growth and
the highly regulated release of neurotransmitter from neurons. Work during
the past decade has established that exocytotic membrane fusion is mediated
by members of conserved protein families including Rab proteins and
SNAREs. SNAREs are probably catalyzing membrane fusion, and major
progress has been made in unraveling their molecular mechanism. In contrast,
less is known about regulatory mechanisms. Here, a brief overview is given
about the current state of knowledge, focusing on SNAREs involved in
neuronal exocytosis.
K
EYWORDS
: SNAREs; neurotransmitter release; syntaxin; SNAP-25; synapto-
brevin; NSF; membrane traffic
INTRODUCTION
Exocytosis is defined as the fusion of an intracellular trafficking vesicle with the
plasma membrane. Every eucaryotic cell is dependent on exocytosis for growth and
differentiation because it is the only mechanism by which a cell can add additional
membrane to the plasmalemma. Furthermore, we distinguish constitutive and regu-
lated exocytosis. Constitutive exocytosis includes all fusion events in which vesicles
are generated, transported, and exocytose continuously, without being subject to
short-term regulation. Regulated exocytosis usually requires that precursor mem-
branes are stored in specialized intracellular pools from which they are mobilized
upon activation of signaling cascades. These membranes often have a composition
different from the plasmalemma and include secretory vesicles and endosome-
derived membrane pools. Regulated exocytosis allows for the controlled delivery of
secretory product such as proteins or small molecules (e.g., neurotransmitters) or for
the regulated incorporation of specialized plasma membrane constituents such as
transporters, enzymes, or channels. In most cases, regulated exocytosis is triggered
by second messengers (predominantly calcium) in response to receptor activation or
membrane depolarization (for a review, see Burgess and Kelly
1
).
Address for correspondence: Reinhard Jahn, Department of Neurobiology, Max-Planck-Insti-
tute for Biophysical Chemistry, Am Fassberg, 37077 Göttingen. Voice: 49-551-201-1635; fax:
49-551-201-1639.
rjahn@gwdg.de

171
JAHN: PRINCIPLES OF EXOCYTOSIS AND MEMBRANE FUSION
PROTEIN FAMILIES PARTICIPATING IN EXOCYTOSIS AND
INTRACELLULAR MEMBRANE FUSION
At the molecular level, exocytosis involves specialized protein families that are
conserved from yeast to humans. Furthermore, it appears that members of the same
protein families are required for all intracellular membrane fusion of the secretory
pathway, that is, with exception of the homotypic fusion of mitochondria and (per-
haps) peroxisomes. These protein families include the SNAREs, the Rab/ypt pro-
teins, and the SM proteins (for a review, see Chen and Scheller
2
and
Jahn
et al.
3
).
Rab/ypt proteins comprise the largest subfamily of ras-related GTPases including 11
members in yeast and more than 60 in mammals.
4
Although among the first non-ras
GTPases to be discovered,
5
an understanding of the molecular role of Rab/ypt pro-
teins has emerged only recently. Rab proteins undergo a GTP-GDP cycle that is as-
sociated with membrane association (GTP form) and membrane dissociation. This
cycle is controlled by accessory proteins that regulate the GTPase activity, mediate
recharging with GTP after hydrolysis, and control membrane association. According
to the emerging picture, rab proteins operate as molecular switches, with the GTP
form being active. Active rabs selectively recruit effector proteins, many of which
are specific for a given rab protein. It is presently believed that protein recruitment
by rab proteins not only defines the specificity of the fusing membranes but also
builds larger “docking” complexes that mediate membrane attachment and prepare
the membranes for subsequent fusion (for review, see Jahn et al.,
3
Zerial and
McBride,
4
and Pfeffer
6
).
SNAREs are represented by a protein superfamily of mostly membrane-anchored
proteins.
7–10
The proteins are small, with molecular masses ranging from 10–35
kDa. The distinguishing feature of SNAREs is a moderately conserved stretch of 60
to 70 amino acids, referred to as SNARE motif. In a typical SNARE, the SNARE
motif is flanked by a single C-terminal transmembrane domain and by an N-terminal
region that either consists of only few amino acids (such as in many members of the
synaptobrevin/VAMP subfamily) or consists of an independently folded domain that
is connected to the SNARE motif by a linker region. The sequences of the N-termi-
nal domains are not conserved, but it appears that their tertiary structures are similar,
with most of them forming antiparallel bundles of three
α
helices. Exceptions from
this general structure include SNAP-25 and its relatives SNAP-29 and SNAP-23,
that contain two SNARE motifs connected by a linker region. In this and some other
cases, transmembrane domains are lacking, and membrane binding may be mediated
by hydrophobic posttranslational modifications.
SNARES: CANDIDATE PROTEINS FOR CATALYZING
MEMBRANE FUSION
An almost overwhelming body of evidence documents that SNARE proteins are
crucial for intracellular fusion events.
3,8,9
Consequently, major efforts have been
made to understand the function of SNAREs at the molecular level. Best character-
ized are the SNAREs functioning in regulated exocytosis of neurons and neuroendo-
crine cells, which were used as paradigms for investigating the molecular properties
of the proteins. They include the SNAREs syntaxin I and SNAP-25, localized prima-
172
ANNALS NEW YORK ACADEMY OF SCIENCES
rily on the plasma membrane, and the SNARE synaptobrevin 2/VAMP-2 that is con-
centrated on the membrane of synaptic and neurosecretory vesicles. These proteins
are also the primary SNAREs in regulated exocytosis of neuroendocrine cells, and,
as far as is known, they are abundantly expressed in neuroendocrine tumors.
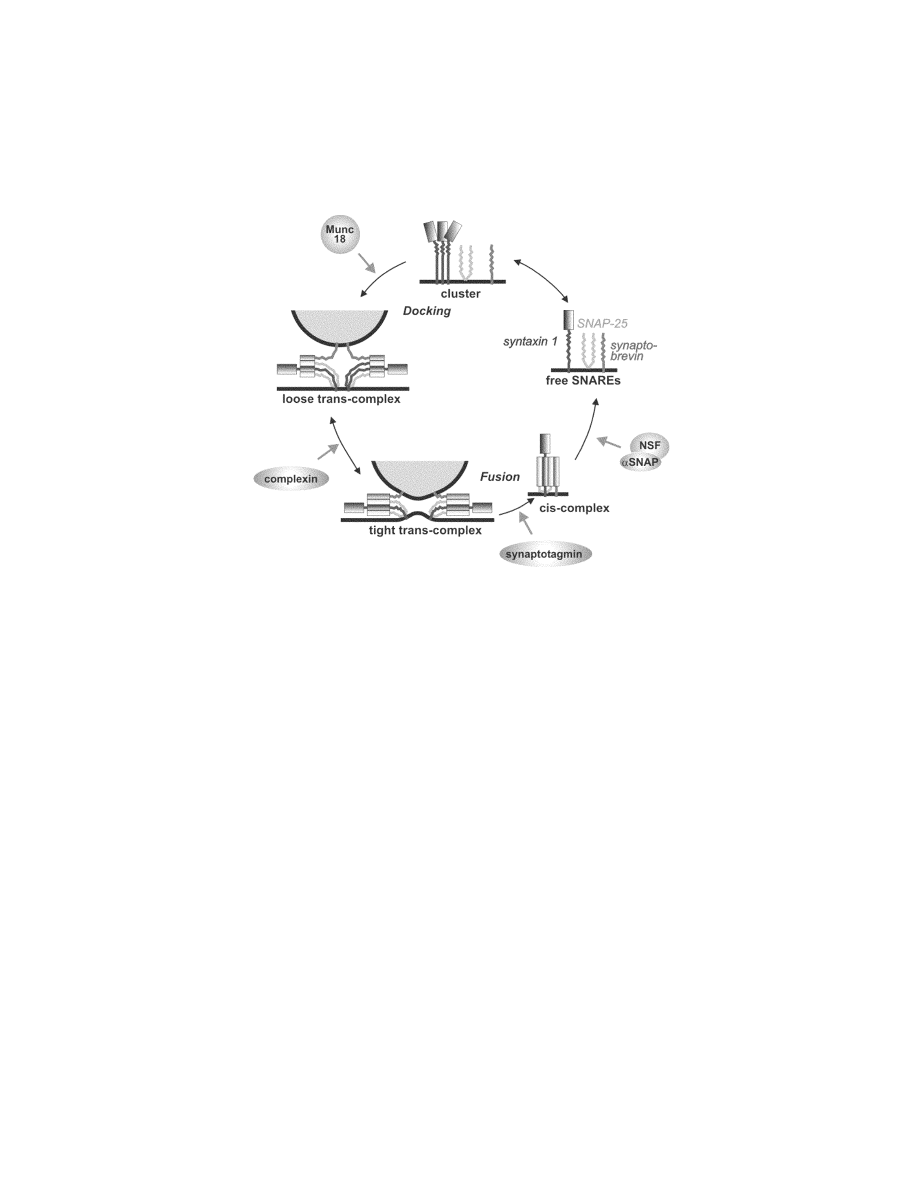
Biochemical experiments based largely on the study of recombinant proteins lacking
their membrane anchors have revealed that SNAREs undergo an association-dissociation
cycle that involves the SNARE motifs and that is accompanied by major conformational
changes (F
IG
. 1) (reviewed in Fasshauer
10
). SNARE motifs are unstructured in solution,
although some of them show a tendency to homooligomerize into helical bundles at high-
er protein concentration. When the three proteins are combined, they assemble into a
tight complex with a 1:1:1 stoichiometry. The neuronal SNARE complex is represented
by an elongated bundle of four
α
helices in which each helix is contributed by one of the
participating SNARE motifs, with the transmembrane domains being located at one end
of the bundle.
11,12
In the core of the bundle, the helices are connected by 16 layers of
mostly hydrophobic amino acid side chains. The assembled SNARE complex is extra-
ordinarily stable. It is resistant to treatment with the detergent sodium dodecyl sulfate
(unless heated),
13
and it unfolds only when heated to high temperature (unfolding transi-
tion at 82°C) or exposed to high concentration of strong denaturants (above 5M guani-
dinium chloride).
14
Sequence comparisons,
15
together with the crystal structure of a second, only dis-
tantly related SNARE complex,
16
revealed common properties that apparently apply
FIGURE 1. Model of the conformational cycle of neuronal SNAREs, its relation to
membrane fusion, and its control by regulatory factors. See text for details. Modified from
Jahn et al.
3

173
JAHN: PRINCIPLES OF EXOCYTOSIS AND MEMBRANE FUSION
to all SNARE complexes functioning in intracellular fusion reactions. Accordingly,
SNAREs (or, more precisely: SNARE motifs) can be classified into four subfamilies
that are designated Qa-, Qb-, Qc-, and R-SNAREs, named after amino acid residues
forming an unusual polar layer in the core of the bundle that are highly conserved.
17
Apparently, each SNARE complex has a QaQbQcR- composition. The situation is
complicated by the fact, however, that many SNAREs tend to form additional com-
plexes of lower stability and of different composition. Best characterized among
these is a complex between SNAP-25 and syntaxin 1. This complex is also represent-
ed by a four-helix bundle in which the position of synaptobrevin is occupied by a
second syntaxin molecule (reviewed in Fasshauer
10
). Currently, it is not known
whether such complexes are of biological significance, although there is some evi-
dence for the formation of such partial complexes being a prerequisite for efficient
fusion.
Although the free energy of SNARE assembly cannot be determined because of
an unusual hysteretic behavior,
14
the reaction is essentially irreversible. Indeed, as-
sembled SNARE complexes do not dissociate at biologically relevant time scales,
and it requires the activity of the chaperone-like ATPase N-ethylmaleimide sensitive
factor (NSF) to dissociate the SNARE motifs.
18
NSF is a hexameric protein that be-
longs to the family of AAA-ATPases (for a review, see Whiteheart et al.
19
). For
SNARE dissociation, binding of cofactors, termed soluble NSF attachment proteins
(SNAPs) is required that, in turn, recruits NSF. It was the binding to SNAPs that led
to the identification of the first SNARE complex (SNARE is an acronym for SNAp
REceptors).
20
NSF is a conserved protein that operates on all SNARE complexes.
Inactivation of a temperature-sensitive NSF variant in Drosophila led to the accumu-
lation of SNARE complexes and loss of neuronal exocytosis,
21
showing that the
molecule is an essential component of the SNARE association-dissociation cycle.
How does the SNARE cycle relate to membrane fusion? It is currently believed
that it is the association of SNAREs that encompasses the functionally relevant
step.
11,22,23
For fusion to occur, complementary sets of SNAREs need to be present
on the two membranes. In neuronal exocytosis, syntaxin 1a and SNAP-25 are pre-
dominantly localized to the presynaptic plasma membrane, whereas synaptobrevin
is predominantly localized to synaptic vesicles. When the membranes are getting
very close to each other, these SNAREs assemble into complexes in trans-configu-
ration. Because in the fully assembled complex the transmembrane domains of ve-
sicular synaptobrevin and plasma membrane-resident syntaxin are adjacent to each
other, assembly pulls the membranes very closely together. Indeed, SNARE assem-
bly may suffice to overcome the energy barrier separating the membranes and ini-
tiate nonbilayer transition states that ultimately lead to fusion. According to this
view, SNAREs are the fusion catalysts. After fusion, the SNARE complexes are in
cis-configuration, with their potential energy spent, and their need recharging by
NSF and relocalization by membrane traffic before they are able to catalyze another
round of membrane fusion.
7–9
Several lines of evidence support the model described above. First, experiments
performed first on the fusion of yeast vacuoles showed that NSF is needed for pre-
paring the membranes for fusion (“priming”) but is expendable during the actual
fusion reaction.
24
These data showed that, in contrast with the original proposal,
25
SNARE disassembly is not involved in fusion. Second, liposomes carrying SNARE
proteins spontaneously fuse with each other.
26
Although the fusion is slow, it shows

174
ANNALS NEW YORK ACADEMY OF SCIENCES
at least some of the properties expected for biological fusion reactions, lending fur-
ther support to the idea that the SNAREs function as fusion catalysts. Third, recent
work has shown that posttranslational modifications of SNAREs, mutations, or
substitutions directly affect the kinetics of release in chromaffin cells.
27,28
These
findings, together with many others, explain why the model just described has
gained wide acceptance.
Despite the supporting evidence and its almost intuitive beauty, the SNARE “zip-
per” model has been challenged on several grounds. For instance, knock-out of
SNAP-25
29
or synaptobrevin
30
caused severe inhibition of neuronal exocytosis but
did not abolish fusion completely, resulting in the proposal that SNAREs may be in-
volved in controlling the real fusion machine but not in catalyzing membrane merger
itself. However, in none of these cases was it possible to exclude that the deleted
SNARE was functionally replaced by one of the closer homologues (see, e.g.,
Sørensen et al.
31
), and personally I am convinced that such redundancy explains
most, if not all, of the phenotypes in which some fusion activity remains after genetic
deletion of individual SNAREs.
A second challenge was derived from studies on the in vitro fusion of yeast vac-
uoles. Homotypic fusion of vacuoles has emerged as a versatile model system for
studying intracellular membrane fusion, because it combines an easy in vitro readout
for fusion with the power of yeast genetics, allowing, for instance, combining vacu-
oles from strains in which individual proteins are deleted or mutagenized (for review,
see Wickner and Haas
32
). Based largely on the effect of externally added compo-
nents on fusion kinetics, it was concluded that SNARE assembly operates upstream
of the actual fusion event.
33
In fact, several factors have been identified that are sup-
posed to operate after the SNAREs have done their job, including calmodulin,
34
pro-
tein phosphatase I,
35
and, surprisingly, the Vo-subunit of the vacuolar ATPase.
36
Again, however, I am not convinced by the evidence supposed to prove that SNARE
assembly does not participate in membrane merger. Most importantly, it is difficult
to interfere with assembly of SNAREs at a specific point in the cycle (e.g., by anti-
bodies, etc.), and it is even more difficult to ascertain that SNARE activity is com-
pletely blocked by the treatment. Furthermore, measurements of “trans” complexes
are almost exclusively based on coimmunoprecipitations that are inherently ambig-
uous because there is no way to differentiate between “trans” and “cis” after deter-
gent solubilization. Nevertheless, the yeast vacuole system has greatly contributed
to the understanding of the complex sequence of events leading up to fusion, and it
has led to the discovery of new proteins that are needed for vesicle docking or for
one of the later steps in the reaction cascade.
37
REGULATION OF SNARES
While the study of purified SNAREs has caused an already well-developed func-
tional molecular model for fusion, it is less well understood how SNAREs are regu-
lated. It would be very dangerous for a cell to have active fusion catalysts in every
membrane without tight control. Thus, there is increasing interest to understand the
mechanisms responsible for controlling membrane fusion, and thus the activity of
SNARE proteins.

175
JAHN: PRINCIPLES OF EXOCYTOSIS AND MEMBRANE FUSION
Obviously, one way to control SNAREs is to team them up with other proteins
that keep them in check and subject them to regulation by intracellular signaling cas-
cades. To identify such proteins, many laboratories have embarked on a search for
SNARE binding proteins. As a result, an almost overwhelming panoply of proteins
were reported to interact with one or another SNARE and to control its function, or
vice versa, that a given SNARE may control the activity of its ligand. For the neu-
ronal SNAREs the number of “specific” binding partners exceeds 50, documenting
the need for establishing rigorous structural and functional standards for the evalua-
tion of such interactions. Particularly the unstructured SNARE motifs are “sticky”
and thus may interact with other proteins in a nonspecific manner. Furthermore, syn-
taxin and SNAP-25 each comprise approximately 1% (!) of total protein in mamma-
lian brain.
38
In other words, the plasma membrane of a neuron probably contains
more copies of each of these SNAREs than of all other membrane proteins com-
bined, including receptors and ion channels. These features may explain why these
SNAREs often are found associated with other membrane proteins in immunopre-
cipitation and pull-down experiments, particularly when detergent extracts are used.
To find out whether a protein regulates SNARE function, one needs to understand in
detail to which of the possible SNARE conformations it binds and how it affects the
SNARE cycle. Evidently, the proposed interaction needs to be thoroughly character-
ized including the determination of affinities, stoichiometries, and conformations.
Unfortunately, these requirements have been met in only few cases.
For the neuronal SNAREs, several proteins have been firmly established as regu-
lators, although the molecular mechanism of the regulation is not understood. These
include synaptotagmin, the Ca
2+
sensor for regulated exocytosis in neurons and neu-
roendocrine cells,
45
the SM protein Munc-18, that controls the function of syntaxins
and/or SNARE assembly in a still unknown manner,
3,9
and complexins, small mol-
ecules that bind to the surface of the assembled neuronal complex and may stabilize
“trans” complexes
39,40
(F
IG
. 1). In addition, two soluble proteins, termed tomosyn
and amisyn, were identified that carry SNARE motifs and that may act as competi-
tive inhibitors of the genuine SNAREs by providing a “wrong” helix for the SNARE
complex.
41,42
In addition, it recently has been demonstrated that the activity of neu-
ronal SNAREs is controlled by protein kinases,
27,28
providing a direct link to intra-
cellular signaling cascades.
A second potential mechanism for regulating SNARE activity recently has
emerged from studies of SNAREs in intact and biologically functional plasma mem-
branes. Apparently, SNAREs are not randomly distributed in the plane of the mem-
brane but rather are clustered in hotspots at which vesicles bind and undergo
exocytosis.
43,44
Intriguingly, these hotspots depend on the presence of cholesterol as
they disintegrate when cholesterol is removed from the membrane. Because unlike
rafts these clusters are not resistant to detergents,
43
their protein composition is un-
known, and thus it remains to be established if these clusters contain proteins that
regulate and or stabilize SNAREs.
CONCLUDING REMARKS
There are still many open questions concerning the function of SNAREs in exo-
cytosis and membrane fusion. For instance, in vitro SNAREs assemble into com-

176
ANNALS NEW YORK ACADEMY OF SCIENCES
plexes rather promiscuously. Particularly, many R-SNAREs appear to be able to
substitute for each other. In the cell, the SNAREs involved for a given fusion reaction
are more specific, although for many of them there appear to be backup systems that
maintain function albeit with lesser efficacy (e.g., substitution of SNAP-25 with
SNAP-23 as recently characterized for exocytosis of bovine chromaffin cells
31
).
Furthermore, there is still a large discrepancy between the fast kinetics of intracellu-
lar fusion reactions and the in vitro assembly of SNAREs or the in vitro fusion of
SNARE containing liposomes that is orders of magnitude slower. The factors gov-
erning release kinetics thus need to be identified. Finally, we are only beginning to
understand the molecular determinants that characterize the specifics of the vast va-
riety of exocytotic events existing in eukaryotic cells, ranging from the constitutive
insertion of plasma membrane to neuronal exocytosis that is tightly regulated and oc-
curs at a millisecond time scale.
ACKNOWLEDGMENTS
I thank the members of my group for many stimulating discussions. This work
was supported by the Gottfried-Wilhelm Leibniz-Program of the Deutsche
Forschungsgemeinschaft.
REFERENCES
1. B
URGESS
, T.L. & R.B. K
ELLY
. 1987. Constitutive and regulated secretion of proteins.
Annu. Rev. Cell Biol. 3: 243–293.
2. C
HEN
, Y.A. & R.H. S
CHELLER
. 2001. SNARE-mediated membrane fusion. Nat. Rev.
Mol. Cell Biol. 2: 98–106.
3. J
AHN
, R., T. L
ANG
& T.C. S
ÜDHOF
. 2003. Membrane fusion. Cell 112: 533.
4. Z
ERIAL
, M. & H. M
C
B
RIDE
. 2001. Rab proteins as membrane organizers. Nat. Rev.
Mol. Cell Biol. 2: 107–117.
5. G
ALLWITZ
, D., C. D
ONATH
& C. S
ANDER
. 1983. A yeast gene encoding a protein
homologous to the human c-has/bas proto-oncogene product. Nature 306: 704–707.
6. P
FEFFER
, S.R. 2001. Rab GTPases: specifying and deciphering organelle identity and
function. Trends Cell Biol. 11: 487–491.
7. J
AHN
, R. & T.C. S
ÜDHOF
. 1999. Membrane fusion and exocytosis. Annu. Rev. Bio-
chem. 68: 863–911.
8. C
HEN
, Y.A. & R.H. S
CHELLER
. 2001. SNARE-mediated membrane fusion. Nat. Rev.
Mol. Cell Biol. 2: 98–106.
9. R
IZO
, J. & T.C. S
ÜDHOF
. 2002. SNAREs and Munc18 in synaptic vesicle fusion. Nat.
Rev. Neurosci. 3: 641–653.
10. F
ASSHAUER
, D. 2003. Structural insights into the SNARE mechanism. Biochim. Bio-
phys. Acta 1641: 87–97.
11. H
ANSON
, P.I., R. R
OTH
, H. M
ORISAKI
, et al. 1997. Structure and conformational
changes in NSF and its membrane receptor complexes visualized by quick-freeze/
deep-etch electron microscopy. Cell 90: 523–535.
12. S
UTTON
, B., D. F
ASSHAUER
, R. J
AHN
& A.T. B
RÜNGER
. 1998. Crystal structure of a core
synaptic fusion complex at 2.4 Å resolution. Nature 395: 347–353.
13. H
AYASHI
, T., S Y
AMASAKI
, S. N
AUENBURG
, et al. 1995. Disassembly of the reconsti-
tuted synaptic vesicle membrane fusion complex in vitro. EMBO J. 14: 2317–2325.
14. F
ASSHAUER
, D., W. A
NTONIN
, V. S
UBRAMANIAM
& R. J
AHN
. 2002. SNARE assembly
and disassembly exhibit a pronounced hysteresis. Nat. Struct. Biol. 9: 144–151.
15. B
OCK
, J.B., H.T. M
ATERN
, A.A. P
EDEN
& R.H. S
CHELLER
. 2001. A genomic perspective
on membrane compartment organization. Nature 409: 839–841.

177
JAHN: PRINCIPLES OF EXOCYTOSIS AND MEMBRANE FUSION
16. A
NTONIN
, W., D. F
ASSHAUER
, S. B
ECKER
, et al. 2002. Crystal structure of the endoso-
mal SNARE complex reveals common structural principles of all SNAREs Nat.
Struct. Biol. 9: 107–111.
17. F
ASSHAUER
, D., B. S
UTTON
, A.T. B
RÜNGER
& R. J
AHN
. 1998. Conserved structural fea-
tures of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-
SNAREs Proc. Natl. Acad. Sci. USA 95: 15781–15786.
18. S
ÖLLNER
, T., M.K. B
ENNET
, S.W. W
HITEHEART
, et al. 1993. A protein assembly-disas-
sembly pathway in vitro that may correspond to sequential steps of synaptic vesicle
docking, activation, and fusion. Cell 75: 409–418.
19. W
HITEHEART
, S.W., T. S
CHRAW
& E.A. M
ATVEEVA
. 2001. N-ethylmaleimide sensitive
factor (NSF) structure and function. Int. Rev. Cytol. 207: 71–112.
20. S
ÖLLNER
, T., S.W. W
HITEHEART
, M. B
RUNNER
, et al. 1993. SNAP receptors implicated
in vesicle targeting and fusion. Nature 362: 318–324.
21. L
ITTLETON
, J.T., E.R. C
HAPMAN
, R. K
REBER
, et al. 1998. Temperature-sensitive para-
lytic mutations demonstrate that synaptic exocytosis requires SNARE complex
assembly and disassembly. Neuron 21: 401–413.
22. P
ELHAM
, H.R., D.K. B
ANFIELD
& M.J. L
EWIS
. 1995. SNAREs involved in traffic
through the Golgi complex. Cold Spring Harb. Symp. Quant. Biol. 60: 105–111.
23. L
IN
, R.C. & R.H. S
CHELLER
. 1997. Structural organization of the synaptic exocytosis
core complex. Neuron 19: 1087–1094.
24. M
AYER
, A., W. W
ICKNER
& A. Haas. 1996. Sec18 (NSF)-driven release of Sec17p (
α
-
SNAP) can precede docking and fusion of yeast vacuoles. Cell 85: 83–94.
25. R
OTHMAN
, J.E. 1994. Mechanisms of intracellular protein transport. Nature 372: 55–63.
26. W
EBER
, T., B.V. Z
EMELMAN
, J.A. M
C
N
EW
, et al. 1998. SNAREpins: Minimal machin-
ery for membrane fusion. Cell 92: 759–772.
27. N
AGY
, G., U. M
ATTI
, R.B. N
EHRING
, et al. 2002. Protein kinase C-dependent phospho-
rylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle
recruitment. J. Neurosci. 22: 9278–9286.
28. S
ØRENSEN
, J.B., U. M
ATTI
, S.H. W
EI
, et al. 2002. The SNARE protein SNAP-25 is linked
to fast calcium triggering of exocytosis. Proc. Natl. Acad. Sci. USA 99: 1627–1632.
29. W
ASHBOURNE
, P., P.M. T
HOMPSON
, M. C
ARTA
, et al. 2002. Genetic ablation of the t-
SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat. Neurosci. 5:
19–26.
30. S
CHOCH
, S., F. D
EAK
, A. K
ÖNIGSTORFER
, et al. 2001. SNARE function analyzed in syn-
aptobrevin/VAMP knockout mice. Science 294: 1117–1122.
31. S
ØRENSEN
, J.B., G. N
AGY
, F. Varoqueaux, et al. 2003. Differential control of the releas-
able vesicle pools by SNAP-25 splice variants and SNAP-23. Cell 114: 75–86.
32. W
ICKNER
, W. & A.H
AAS
. 2000. Yeast homotypic vacuole fusion: a window on
organelle trafficking mechanisms. Annu. Rev. Biochem. 69: 247–275.
33. U
NGERMANN
, C., K. S
ATO
& W. W
ICKNER
. 1998. Defining the functions of trans-
SNARE pairs. Nature 396: 543–548.
34. P
ETERS
, C. & A. M
AYER
. 1998. Ca
2
+
/calmodulin signals the completion of docking and
triggers a late step of vacuole fusion. Nature 396: 575–580.
35. P
ETERS
, C., P.D. A
NDREWS
, M.J. S
TARK
, et al. 1999. Control of the terminal step of
intracellular membrane fusion by protein phosphatase 1. Science 285: 1084–1087.
36. P
ETERS
, C., M.J. B
AYER
, S. B
UHLER
, et al. 2001. Trans-complex formation by proteo-
lipid channels in the terminal phase of membrane fusion. Nature 409: 581–588.
37. W
ICKNER
, W. & A. H
AAS
. 2000. Yeast homotypic vacuole fusion: a window on
organelle trafficking mechanisms. Annu. Rev. Biochem. 69: 247–275.
38. W
ALCH
-S
OLIMENA
, C., J. B
LASI
, L. E
DELMANN
, et al. 1995. The t-SNAREs syntaxin 1
and SNAP-25 are present on organelles that participate in synaptic vesicle recycling.
J. Cell Biol. 128: 637–645.
39. R
EIM
, K., M. M
ANSOUR
, F. V
AROQUEAUX
, et al. 2001. Complexins regulate a late step in
Ca2+-dependent neurotransmitter release. Cell 104: 71–81.
40. C
HEN
, X., D.R. T
OMCHICK
, E. K
OVRIGIN
, et al. 2002. Three-dimensional structure of
the complexin/SNARE complex. Neuron 33: 397–409.
41. M
ASUDA
, E.S., B.C.B. H
UANG
, J.M. F
ISHER
, et al. 1998. Tomosyn binds t-SNARE pro-
teins via a VAMP-like coiled coil. Neuron 21: 479–480.

178
ANNALS NEW YORK ACADEMY OF SCIENCES
42. S
CALES
, S.J., B.A. H
ESSER
, E.S. M
ASUDA
& R.H. S
CHELLER
. 2002. Amisyn, a novel
syntaxin-binding protein that may regulate SNARE complex assembly. J.Biol. Chem.
277: 28271–28279.
43. L
ANG
, T., D. B
RUNS
, D. W
ENZEL
, et al. 2001. SNAREs are concentrated in cholesterol-
dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 20:
2202–2213.
44. C
HAMBERLAIN
, L.H., R.D. B
URGOYNE
& G.W. G
OULD
. 2001. SNARE proteins are
highly enriched in lipid rafts in PC12 cells: implications for the spatial control of
exocytosis. Proc. Natl. Acad. Sci. USA 98: 5619–5624.
45. C
HAPMAN
, E.R. 2002. Synaptotagmin: a Ca
2+
-sensor that triggers exocytosis? Nat.
Rev. Mol. Cell Biol. 3: 613–618.
Wyszukiwarka
Podobne podstrony:
egzocytoza2000 5fantastic pl
egzocytoza2000 5fantastic pl
2011Wykład1 chemii ogólnej 5fantastic pl
fizjologia 5fantastic pl
POPRAWA WSZYSTKICH KOLOKWIËW. 5fantastic.pl , Ćwiczenia
Osocze a mocz. 5fantastic.pl , Ćwiczenia
Zjazd5s1 v2. 5fantastic.pl , Ćwiczenia
wykład 1. 5fantastic.pl , Ćwiczenia
podstawy produkcji roślinnej. 5fantastic.pl , Ćwiczenia(2)
Dobrostan owiec. 5fantastic.pl , Wykłady(1)
kolos 2. 5fantastic.pl , Ćwiczenia(1)
MODU 0 MODU Y SZCZEG Y. 5fantastic.pl , Wykłady(1)
sprawozdanie. 5fantastic.pl , Ćwiczenia
Chwasty1. 5fantastic.pl , Ćwiczenia
więcej podobnych podstron