Postępy Biochemii 58 (4) 2012
429
Anna Cmoch
1,*
Patrick Groves
2
Małgorzata Palczewska
2
Sławomir Pikuła
1
1
Department of Biochemistry, Nencki Insti-
tute of Experimental Biology, Polish Acad-
emy of Sciences, 02-093 Warsaw, Poland
2
Department of Biological Chemistry, Instituto
de Tecnologia Quimica e Biologica, Universi-
dade Nova de Lisboa, Av. da Republica, 2780-
157 Oeiras, Portugal
*
Department of Biochemistry, Nencki Institute
of Experimental Biology, Polish Academy of
Sciences, 3 Pasteura Street, 02-093 Warsaw,
Poland; e-mail: a.cmoch@nencki.gov.pl
Received: October 10, 2012
Accepted: October 29, 2012
Key words: S100, annexins, signal transduc-
tion, calcium ions, calcium binding proteins
Abbreviations: AnxA — vertebrate annexin;
[Ca
2+
] — Ca
2+
concentration; CaBPs — calcium
binding proteins; ER — endoplasmic reticulum
Acknowledgements: This work was sup-
ported by a grant N N401 140639 from the Pol-
ish Ministry of Science and Higher Education
and by Polish-Portugal Executive Program for
years 2011-2012 (project 760) sponsored by the
Polish Ministry of Science and Higher Educa-
tion and by Portuguese Fundação para a Ciên-
cia e a Tecnologia.
S100A proteins in propagation of a calcium signal in norm and pathology
ABSTRACT
C
alcium ions are essential factors controlling the balance between cell survival, growth,
differentiation and metabolism. Ca
2+
acts as a global second messenger involved in the
regulation of all aspects of cell function. Fluctuations in the intra- and extracellular Ca
2+
con-
centration [Ca
2+
] in response to different environmental stimuli drive most cellular func-
tions. Therefore, sustenance of calcium homeostasis requires perfect organization in time
and space that is achieved by calcium binding proteins (CaBPs). These proteins are involved
in sensing and transforming calcium signals to downstream cellular responses. Growing
number of evidence suggests than many human disorders, including cancer progression, are
related to deregulation of cellular calcium homeostasis and defects in CaBPs functions. In
this review we will focus on the roles of S100A proteins in intracellular and extracellular
calcium signalling and homeostasis. The S100A subfamily is among the most distinctive of
EF-hand CaBPs and are found exclusively in vertebrates. They are believed to have evolved
to enable activation of specific biochemical pathways in parallel to the activity of Ca
2+
sen-
sors such as calmodulin and/or annexins. The importance of S100 proteins is underscored by
their deregulated expression in neurodegenerative and inflammatory disorders, myopathies
and cancer. In addition, S100 proteins serve as diagnostic markers in the clinic and are under
constant investigation. Their roles and the roles of the S100A protein partners in normal and
pathology will be also discussed.
InTroduCTIon
The role of Ca
2+
as a key and pivotal second messenger in cells depends largely
on a wide number of heterogeneous calcium binding proteins. The S100 family
of proteins comprises 25, homologous, acidic calcium binding proteins with the
EF-hand type (helix-loop-helix motif) calcium binding domain. The canonical
C-terminal calcium-binding EF- hand is common to all EF-hand proteins, whi-
le the N-terminal EF-hand is non-canonical. The N-terminal EF-hand exhibits a
different architecture with a specific 14 amino acid motif flanked by helices HI
and HII. This motif is characteristic for S100 proteins and therefore it is often cal-
led ‘S100-specific’ or ‘pseudo EF-hand’. S100 proteins are characterized by low
molecular weights (9–13 kDa as monomers). They possess broadly known abi-
lity to form oligomers (homodimers, heterodimers and oligomeric assemblies)
and are characterized by tissue and cell-specific expression [1-4]. There is a great
diversification of the identified S100 proteins, but solely they are present only in
vertebrates. S100 genes were not found in such model organism as Arabidopsis
thaliana, Drosophila melanogaster, Caenorhabditis elegans or Saccharomyces cerevisiae.
In evolutionary terms, the lowest organism reported thus far containing a pseu-
do EF- hand protein closely related to S100A is a chondrichthye (dogfish Squalus
acanthias) [3].
The adopted nomenclature designates the S100 genes in the chro-
mosomal cluster 1q 21 as S100A followed by Arabic numbers (S100A1, S100A2
etc.). Several S100 proteins are present in human, but absent in rat and mouse
(S100A2, S100A12). There is also gene duplication (human S100A7) supporting
the hypothesis of the rapid evolution and expansion of the S100 family of pro-
teins [5].
S100A proteins possess ability to form higher complexes (homodimers, hete-
rodimers and oligomeric assemblies) and are characterized by tissue and cell-
-specific expression [3,4]. Up to date, several heterodimeric S100 proteins have
been reported: S100B forms heterodimers with S100A1, S100A6 and S100A11;
S100A1 with S100A4, S100P and S100A7 with S100A10. Noncovalent multimers
were observed for S100A12, S100A8 ⁄A9, S100A4 and a Zn
2+
-dependent tetramer
for S100A2 [3].
The conformation, folding and oligomerization state of S100s are responsive
to their metal-binding properties and have a pivotal influence on their function.
S100 proteins exert their actions usually through Ca
2+
binding, although Zn
2+
and Cu
2+
have also been shown to regulate their biological activity. Binding of
430
www.postepybiochemii.pl
Ca
2+
to S100s exposes hydrophobic sites, which enable them
to interact with specific target proteins or membranes. Bin-
ding of the target protein in the presence of calcium often
results in an increase in calcium affinity of the S100 prote-
in as well [6-8]. Most S100 proteins are directly involved in
intracellular calcium signal transduction, Ca buffering and
in Ca uptake and transport. At low cytosolic [Ca
2+
], as in
the resting state of the cell, S100 proteins possess a closed,
relatively hydrophilic conformation. During cell activation
the cytosolic [Ca
2+
] increases due to Ca
2+
influx via plasma
membrane Ca
2+
channels and exchangers or due to their
release from intracellular Ca
2+
stores such as endoplasmic
reticulum (ER) and mitochondria. S100 proteins are charac-
terized by affinites for Ca
2+
, e.g. in a range that allows them
to respond to changes of cytosolic [Ca
2+
] (with one exception
of S100A10, which is Ca
2+
insensitive) [9-11]. The resulting,
Ca
2+
-dependent structural changes largely affect helix III
[12,13].
Intracellularly, S100 proteins act as Ca
2+
sensors, transla-
ting increases of cytosolic Ca
2+
level into a cellular respon-
se. S100 proteins display a relatively large range of calcium
binding affinity (K
D
20–500 µM). The binding of S100 pro-
teins to their targets is typically calcium-dependent, but
calcium-independent interactions have also been described
(S100A10-AnxA2). Evidence exists that Ca
2+
binding dicta-
tes the membrane binding affinity of S100A. Interestingly,
in some cases the interaction with membranes is weaker for
Ca
2+
bound S100A13 than in apo-S100A13 [14].
S100A2, S100A3, S100A6, S100A7, S100A8⁄9 and S100A12
bind Zn
2+
in specific structural sites. The binding of diffe-
rent metal ions results in conformational adjustments and
modulation of S100 protein folding and function in cells.
In the case of S100A12, Zn
2+
binding leads to an increase in
Ca
2+
affinity, whereas in S100A2 the opposite
effect was observed. On the other hand, Zn
2+
and Ca
2+
binding to some of S100 proteins
are both required for their interaction with
receptors such as RAGE (the receptor for ad-
vanced glycation end-products). The S100A5,
S100A12 and S100A13 binds Cu
2+
at the same
sites to which Zn
2+
binds [3].
InTrA- And EXTrACELLuLAr
PArTnErS oF S100A ProTEInS
Members of the S100 family of proteins, in
the calcium dependent or independent man-
ner, interact with a variety of target proteins
including enzymes, cytoskeletal subunits,
receptors, transcription factors and nucleic
acids. Several S100 proteins exhibit Ca
2+
-de-
pendent interactions with metabolic enzymes
(S100A1 with aldolase C), with cytoskeletal
proteins (S100A1 with tubulin or with DNA-
-binding proteins, S100A2 and S100A4 inte-
ract with p53) [15].
S100 proteins are known to interact with
members of the other large family of cal-
cium binding proteins - annexins -(AnxA2
with S100A4, S100A6, S100A10 or S100A11
and AnxA6 with S100B, S100A6, S100A11,
S100A1) to form complexes that exhibit biological activities
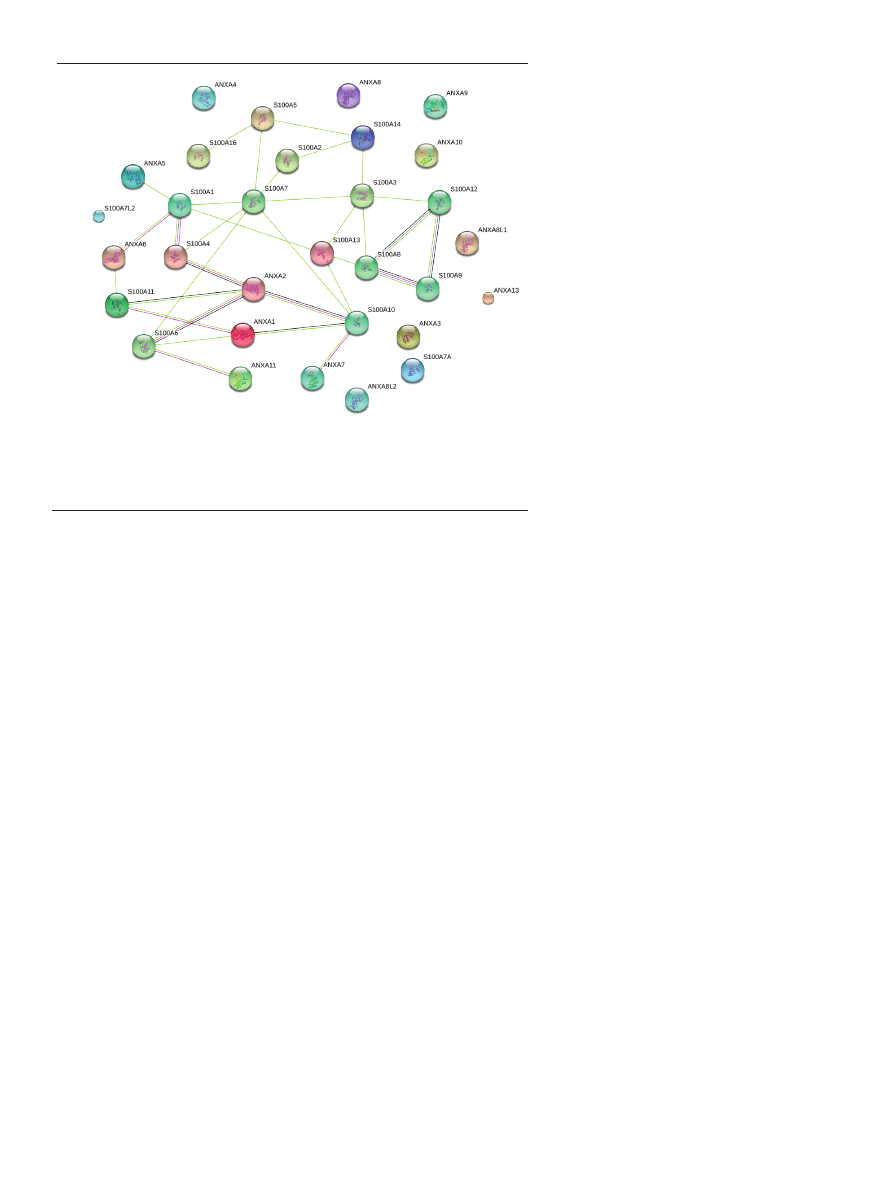
[16-18] (Fig. 1).
S100A10 interacts not only with AnxA2 but also with
multiple proteins: serotonin receptor 5-HT1B, NaV1.8 so-
dium channel, TASK-1 potassium channel, ASIC-1 chan-
nels; TRPV5/TRPV6 channels, cytosolic phospholipase A
2
,
BAD (Bcl2-antagonist of cell death), AHNAK (neuroblast
differentiation-associated protein), cathepsin B, plasmi-
nogen activator, transglutaminase, S100A7, S100A8 [19].
Many functional consequences of the interactions between
S100A10 and its partners have been reported. There is accu-
mulating evidence that S100A10 interacts with a diverse set
of target proteins and regulates various biological functions
in different cellular compartments.
Most researchers concentrate on S100A10-annexin A2
heterotetramer formation and functions inside and outside
of the cell. Typically, S100A10 is found in most cells bound
to its annexin A2 ligand as the heterotetrameric S100A-
10
2
AnxA2
2
complex, AIIt. In addition to an intracellular di-
stribution, S100A10 is present on the extracellular surface
of many cells. It was indicated that it facilitates the translo-
cation of TRPV5 and TRPV6 channels towards the plasma
membrane in endothelial cells [20]. TRP (transient recep-
tor potential) channels constitute a superfamily of sensory
channels whose functions range from phototransduction
(where they were first described), olfaction, heat, cold sen-
sation etc., to Ca
2+
sensors/transporters. The TRP Ca
2+
chan-
nels are important for absorption of Ca
2+
into kidney, bone,
placenta, or intestine to maintain systemic Ca
2+
homeostasis.
The S100A10-annexin 2 complex specifically associates with
the C-terminal of TRP channels and is suggested to play a
Figure 1. STRING 9.0 analysis of direct (physical) or indirect (functional) associations between human
annexins and S100s proteins. The lines represent the existence of the several types of evidence used in
predicting the associations (high confidence score 0.7). The interactions are shown in different colors:
black is co-expression, dark blue is co-occurrence, purple is experimental evidence, light green is text
mining.
Postępy Biochemii 58 (4) 2012
431
role in guiding and localizing channels to the plasma mem-
brane and/or in the modulation of channel activity. It ap-
pears to act as a scaffolding protein that conjugates appro-
priate proteins at the plasma membrane. S100A10-annexin 2
also interacts with acid-sensing ion channels (ASIC1a) [21].
S100A10 has been reported to modulate the activity of
NaV1.8 (tetrodotoxin-resistant voltage-gated sodium chan-
nel), which is involved in the transmission of nociceptive
information from sensory neurons to the central nervous
system in nociceptive and neuropathic pain conditions.
NaV1.8 requires S100A10 accessory proteins for its functio-
nal expression on the plasma membrane [22].
The AnxA2-S100A10 complex formation in some types of
cells leads to plasminogen activation, either tissue-type pla-
sminogen activator (tPA) or urokinase-type plasminogen
activator (uPA), facilitating the conversion of plasminogen
to plasmin. The formation of a ternary complex between
tPA, plasminogen, and S100A10 provides a mechanism to
localize the proteolytic activity of plasmin to the cell sur-
face. Active plasmin both degrades fibrin directly and ac-
tivates members of the matrix metalloproteases family,
creating a localized proteolytic hub during angiogenesis or
tumor growth [23,24]. Annexin A2 plays an important role
in plasminogen regulation by controlling the levels of extra-
cellular S100A10 and by acting as a plasmin reductase. The
mechanism by which annexin A2 regulates the extracellular
levels of S100A10 is unknown.
Some ion channels that are regulated by calmodulin
may in fact be modulated by S100 proteins, either under
resting conditions or under special circumstances. Whether
the effect of S100 proteins is direct or indirect, knowledge
of the molecular basis for calmodulin interactions with ion
channels may be helpful in discerning how S100 proteins
modulate ion channels, in particular since there may well
be a similarity in their mode of action [25]. Some K
+
, Na
+
,
and Cl
−
channels are activated or modulated by intracellular
Ca
2+
signals giving rise to the notion that Ca
2+
binding pro-
teins may play a role in regulating channel gating function.
There is growing evidence for modulatory roles played by
the S100 proteins in the regulation of those types of chan-
nels. External binding of S100 proteins to ion channels has,
however, not been reported so far [26].
S100A4, the recently recognized novel binding partner of
AnxA2, manages various functions dependent on cellular
compartmentalization. Intracellular S100A4 exists as a sym-
metric homodimer that facilitates the binding of its target
proteins (actin, nonmuscle myosin IIA and IIB, tropomy-
osin). Extracellular S100A4 interacts with AnxA2, MMP-13,
RAGE or epidermal growth factor (EGF) receptor ligands.
Through these interactions, S100A4 regulates cell mobility,
invasion, and angiogenesis [27,28]. S100A4 was reported
as involved in the regulation of osteoblastic transcription
factors Runx2/Cbfa1 and Osx. S100A4 plays an important
role in matrix remodeling by up-regulating the expression
of crucial matrix metalloproteinases (MMPs) of bones [29].
S100A1 is known to increase the L-type Ca
2+
channel (L-
-type Ca
2+
channels located in the SR) current at nanomolar
Ca
2+
concentrations and enhances Ca
2+
release in skeletal
and in cardiac muscle. S100A1 was shown to directly in-
teract with PKA and this complex appears to affect Ca
2+
channels. At the molecular level, S100A1 was shown to
interact in a Ca
2+
-dependent manner with the cardiac iso-
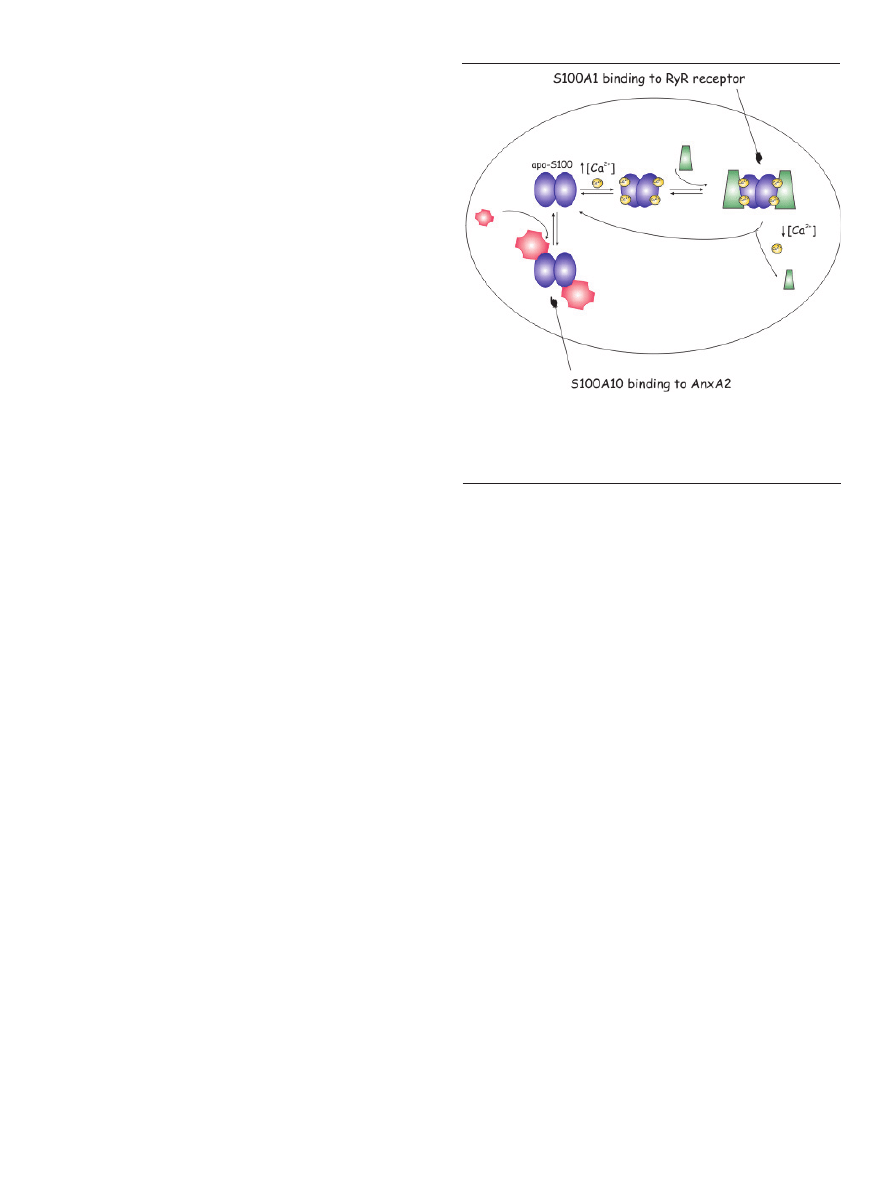
forms of RYR2 (ryanodine receptor, isoform 2) (Fig. 2) [30],
SERCA2A (sarco/endoplasmic reticulum calcium ATPase,
isoform 2A) [31], phospholamban (PLN), titin, and the mi-
tochondrial F1-ATP synthase in complex V of the respira-
tory chain [5].
S100A proteins have been also intensively studied for
their interaction with heat shock-regulated proteins. For
example, S100A6 mediates nuclear translocation of Sgt1
and its interaction with Hsp-90 [32,33]. S100A1 and S100A2
proteins regulate the Hsp-90 interaction with target proteins
[34]. S100A1 is also known to be a component of the Hsp70/
Hsp90 multichaperone complex [35].
CELLuLAr FunCTIonS oF S100A ProTEInS
A richness of possible targets for S100 proteins is in
accordance with their multiple functions. Therefore, S100
proteins regulate a diverse array of cellular activities,
including the differentiation [36] and apoptosis [37,38],
motility, membrane–cytoskeleton interactions and cyto-
skeleton dynamics [39,40], cellular Ca
2+
homeostasis [41],
transduction of intracellular Ca
2+
signals, innate and ada-
ptive immunity [1,2,25,42] and are predicted to partici-
pate in mineralization [43]. Up to now, only S100A4 and
S100A8/A9 have been considered as factors involved in
mineralization control [44-46]. They have been also re-
ported as cellular protectors from oxidative cell damage,
regulators of protein phosphorylation [47], secretion and
transcriptional factors [1-3]. S100A8/A9 complex, was re-
ported as a Ca
2+
sensor which controls the interplay be-
Figure 2. Molecular mechanisms of S100 target protein interactions. In the presen-
ce of elevated Ca
2+
concentrations, apo-S100 undergoes a conformational change
and interacts with target proteins in a Ca
2+
-dependent pathway. The interaction
of S100A1 and RyR receptors is shown as a specific example. In contrast, S100A10
interacts with annexin A2 independently of Ca
2+
.
432
www.postepybiochemii.pl
and vascular smooth muscle cells, neurons, astrocytes,
Schwann cells, epithelial cells, myoblasts and cardiomy-
ocytes [49]. Multimeric assemblies seem to be necessary for
the extracellular functions of S100 proteins and have been
reported for S100A12, S100A4, S100B and S100A8/A9 [3].
S100-associated cell signalling may be promiscuous. This
can be best exemplified through S100A8 ⁄A9, which pro-
motes RAGE-dependent cell survival as well as multiple
RAGE-independent cell death pathways.
This diversity in function of the S100 provides evidence
of very extensive evolutionary optimization of the fit be-
tween the EF-hand CaBPs fold and the calcium ion. S100
proteins form a phylogenetically young group among the
EF-hand proteins (present only in verterbrates). Future rese-
arch in the coming years will certainly contribute to clarify
some of these and other questions and will ultimately bring
us to a higher level of understanding the biology of tumour
and degeneration and enable to use our acquired knowled-
ge of S100 structure and functions in developing strategies
to modulate their activity for therapeutic purposes [51].
rEGuLATIon oF S100A FunCTIonS BY
PoSTTrAnSLATIonAL ModIFICATIonS
A number of posttranslational protein modifications have
been described. Posttranslational protein modifications may
result in physiochemical changes of the protein with respect
to mass, charge, structure and conformation. S100 proteins
may be modified by various post-translational modifica-
tions, including phosphorylation, methylation, acetylation,
and oxidation. These modifications may alter their ion-bin-
ding properties, interactions with target proteins, transloca-
tion within cell compartments, degradation, protein-protein
interactions and extracellular functions.
tween extracellular Ca
2+
entry and intraphagosomal ROS
production [48].
Within the cells, S100 proteins often translocate from
one compartment to another (nucleus, cytoplasm) in re-
sponse to changes in calcium concentration or concentra-
tion of extracellular S100 using different translocation pa-
thways. In addition to their intracellular functions, S100
proteins can also be secreted and may exert the role of
cytokines (e.g. S100A4, S100A8, S100A9) through the ac-
tivation of various cell surface receptors in an autocrine
and paracrine manner through various receptors RAGE
(Fig. 3), toll-like receptor 4 (TLR-4) (Fig. 4), G-protein-co-
upled receptors, scavenger receptors or heparan sulfate
proteoglycans and N-glycans
[25]. S100B, S100P, S100A4,
S100A6, S100A8/A9, S100A11 and S100A12 are known to
interact with RAGE [49], while S100A8/A9 also bind Toll-
-like receptors or TLRs [50]. Some S100 proteins, including
S100A4, S100A7, S100A8 ⁄A9, S100A11, S100A12, S100B
and others, are commonly secreted, exhibiting cytokine-
-like and chemotactic activity. When S100A7, S100A8,
S100A9, S100A12 or S100B are secreted in response to cell
damage or activation, they act as alarmins (cellular stress
signals), activating other immune and endothelial cells.
Frequently, S100 poroteins are secreted upon Ca
2+
signa-
ling via vesicle fusion with the cell membrane into the
extra cellular space, where they might acquire oligomeric
structures specialized for extracellular functions [5].
S100 proteins can also be released into the extracellular
space in response to stimuli, or during cell damage, and
they promote responses including neuronal survival and
extension (S100B), apoptosis (S100A4 and S100A6), inflam-
mation (S100B, S100A8/A9, S100A11 and S100A12), au-
toimmunity (S100A8/A9), chemotaxis (S100A8/A9) and
cell proliferation and survival (S100P, S100A7), effectively
functioning as paracrine and autocrine mediators. Thus,
extracellular S100 proteins exert regulatory activities on
monocytes/macrophages/microglia, neutrophils, lym-
phocytes, mast cells, articular chondrocytes, endothelial
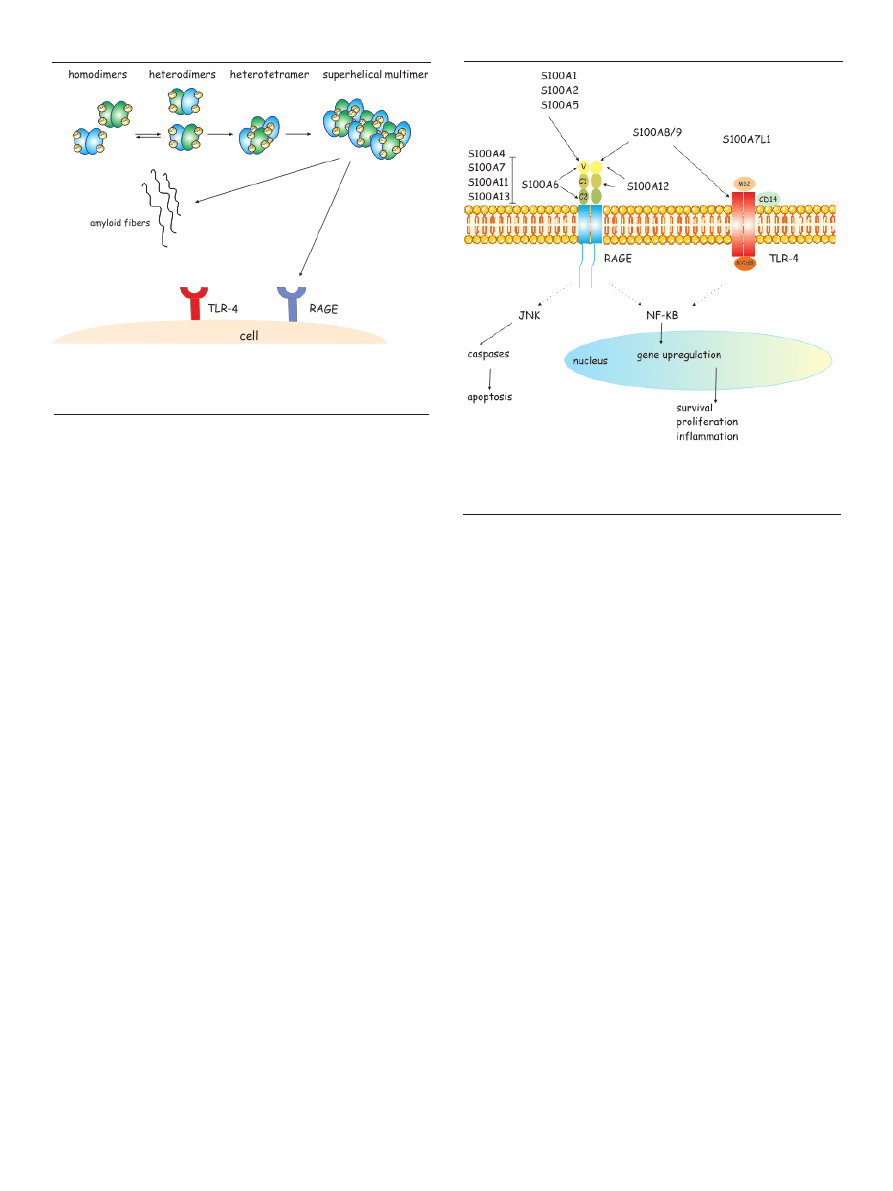
Figure 3. Native states and oligomerization pathways in S100 proteins. A scheme
outlining possible routes of oligomerization pathways for S100A8 and S100A9
proteins. From Fritz et al. [3], modified.
Figure 4. Binding of S100A to receptors in human disorders. Activation of RAGE
by S100 proteins leads to cell survival and proliferation. S100A8/A9 complex ac-
tivates TLR-4 receptors to induce inflammation. S100A8/A9 and S100A12 interact
with scavenger receptor. From LeClerc et al. [4], modified
Postępy Biochemii 58 (4) 2012
433
S100 proteins can be modified post-translationally by
phosphorylation (S100A8/A9, S100A11), nitrosylation
(S100A1, S100B, S100A8), citrullination (S100A3) (34), car-
boxymethylation (S100A8/A9), glutathionylation, trans-
amidation (S100A11) or sumoylation. These modifications
often modulate the interaction with calcium or target pro-
teins. Additionally, the promoters of several S100 proteins
have been found hypo- or hyper-methylated, resulting in
epigenetic changes in protein expression [25] (Fig. 5).
Extracellular functions of several S100s may be regula-
ted by oxidative modifications. Many redox-based signa-
ling pathways are regulated by reversible modifications
such as intra- and interprotein disulfides, S-nitrosylation
and glutathionylation. Cysteine S-nitrosylation is a new fac-
tor responsible for increasing functional diversity of S100A
proteins and helps explain the role of S100A as a Ca
2+
si-
gnal transmitter sensitive to NOS/redox state within cells.
S100A1 has been recently reported in PC12 cells to be endo-
genously S-nitrosylated at site important for target binding
[52]. S100A8 and S100A9 are S-nitrosylated by NO donors
including GSNO, the physiological regulator of NO trans-
port and signaling. S100A8 is preferentially nitrosylated in
the S100A8/A9 complex. S-nitrosylation of S100A9 is cal-
cium-dependent, whereas S100A8 is not. In contrast to the
proposed, protective role of extracellular S100A8 and to
some extent, S100A9, these proteins regulate intracellular
NADPH oxidase activity and thus, ROS (reactive oxygen
species) generation. Thus, there is a paradigm on dual roles
of S100A8/A9 in redox balance. Oxidation may represent a
switch, whereby the modified proteins display their func-
tions [53].
Phosphorylation of Ser and Thr residues increased the af-
finity of S100A for the p53 TAD domains. Conversely, acety-
lation and phosphorylation of the C-terminus of p53 decre-
ased the affinity for S100A2 [15]. S100A9 protein has been
also reported as phosphorylation target for MAPK [54].
Phosphorylation of that protein leads to S100A8/A9 hete-
rotetramer translocation and NOX2 activation. Kouno et al.
[55] proposed phosphorylated S100A11 to be recognized by
its target protein, nucleolin and in such complex transloca-
ted to the nucleus.
Sumoylation of two lysines in S100A4 molecule results in
nuclear translocation of this protein and its action as trans-
cription factor bound to the promoter region of MMP-13
[29,53]. Up to date S100 proteins have not been described to
be glycosylated.
dErEGuLATIon oF S100A GEnES EXPrESSIon
Deregulated expression of genes encoding members of
the S100 family of calcium-binding proteins has been asso-
ciated with multiple tumor types. S100A2, S100A4, S100A6
and S100A10 genes alterations have been associated with
brain tumors. Furthmore, the methylation/demethylation
of these genes plays a role in the control of their tumor gene-
rating potency [56]. S100A1 is thought to modulate the Ca
2+
sensitivity of the sarcoplasmic reticulum (SR) Ca
2+
release
channels (ryanodine receptors or RyRs) and chronic absence
of S100A1 results in enhanced L-type Ca
2+
channel activity
combined with a blunted SR Ca
2+
release amplification in
cardiomyocytes [57]. The loss of S100A10 from the extracel-
lular surface of cancer cells results in a significant loss in
plasmin generation. In addition, S100A10 knock-down cells
demonstrate a dramatic loss in extracellular matrix degra-
dation and invasiveness as well as reduced metastasis [58].
The specific knockdown of S100A4 strongly suppressed cell
growth, migration and invasion activities in cancer cells,
therefore S100A4 may positively regulate tumor cell proli-
feration, invasion and metastasis associated with multiple
molecules [59].
S100A ProTEInS In HuMAn dISEASES
— CLInICAL SIGnIFICAnCE
Members of the S100 protein family have been shown to
posess pathophysiologic implications. In addition, members
of the S100 protein family are extensively tested as useful
biomarkers of certain diseases and potential targets of clini-
cal therapies [60]. There is growing evidence that expression
of S100 proteins is altered in pathologies. The S100 protein
levels are associated with a wide array of pathological con-
ditions like chronic inflammation [1,2,61-65], immunode-
fence [27], cardiomyopathies [30,66-68], atherosclerosis [69],
rheumatoid arthritis [70,71], cystic fibrosis [72] and cancer
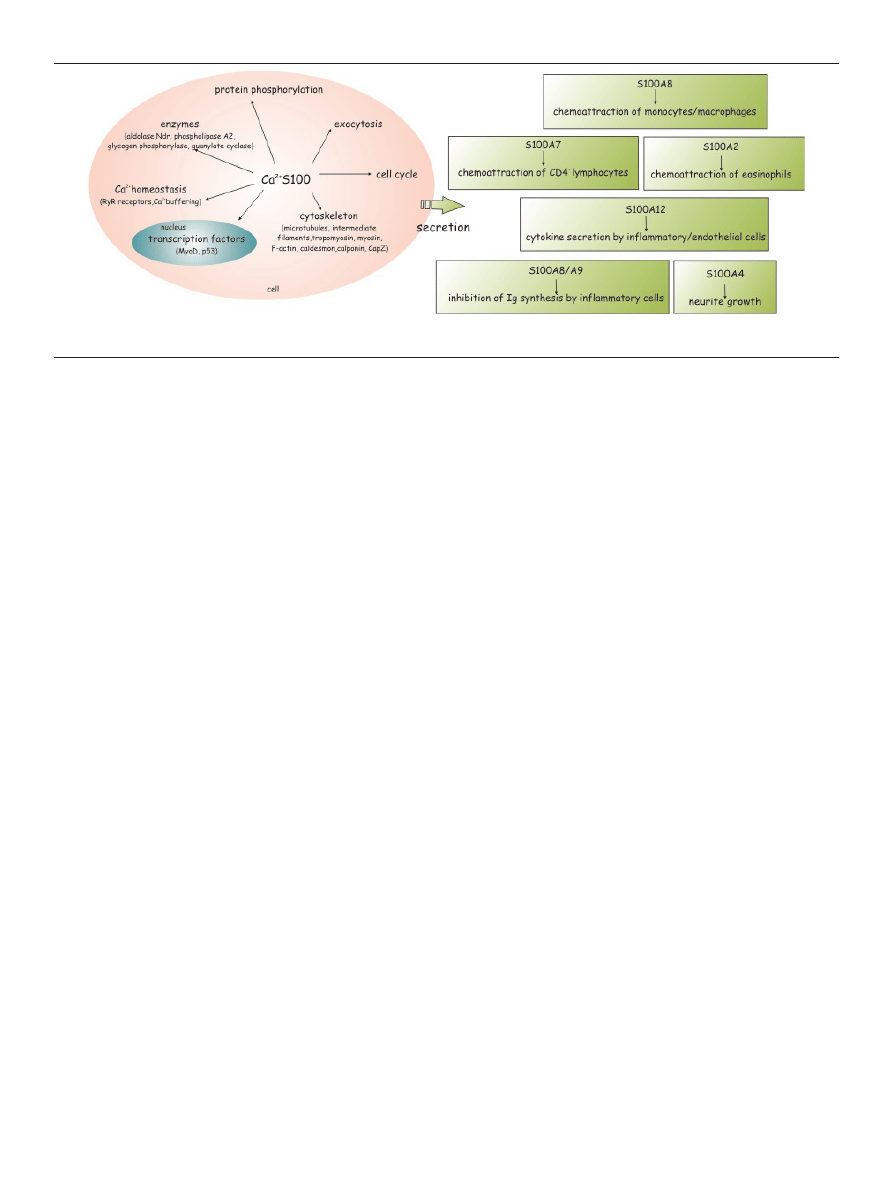
Figure 5. Intracellular and extracellular roles of S100A proteins. Shown are the best known targets/activities putatively regulated by S100A proteins in a Ca
2+
-dependent
and Ca
2+
-independent manner. From Donato et al. [1], modified.

434
www.postepybiochemii.pl
[1,2,61,73-89]. S100 proteins are thought to be also associa-
ted with diabetes and its complications [80], neurodege-
neration and Alzheimer disease [90,91] and posttraumatic
stress disorder [92].One of the most intringuing directions
in research on S100A proteins is their involvement in neuro-
nal plasticity regulation and depression [19].
Functions of some S100A proteins in cancer progression
are opposite to others, for example down-regulation of
S100A6 and S100A10 in breast cancer, irrespective of patho-
logical stage, was observed whereas S100A7, S100A8 and
S100A9 were strongly up-regulated only in some type of
breast cancer [93]. There are many factors that can modu-
late S100A expression in biological systems. For example,
the S100A10 coding gene is regulated by various factors:
dexamethasone, TGF-α, EGF, NO donors, interferon-γ, vita-
min D, retinoic acid, NGF, electroconvulsive treatment [19].
Up regulation of S100A10 occurs also in response to AnxA2
upregulation. Down-regulation of AnxA2 is coordinated
with lack of S100A10 protein, but the specificity of mecha-
nism which is responsible for that phenomenon still rema-
ins unknown. Here we provided the examples of complica-
ted regulation of S100A proteins functions and expression.
ConCLudInG rEMArKS And FuTurE PErSPECTIVES
The thesis is emerging that S100 proteins are phyloge-
netically new proteins displaying the unusual property of
acting both within cells as Ca
2+
sensor proteins implicated
in Ca
2 +
signal transduction, and outside cells as ligands for
specific cell surface receptors on an increasingly larger num-
ber of cell types. Therefore the grand challenge ahead is de-
termining the role of particular S100A proteins in diseases
related to Ca
2+
homeostasis disorder such as neurological
diseases and artheriosclerosis.
LITErATurE
1. Donato R (2001) S100: a multigenic family of calcium-modulated pro-
teins of the EF-hand type with intracellular and extracellular functio-
nal roles. Int J Biochem Cell Biol 33: 637-668
2. Heizmann CW, Fritz G, Schäfer BW (2002) S100 proteins: structure,
functions and pathology. Front Biosci 7: 1356-1368
3. Fritz G, Botelho HM, Morozova-Roche LA, Gomes CM (2010) Natural
and amyloid self-assembly of S100 proteins: structural basis of functio-
nal diversity. FEBS J 277: 4578-4590
4. Leclerc E, Heizmann CW (2011) The importance of Ca
2+
/Zn
2+
signa-
ling S100 proteins and RAGE in translational medicine. Front Biosci
3: 1232-1262
5. Schaub MC, Heizmann CW (2008) Calcium, troponin, calmodulin,
S100 proteins: from myocardial basics to new therapeutic strategies.
Biochem Biophys Res Commun 369: 247-264
6. Bhattacharya S, Bunick CG, Chazin WJ (2004) Target selectivity in EF-
-hand calcium binding proteins. Biochim Biophys Acta 1742: 69-79
7. Streicher WW, Lopez MM, Makhatadze GI (2009) Annexin I and an-
nexin II N-terminal peptides binding to S100 protein family members:
specificity and thermodynamic characterization. Biochemistry 48:
2788-2798
8. Streicher WW, Lopez MM, Makhatadze GI (2010) Modulation of qu-
aternary structure of S100 proteins by calcium ions. Biophys Chem
151: 181-186
9. Otterbein LR, Kordowska J, Witte-Hoffmann C, Wang CLA, Domin-
guez R (2002) Crystal structures of S100A6 in the Ca
2+
-free and Ca
2+-
-bound states: The calcium sensor mechanism of S100 proteins reve-
aled at atomic resolution structure. Structure 10: 557-567
10. Zimmer DB, Wright Sadosky P, Weber DJ (2003) Molecular mechani-
sms of S100-target protein interactions. Microsc Res Tech 60: 552-559
11. Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS (2006) Calcium-
-dependent and -independent interactions of the S100 protein family.
Biochem J 396: 201-214
12. Groves P, Finn BE, Kuznicki J, Forsén S (1998) A model for target pro-
tein binding to calcium-activated S100 dimers. FEBS Lett 421: 175-179
13. Smith SP, Shaw GS (1998) A change-in-hand mechanism for S100 si-
gnalling. Biochem Cell Biol 76: 324-333
14. Kathir KM, Ibrahim K, Rajalingam D, Prudovsky I, Yu C, Kumar TK
(2007) S100A13-lipid interactions-role in the non-classical release of the
acidic fibroblast growth factor. Biochim Biophys Acta 1768: 3080-3089
15. Van Dieck J, Teufel DP, Jaulent AM, Fernandez-Fernandez MR, Ru-
therford TJ, Wyslouch-Cieszynska A, Fersht AR (2009) Posttranslatio-
nal modifications affect the interaction of S100 proteins with tumor
suppressor p53. J Mol Biol 394: 922-930
16. Miwa N, Uebi T, Kawamura S (2008) S100-annexin complexes - biolo-
gy of conditional association. FEBS J 275: 4945-4955
17. Rintala-Dempsey AC, Rezvanpour A, Shaw GS (2008) S100-annexin
complexes - structural insights. FEBS J 275: 4956-4966
18. Rezvanpour A, Santamaria-Kisiel L, Shaw GS (2011) The S100A10-an-
nexin A2 complex provides a novel asymmetric platform for membra-
ne repair. J Biol Chem 286: 40174-40183
19. Svenningsson P, Greengard P (2007) p11 (S100A10) - an inducible ada-
ptor protein that modulates neuronal functions. Curr Opin Pharmacol
7: 27-32
20. Van de Graaf SJ, Hoenderop JGJ, Bindels RJM (2006) Regulation of
TRPV5 and TRPV6 by associated proteins. Am J Physiol Renal Physiol
290: F1295-F1302
21. Donier E, Rugiero F, Okuse K, Wood JN (2005) Annexin II light chain
p11 promotes functional expression of acid-sensing ion channel ASI-
C1a. J Biol Chem 280: 38666-38672
22. Swanwick RS, Pristerá A, Okuse K (2010) The trafficking of Na(V)1.8.
Neurosci Lett 486: 78-83
23. O’Connell PA, Surette AP, Liwski RS, Svenningsson P, Waisman DM
(2010) S100A10 regulates plasminogen-dependent macrophage inva-
sion. Blood 116: 1136-1146
24. Phipps KD, Surette AP, O’Connell PA, Waisman DM (2011) Plasmino-
gen receptor S100A10 Is essential for the migration of tumor-promo-
ting macrophages into tumor sites. Cancer Res 71: 6676-6683
25. Hermann A, Donato R, Weiger TM, Chazin WJ (2012) S100 calcium
binding proteins and ion channels. Front Pharmacol 3: 67
26. Chazin WJ (2011) Relating form and function of EF-hand calcium bin-
ding proteins. Acc Chem Res 44: 171-179
27. Bian L, Strzyz P, Jonsson IM, Erlandsson M, Hellvard A, Brisslert
M, Ohlsson C, Ambartsumian N, Grigorian M, Bokarewa M (2011)
S100A4 deficiency is associated with efficient bacterial clearance and
protects against joint destruction during staphylococcal infection. J In-
fect Dis 204: 722-730
28. Semov A, Moreno MJ, Onichtchenko A, Abulrob A, Ball M, Ekiel I,
Pietrzynski G, Stanimirovic D, Alakhov V (2005) Metastasis-associated
protein S100A4 induces angiogenesis through interaction with anne-
xin II and accelerated plasmin formation. J Biol Chem 280: 20833-20841
29. Miranda KJ, Loeser RF, Yammani RR (2010) Sumoylation and nuclear
translocation of S100A4 regulate IL-1beta-mediated production of ma-
trix metalloproteinase-13. J Biol Chem 285: 31517-31524
30. Prosser BL, Hernández-Ochoa EO, Schneider MF (2011) S100A1 and
calmodulin regulation of ryanodine receptor in striated muscle. Cell
Calcium 50: 323-331
31. Kranias EG, Hajjar RJ (2012) Modulation of cardiac contractility by the
hospholamban/SERCA2a regulatome. Circ Res 110: 1646-1660
32. Filipek A, Michowski W, Kuznicki J (2007) Involvement of S100A6
(calcyclin) and its binding partners in intracellular signaling path-
ways. Advan Enzyme Regul 48: 225-239
33. Prus W, Filipek A (2011) S100A6 mediates nuclear translocation of
Sgt1: a heat shock-regulated protein. Amino Acids 41: 781-787

Postępy Biochemii 58 (4) 2012
435
34. Shimamoto S, Kubota Y, Tokumitsu H, Kobayashi R (2010) S100 pro-
teins regulate the interaction of Hsp90 with cyclophilin 40 and FKBP52
through their tetratricopeptide repeats. FEBS Lett 584: 1119-1125
35. Okada M, Hatakeyama T, Itoh H, Tokuta N, Tokumitsu H, Kobayashi
R (2004) S100A1 is a novel molecular chaperone and a member of the
Hsp70/Hsp90 multichaperone complex. J Biol Chem 279: 4221-4233
36. Kizawa K, Takahara H, Unno M, Heizmann CW (2011) S100 and S100
fused-type protein families in epidermal maturation with special focus
on S100A3 in mammalian hair cuticles. Biochimie 93: 2038-2047
37. Li C, Chen H, Ding F, Zhang Y, Luo A, Wang M, Liu Z (2009) A novel
p53 target gene, S100A9, induces p53-dependent cellular apoptosis
and mediates the p53 apoptosis pathway. Biochem J 422: 363-372
38. Atallah M, Krispin A, Trahtemberg U, Ben-Hamron S, Grau A, Ver-
bovetski I, Mevorach D (2012) Constitutive neutrophil apoptosis: reg-
ulation by cell concentration via S100 A8/9 and the MEK-ERK path-
way. PLoS One 7: e29333
39. Benaud C, Gentil BJ, Assard N, Court M, Garin J, Delphin C, Baudier
J (2004) AHNAK interaction with the annexin 2/S100A10 complex
regulates cell membrane cytoarchitecture. J Cell Biol 164: 133-144
40. Jung MJ, Murzik U, Wehder L, Hemmerich P, Melle C (2010) Regula-
tion of cellular actin architecture by S100A10. Exp Cell Res 316: 1234-
1240
41. Donato R (2003) Intracellular and extracellular roles of S100 proteins.
Microsc Res Tech 60: 540-551
42. Woodham AW, Da Silva DM, Skeate JG, Raff AB, Ambroso MR, Brand
HE, Isas JM, Langen R, Kast WM (2012) The S100A10 subunit of the
annexin A2 heterotetramer facilitates L2-mediated human Papilloma-
virus infection. PLoS One 7: e43519
43. Cmoch A, Strzelecka-Kiliszek A, Palczewska M, Groves P, Pikula S
(2011) Matrix vesicles isolated from mineralization-competent Saos-2
cells are selectively enriched with annexins and S100 proteins. Bio-
chem Biophys Res Commun 412: 683-687
44. Duarte WR, Shibata T, Takenaga K, Takahashi E, Kubota K, Ohya K,
Ishikawa I, Yamauchi M, Kasugai S (2003) S100A4: A novel negative
regulator of mineralization and osteoblast differentiation. J Bone Min-
er Res 18: 493-501
45. McCormick MM, Rahimi F, Bobryshev YV, Gaus K, Zreiqat H, Cai
H, Lord RSA, Geczy CL (2005) S100A8 and S100A9 in human arterial
wall, implications for artherogenesis. J Biol Chem 280: 41521-41529
46. Zreiqat H, Howlett CR, Gronthos S, Hume D, Geczy CL (2007)
S100A8/S100A9 and their association with cartilage and bone. J Mol
Histol 38: 381-391
47. Yamaguchi F, Umeda Y, Shimamoto S, Tsuchiya M, Tokumitsu H, To-
kuda M, Kobayashi R (2012) A link between Ca
2+
signal transduction
and protein dephosphorylation. J Biol Chem 287: 13787-13798
48. Steinckwich N, Schenten V, Melchior C, Bréchard S, Tschirhart EJ
(2011) An essential role of STIM1, Orai1, and S100A8-A9 proteins for
Ca
2+
signaling and FcγR-mediated phagosomal oxidative activity. J
Immunol 186: 2182-2191
49. Leclerc E, Sturchler E, Vetter SW, Heizmann CW (2009) Crosstalk be-
tween calcium, amyloid beta and the receptor for advanced glycation
endproducts in Alzheimer’s disease. Rev Neurosci 20: 95-110
50. Källberg E, Vogl T, Liberg D, Olsson A, Björk P, Wikström P, Bergh
A, Roth J, Ivars F, Leanderson T (2012) S100A9 interaction with TLR4
promotes tumor growth. PLoS One 7: e34207
51. Rezvanpour A, Shaw GS (2009) Unique S100 target protein interac-
tions. Gen Physiol Biophys 28: F39-F46
52. Lenarcic Zivkovic M, Zareba-Koziol M, Zhukova L, Poznanski J,
Zhukov I, Wyslouch-Cieszynska A (2012) Post-translational S-nitro-
sylation is an endogenous factor fine-tuning the properties of human
S100A1 protein. J Biol Chem, in press
53. Lim SY, Raftery MJ, Goyette J, Hsu K, Geczy CL (2009) Oxidative mod-
ifications of S100 proteins: functional regulation by redox. J Leukoc
Biol 86: 577-587
54. Pavón EJ, García-Rodríguez S, Zumaquero E, Perandrés-López R,
Rosal-Vela A, Lario A, Longobardo V, Carrascal M, Abián J, Callejas-
Rubio JL, Ortego-Centeno N, Zubiaur M, Sancho J (2012) Increased
expression and phosphorylation of the two S100A9 isoforms in mono-
nuclear cells from patients with systemic lupus erythematosus: a
proteomic signature for circulating low-density granulocytes. J Pro-
teomics 75: 1778-1791
55. Kouno T, Mizuguchi M, Sakaguchi M, Makino E, Mori Y, Shinoda H,
Aizawa T, Demura M, Huh NH, Kawano K (2008) The structure of
S100A11 fragment explains a local structural change induced by phos-
phorylation. J Pept Sci 14: 1129-1138
56. Lindsey JC, Lusher ME, Anderton JA, Gilbertson RJ, Ellison DW, Clif-
ford SC (2007) Epigenetic deregulation of multiple S100 gene fam-
ily members by differential hypomethylation and hypermethylation
events in medulloblastoma. Br J Cancer 97: 267-274
57. Gusev K, Ackermann GE, Heizmann CW, Niggli E (2009) Ca
2+
signa-
ling in mouse cardiomyocytes with ablated S100A1 protein. Gen Phy-
siol Biophys 28: 371-383
58. Kwon M, MacLeod TJ, Zhang Y, Waisman DM (2005) S100A10, an-
nexin A2, and annexin A2 heterotetramer as candidate plasminogen
receptors. Front Biosci 10: 300-325
59. Huang L, Xu Y, Cai G, Guan Z, Cai S (2012) Downregulation of S100A4
expression by RNA interference suppresses cell growth and invasion
in human colorectal cancer cells. Oncol Rep 27: 917-922
60. Sedaghat F, Notopoulos A (2008) S100 protein family and its applica-
tion in clinical practice. Hippokratia 12: 198-204
61. Srikrishna G, Freeze HH (2009) Endogenous damage-associated mole-
cular pattern molecules at the crossroads of inflammation and cancer.
Neoplasia 11: 615-628
62. Goyette J, Geczy CL (2011) Inflammation-associated S100 proteins:
new mechanisms that regulate function. Amino Acids 41: 821-842
63. Lim SY, Raftery MJ, Geczy CL (2011) Oxidative modifications of
DAMPs suppress inflammation: the case for S100A8 and S100A9. An-
tioxid Redox Signal 15: 2235-2248
64. Meijer B, Gearry RB, Day AS (2012) The role of S100A12 as a systemic
marker of inflammation. Int J Inflam, in press
65. Nasser MW, Qamri Z, Deol YS, Ravi J, Powell CA, Trikha P, Schwen-
dener RA, Bai XF, Shilo K, Zou X, Leone G, Wolf R, Yuspa SH, Ganju
RK (2012) S100A7 enhances mammary tumorigenesis through upre-
gulation of inflammatory pathways. Cancer Res 72: 604-615
66. Kraus C, Rohde D, Weidenhammer C, Qiu G, Pleger ST, Voelkers M,
Boerries M, Remppis A, Katus HA, Most P (2009) Kraus S100A1 in car-
diovascular health and disease: closing the gap between basic science
and clinical therapy. J Mol Cell Cardiol 47: 445-455
67. Rohde D, Ritterhoff J, Voelkers M, Katus HA, Parker TG, Most P (2010)
S100A1: a multifaceted therapeutic target in cardiovascular disease. J
Cardiovasc Transl Res 3: 525-537
68. Ritterhoff J, Most P (2012) Targeting S100A1 in heart failure. Gene Ther
19: 613-621
69. Abbas A, Aukrust P, Dahl TB, Bjerkeli V, Sagen EB, Michelsen A, Rus-
sell D, Sørensen KK, Holm S, Skjelland M, Halvorsen B (2012) High
levels of S100A12 are associated with recent plaque symptomatology
in patients with carotid atherosclerosis. Stroke 43: 1347-1353
70. Grevers LC, de Vries TJ, Vogl T, Abdollahi-Roodsaz S, Sloetjes AW,
Leenen PJM, Roth J, Everts V, van den Berg WB, van Lent PLE (2011)
S100A8 enhances osteoclastic bone resorption in vitro through activa-
tion of toll-like receptor 4. Implications for bone destruction in murine
antigen-induced arthritis. Arthritis Rheum 63: 1365-1375
71. Nishioku T, Furusho K, Tomita A, Ohishi H, Dohgu S, Shuto H,
Yamauchi A, Kataoka Y (2011) Potential role for S100A4 in the disrup-
tion of the blood-brain barrier in collagen-induced arthritic mice, an
animal model of rheumatoid arthritis. Neuroscience 189: 286-292
72. Borthwick LA, Riemen C, Goddard C, Colledge WH, Mehta A, Ger-
ke V, Muimo R (2008) Defective formation of PKA/CnA-dependent
annexin 2-S100A10/CFTR complex inΔF508 cystic fibrosis cells. Cell
Signal 20: 1073-1083
73. Emberley ED, Murphy LC, Watson PH (2004) S100 proteins and their
influence on pro-survival pathways in cancer. Biochem Cell Biol 82:
508-515

436
www.postepybiochemii.pl
udział białek S100A w przekazywaniu sygnału
wapniowego w normie i patologii
Anna Cmoch
1,*
, Patrick Groves
2
, Małgorzata Palczewska
2
, Sławomir Pikuła
1
1
Zakład Biochemii, Instytut Biologii Doświadczalnej PAN im. M. Nenckiego, Warszawa, Polska
2
Zakład Chemii Biologicznej, Instytut Technologii Chemicznej i Biologicznej, Oeiras, Portugalia
*
e-mail: a.cmoch@nencki.gov.pl
Słowa kluczowe: białka S100, aneksyny, przekazywanie sygnałów, jony wapnia, białka wiążące wapń
STrESZCZEnIE
Jony wapnia są niezbędne w utrzymaniu równowagi pomiędzy procesami wzrostu, przeżywalności, różnicowania i metabolizmu komórki.
Jony wapnia odgrywają rolę przekaźnika II rzędu w niemal wszystkich procesach komórkowych. Zmiany zewnątrz- i wewnątrzkomórkowe-
go stężenia jonów wapnia, w odpowiedzi na pobudzenie, wpływają na funkcje komórek. utrzymanie homeostazy wapnia wymaga właściwej
organizacji wewnątrzkomórkowego rozmieszczenia wapnia, w czym uczestniczą białka wiążące jony wapnia. Białka te przekształcają sygnał
wapniowy w odpowiedź komórkową. Coraz większa liczba obserwacji świadczy o tym, że zaburzona homeostaza wapnia i nieprawidło-
wa funkcja białek wiążących jony wapnia jest przyczyną wielu chorób człowieka, w tym rozwoju nowotworów. W niniejszym artykule
przeglądowymi, omówiono funkcje białek S100A w procesie przekazywania sygnału wapniowego. Białka z tej rodziny występują tylko w
organizmach kręgowców. uczestniczą w specyficznych procesach komórkowych, równolegle z kalmoduliną i aneksynami. Ich znaczenie
podkreślają obserwacje świadczące, że ich poziom ulega zmianom w chorobach neurodegeneracyjnych, stanach zapalnych, miopatiach i w
różnych typach nowotworów. Białka S100A są również postrzegane jako wskaźniki kliniczne różnych chorób, co jest nadal przedmiotem
intensywnych badań. W przedstawionym artykule omówiono również białka partnerskie białek z rodziny S100A.
74. Wang G, Zhang S, Fernig DG, Martin-Fernandez M, Rudland PS, Bar-
raclough R (2005) Mutually antagonistic actions of S100A4 and S100A1
on normal and metastatic phenotypes. Oncogene 24: 1445-1454
75. Rust R, Visser L, van der Leij J, Harms G, Blokzijl T, Deloulme JC,
van der Vlies P, Kamps W, Kok K, Lim M, Poppema S, van den Berg
A (2005) High expression of calcium-binding proteins, S100A10,
S100A11 and CALM2 in anaplastic large cell lymphoma. Br J Haema-
tol 131: 596-608
76. Wang G, Wang X, Wang S, Song H, Sun H, Yuan W, Cao B, Bai J, Fu
S (2008) Colorectal cancer progression correlates with upregulation of
S100A11 expression in tumor tissues. Int J Colorectal Dis 23: 675-682
77. Tsuna M, Kageyama S, Fukuoka J, Kitano H, Doki Y, Tezuka H, Ya-
suda H (2009) Significance of S100A4 as a prognostic marker of lung
squamous cell carcinoma. Anticancer Res 29: 2547-2554
78. Boye K, Maelandsmo GM (2010) S100A4 and metastasis: a small actor
playing many roles. Am J Pathol 176: 528-535
79. Salama I, Malone PS, Mihaimeed F, Jones JL (2008) A review of the
S100 proteins in cancer. Eur J Surg Oncol 34: 357-364
80. Basso D, Greco E, Padoan A, Fogar P, Scorzeto M, Fadi E, Bozzato D,
Moz S, Navaglia F, Zambon CF, Seraglia R, De Carlo E, Valerio A, Reg-
giani C, Pedrazzoli S, Plebani M (2011) Altered intracellular calcium
fluxes in pancreatic cancer induced diabetes mellitus: relevance of the
S100A8 N-terminal peptide (NT-S100A8). J Cell Physiol 226: 456-468
81. Fujiwara M, Kashima TG, Kunita A, Kii I, Komura D, Grigoriadis
AE, Kudo A, Aburatani H, Fukayama M (2011) Stable knockdown of
S100A4 suppresses cell migration and metastasis of osteosarcoma. Tu-
mor Biol 32: 611-622
82. Li J, Riau AK, Setiawan M, Mehta JS, Ti SE, Tong L, Tan DT, Beuer-
man RW (2011) S100A expression in normal corneal-limbal epithelial
cells and ocular surface squamous cell carcinoma tissue. Mol Vis 17:
2263-2271
83. Yang X, Popescu NC, Zimonjic DB (2011) DLC1 interaction with
S100A10 mediates inhibition of in vitro cell invasion and tumorigenic-
ity of lung cancer cells through a RhoGAP-independent mechanism.
Cancer Res 71: 2916-2925
84. Elsner M, Rauser S, Maier S, Schöne C, Balluff B, Meding S, Jung G,
Nipp M, Sarioglu H, Maccarrone G, Aichler M, Feuchtinger A, Langer
R, Jütting U, Feith M, Küster B, Ueffing M, Zitzelsberger H, Höfler H,
Walch A (2012) MALDI imaging mass spectrometry reveals COX7A2,
TAGLN2 and S100-A10 as novel prognostic markers in Barrett’s ad-
enocarcinoma. J Proteomics 75: 4693-4704
85. Fleming JM, Ginsburg E, Oliver SD, Goldsmith P, Vonderhaar BK
(2012) Hornerin, an S100 family protein, is functional in breast cells
and aberrantly expressed in breast cancer. BMC Cancer 12: 266
86. Grebhardt S, Veltkamp C, Ströbel P, Mayer D (2012) Hypoxia and
HIF-1 increase S100A8 and S100A9 expression in prostate cancer. Int
J Cancer, in press
87. Kahn N, Meister M, Eberhardt R, Muley T, Schnabel PA, Bender C, Jo-
hannes M, Keitel D, Sültmann H, Herth FJ, Kuner R (2012) Early detec-
tion of lung cancer by molecular markers in endobronchial epithelial-
lining fluid. J Thorac Oncol 7: 1001-1008
88. Lukanidin E, Sleeman JP (2012) Building the niche; the role of the S100
proteins in metastatic growth. Semin Cancer Biol 22: 216-225
89. Nipp M, Elsner M, Balluff B, Meding S, Sarioglu H, Ueffing M, Rauser
S, Unger K, Höfler H, Walch A, Zitzelsberger H (2012) S100-A10, thio-
redoxin, and S100-A6 as biomarkers of papillary thyroid carcinoma
with lymph node metastasis identified by MALDI imaging. J Mol Med
(Berl) 90: 163-174
90. Chang K, Kim HJ, Suh JH (2012) The role of S100a9 in the pathogenesis
of Alzheimer’s disease: the therapeutic effects of S100A9 knockdown
or knockout. Neurodegenerative Dis 10: 27-29
91. Vogl T, Gharibyan AL, Morozova-Roche LA (2012) Pro-Inflammatory
S100A8 and S100A9 Proteins: self-assembly into multifunctional na-
tive and amyloid complexes. Int J Mol Sci 13: 2893-2917
92. Zhang L, Ursano RJ, Li H (2012) P11: a potential biomarker for post-
traumatic stress disorder. Methods Mol Biol 829: 453-468
93. Carlsson H, Petersson S, Enerbäck C (2005) Cluster analysis of S100
gene expression and genes correlating to psoriasin (S100A7) expres-
sion at different stages of breast cancer development. Int J Oncol 27:
1473-1481
Wyszukiwarka
Podobne podstrony:
Dz U 2006 nr 60 poz 429
Zróżnicowana składka na ubezpieczenie społeczne z tytułu wypadków i chorób 08 73 436
429
pedagogika, system oswiatowy we wloszech, Włochy liczą 57 576 429 mieszkańców zamieszkujących teryto
429
436 a
32 425 436 Ifluence of Vacuum HT on Microstructure and Mechanical Properties of HSS
436-444, materiały ŚUM, IV rok, Patomorfologia, egzamin, opracowanie 700 pytan na ustny
436 437
429
429
429
429
429
436
kpk, ART 429 KPK, WZ 73/05 - postanowienie z dnia 17 listopada 2005 r
PSYCHOLOGIA PAMIĘCI I UCZENIA ćw nr 3 Zniekształcenia pamięci Jagodzińska [436 443]
więcej podobnych podstron