Student: Anna Nowicka Wrocław, 17.06.2014
Student number: 185147
Medicinal chemistry
Project I
Spectroscopic methods in medicinal chemistry
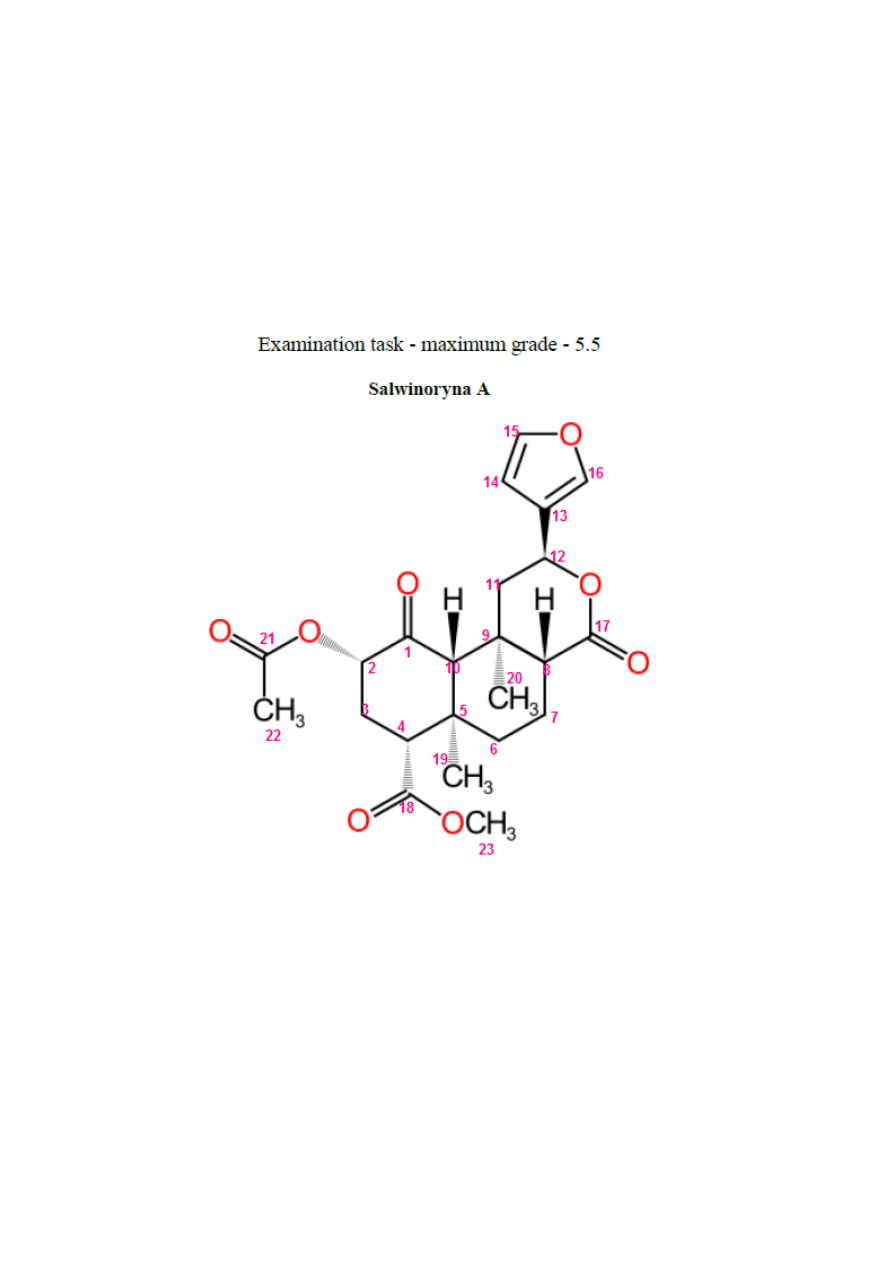
Numbering of atoms: every hydrogen has the same number as carbon atom, to which it’s
attached. If there is more than one, than they are letters (for example H11a, H11b).
1.
ONE DIMENSIONAL H
1
NMR SPECTRUM
Explenation:
15,16 – double bonds between carbons, next to oxygen: ~7
14 – double bond between carbons, but away from oxygen: ~ 6,4
12 – the nearest (hydrogen) atom to furan ring (with hydrogen atom)
23- integration ~3, directly connected with oxygen, so shift is the biggest (from all CH
3
groups)
20- integration ~3, nearer to furan ring than 19 – bigger shift
19- integration ~3, farer to furan ring than 20 – lower shift
22- last –CH
3
group, last integration equal or bigger than 3
4 – nearly connected with 2 oxygen, bigger shift in alkyl region
2 – directly connected with oxygen and next to carbonyl group, regular triplet
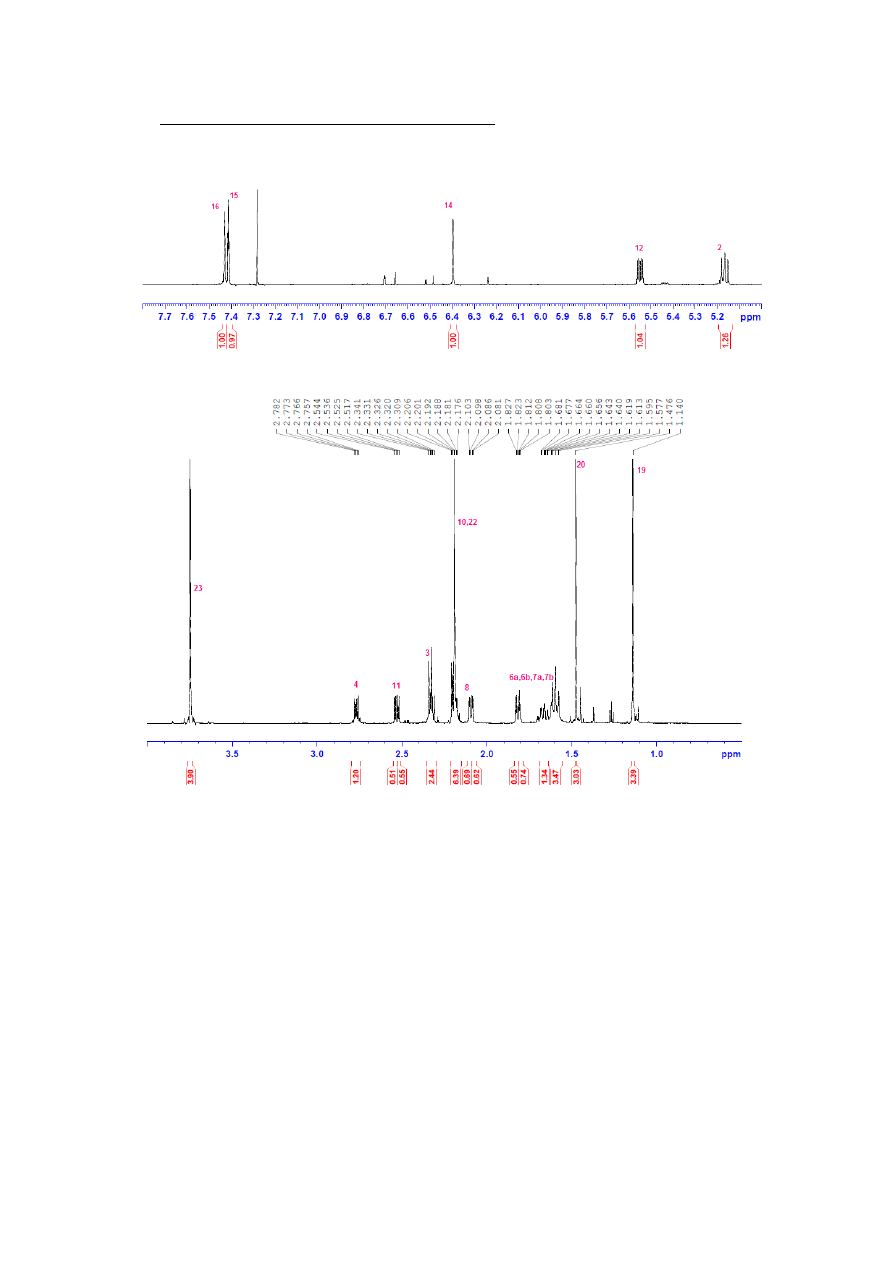
2.
COSY SPECTRUM
I put all known signals on the spectrum. I found all signals derived from them:
H14-H15, -it allows me to distinguish H15 and H16 atoms
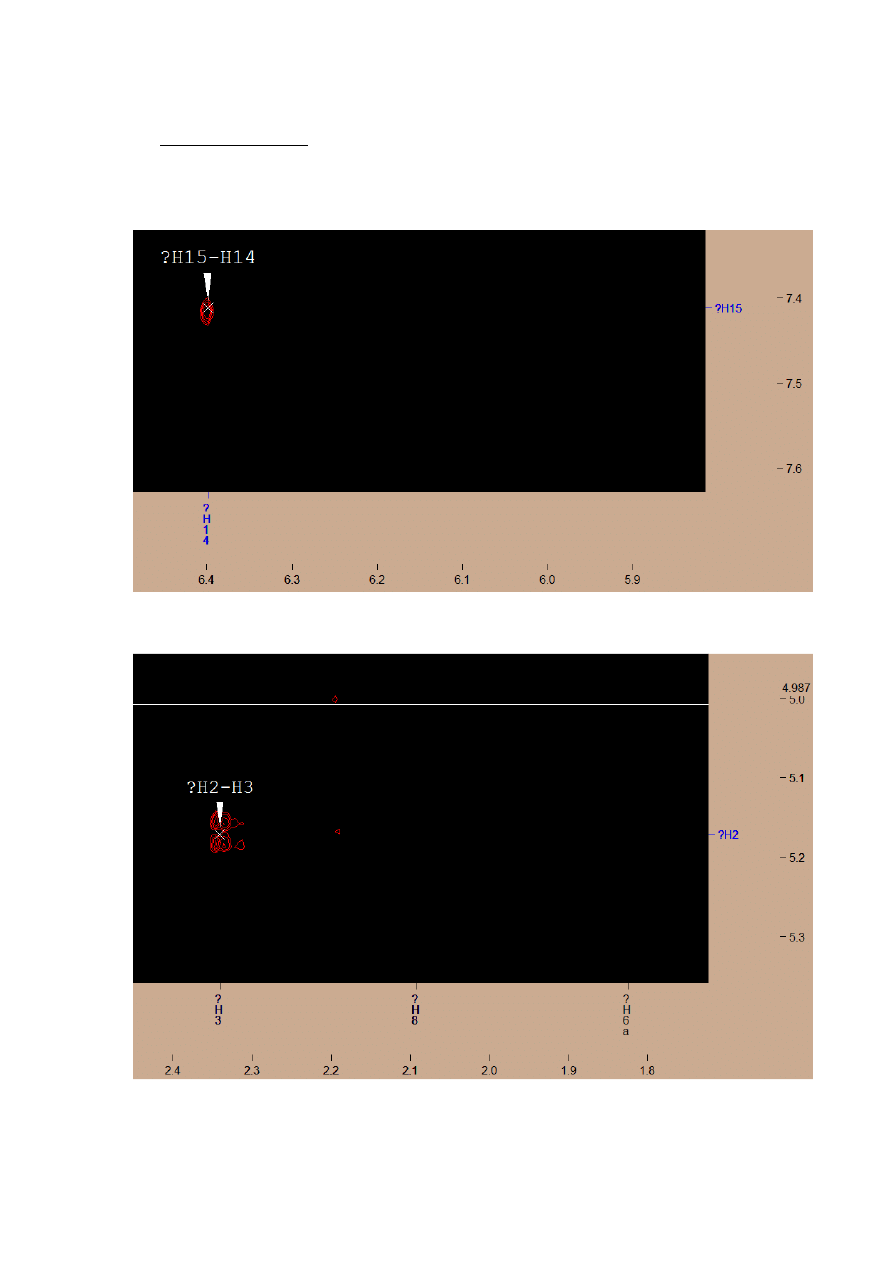
I found signal with chemical shift coupling with H2 and H4 – it’s a signal from two H3
atoms.
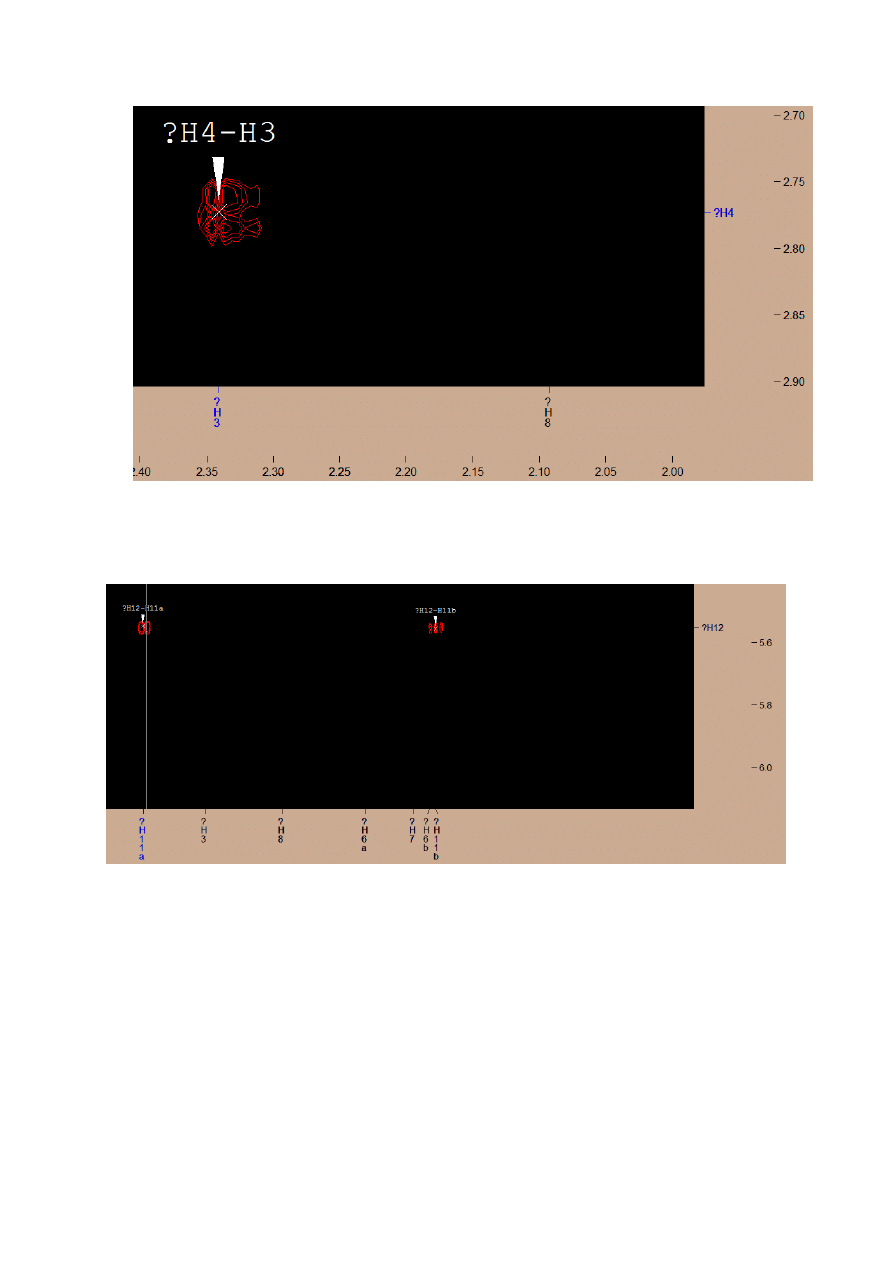
Next, I found two signals connected with H12 – this are H11a and H11b atoms.
The difference may be due to arrangement of the atoms in space.
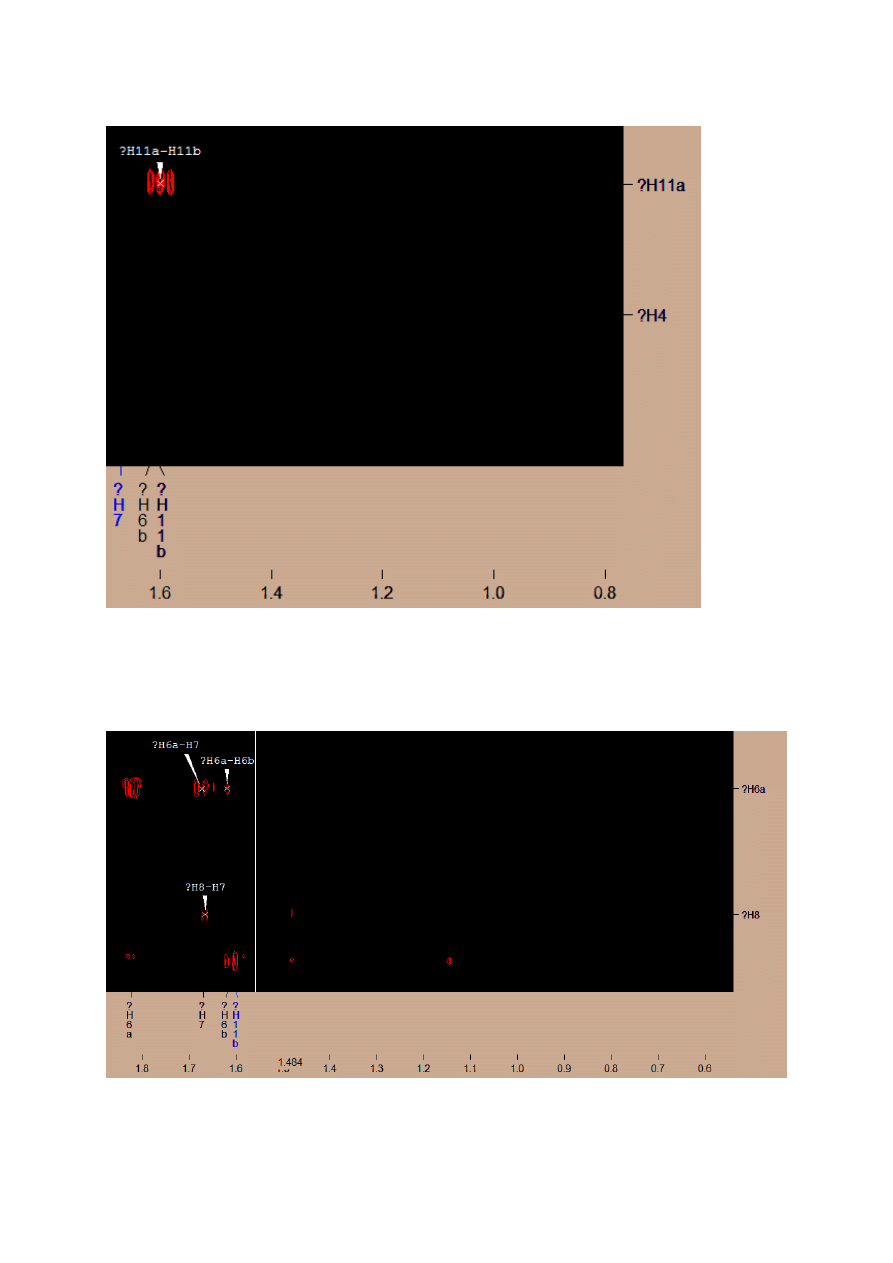
Also, I found the signal from both H11 atoms (a and b)
I found 3 last signals from 3 last hydrogen groups, which should give a signal on COSY
(6,7,8). Only H8 group has integration ~1, so I supposed that is the signal with shift near to
2.1ppm. H6 and H7 group gave 3 clear signals, so one of them gave two separate signals-
I guessed that it was H6 group, because of it position, but I wasn’t sure.
I found signal from H7 and H6b after big change of resonance, when a lot of interferences
was visible, so I didn’t find this signal reasonable.
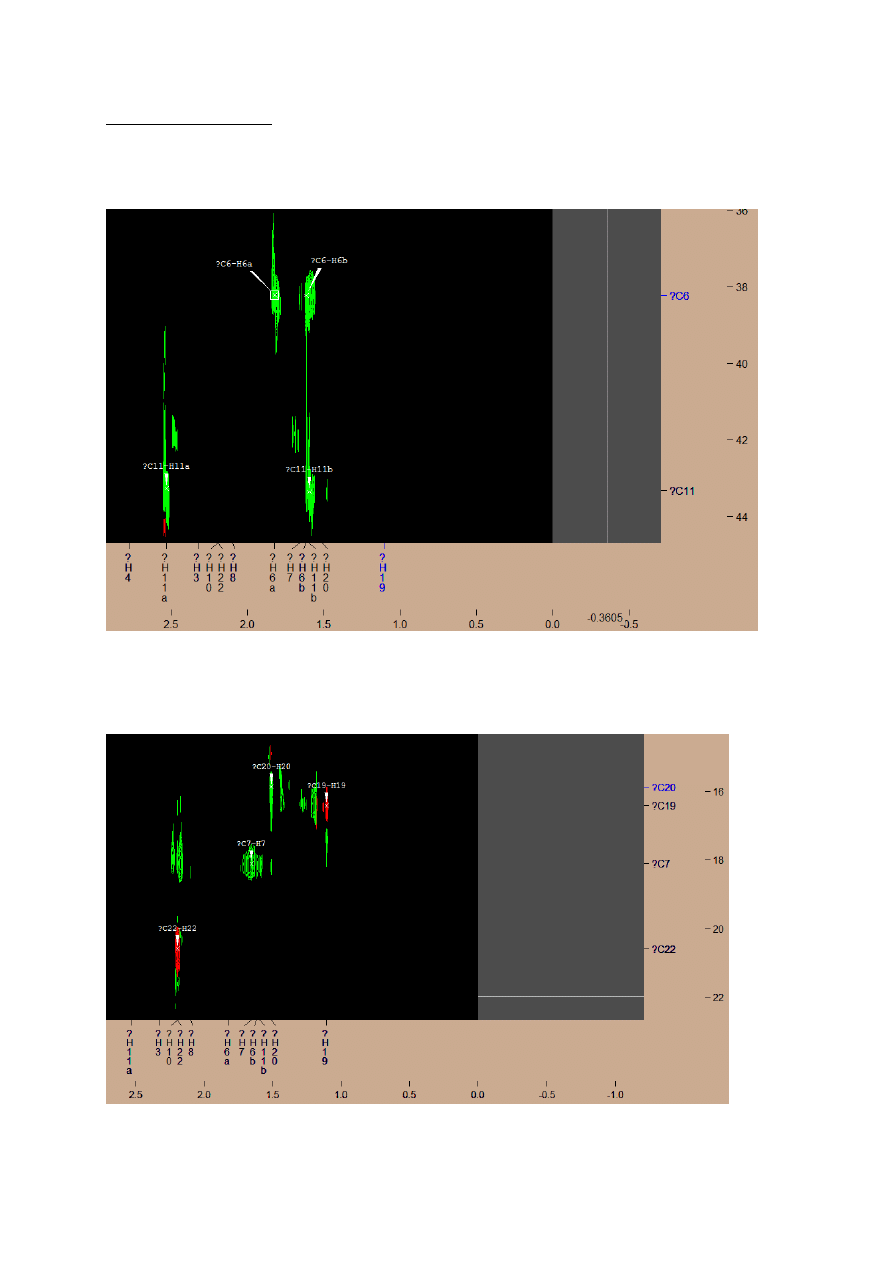
2. HSQC SPECTRUM
Firstly, I found signals from –CH
2
- groups, in which hydrogens gives separated signals but
close to each other (H11, H6) – green signals in aliphatic region of C
13
NMR.
Next, I found the positive signals from -CH
3
groups C20, C19, C22 ( C20 was labeled
wrongly, it should be the little red signal, which is closed to labeled one):
There is big, clear negative signal, which fit to H7 (–CH
2
- group), so I decided that this is
signal from C7. Then I looked for signals one by one, taking into account the color of the
signal (red for –CH- and -CH
3
groups, green for -CH
2
-) and tried to find the clearest, lying in
the range appropriate for the structure – for example carbon atoms. Some of the signals which
should be positive, are negative (or vice versa), but after the change in the resonance range
signals are visible, and they have been selected.
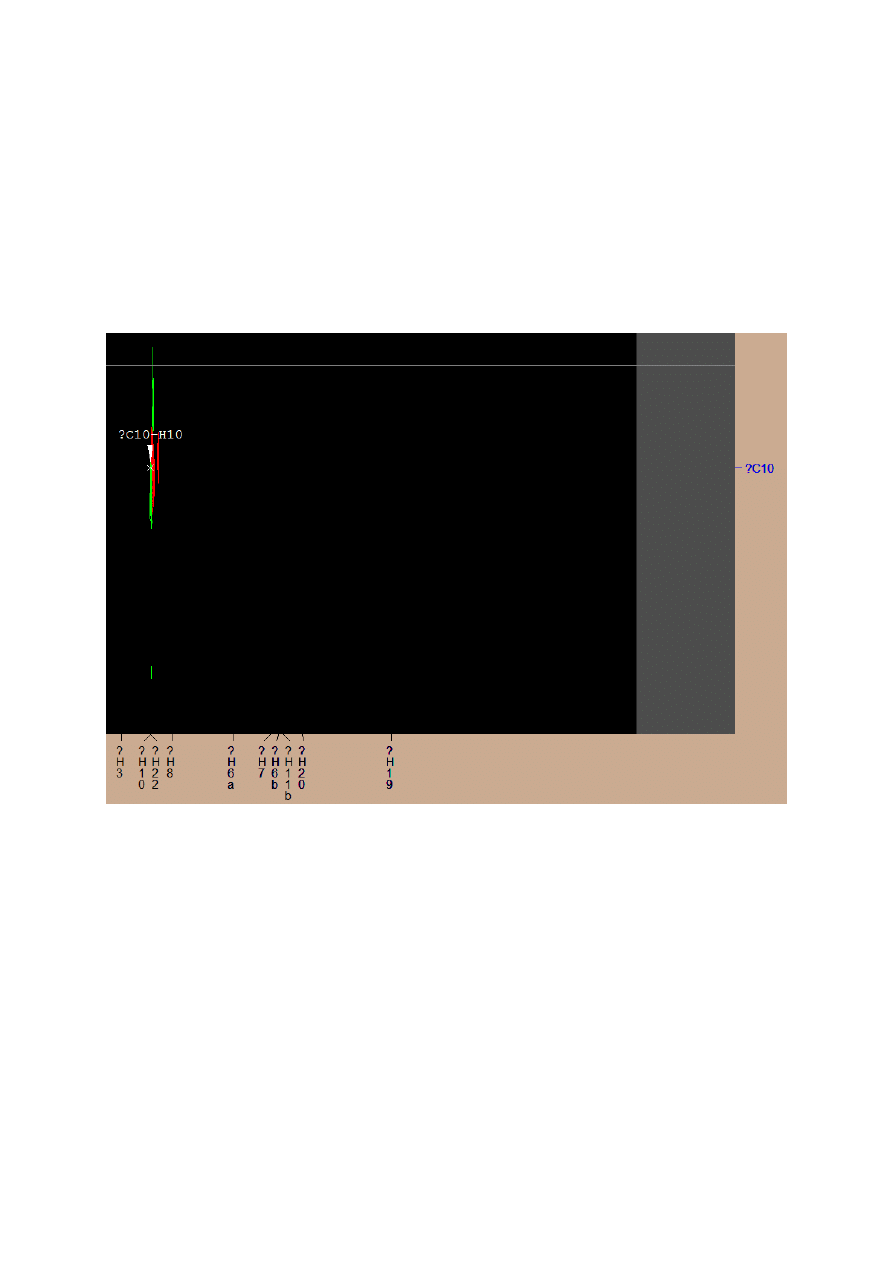
The only unmarked hydrogen was H10. Integration of the H22 signal was very high and
inadequate for this group, so I came to the conclusion that there has been imposing of these
two signals. To confirm this, I was looking for a positive signal in the carbon spectrum
matches the shift of atom H10.
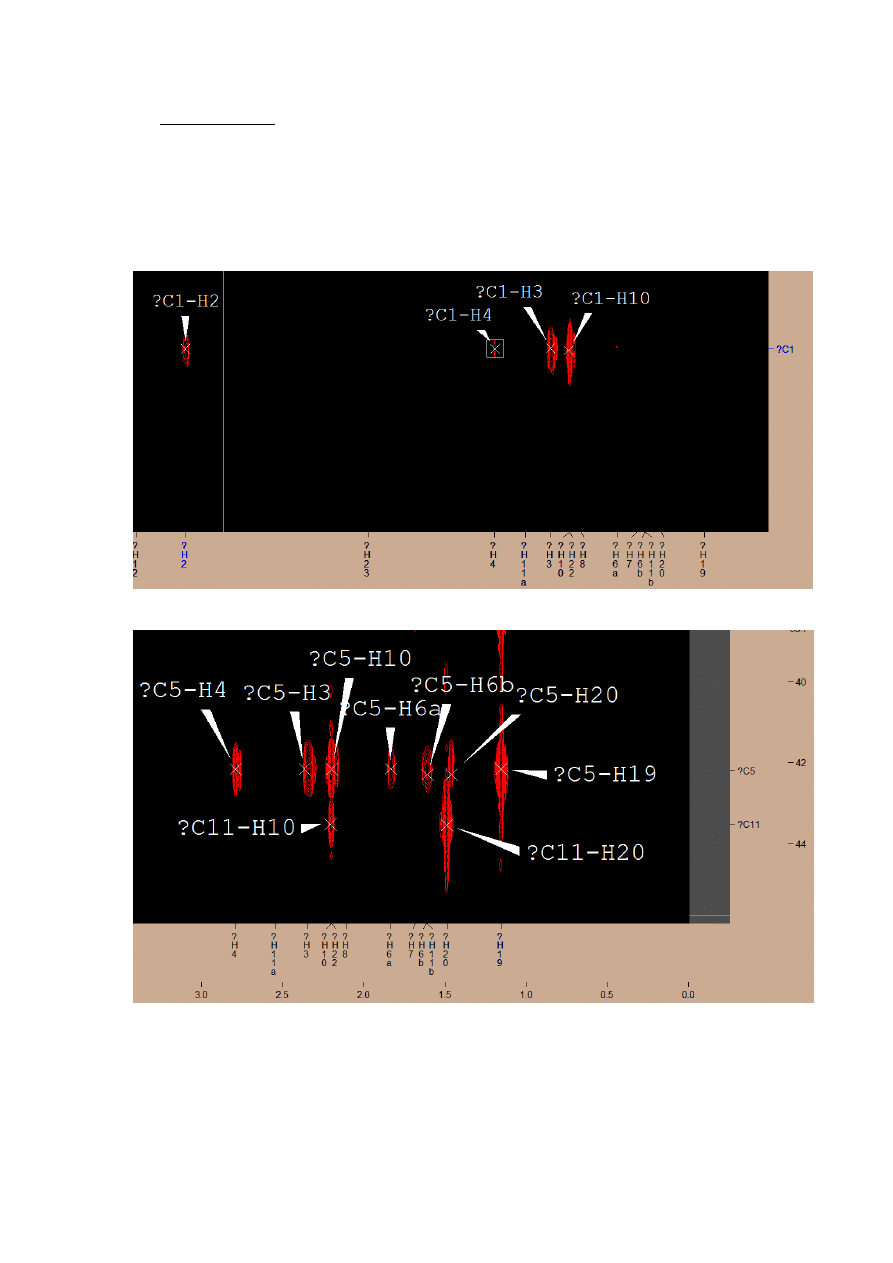
3.
CIGAR-HMBC
On the spectrum I have indicated all coupling for previously labeled atoms. There are still
few groups of signals, which creates horizontal lines – these were lines of unsubstituted
by hydrogens carbon atoms:
Carbon atom connected with H2, H3, H4, H10 -> C1
Connected with H4, H3, H10, H6, H20, H19 -> C5
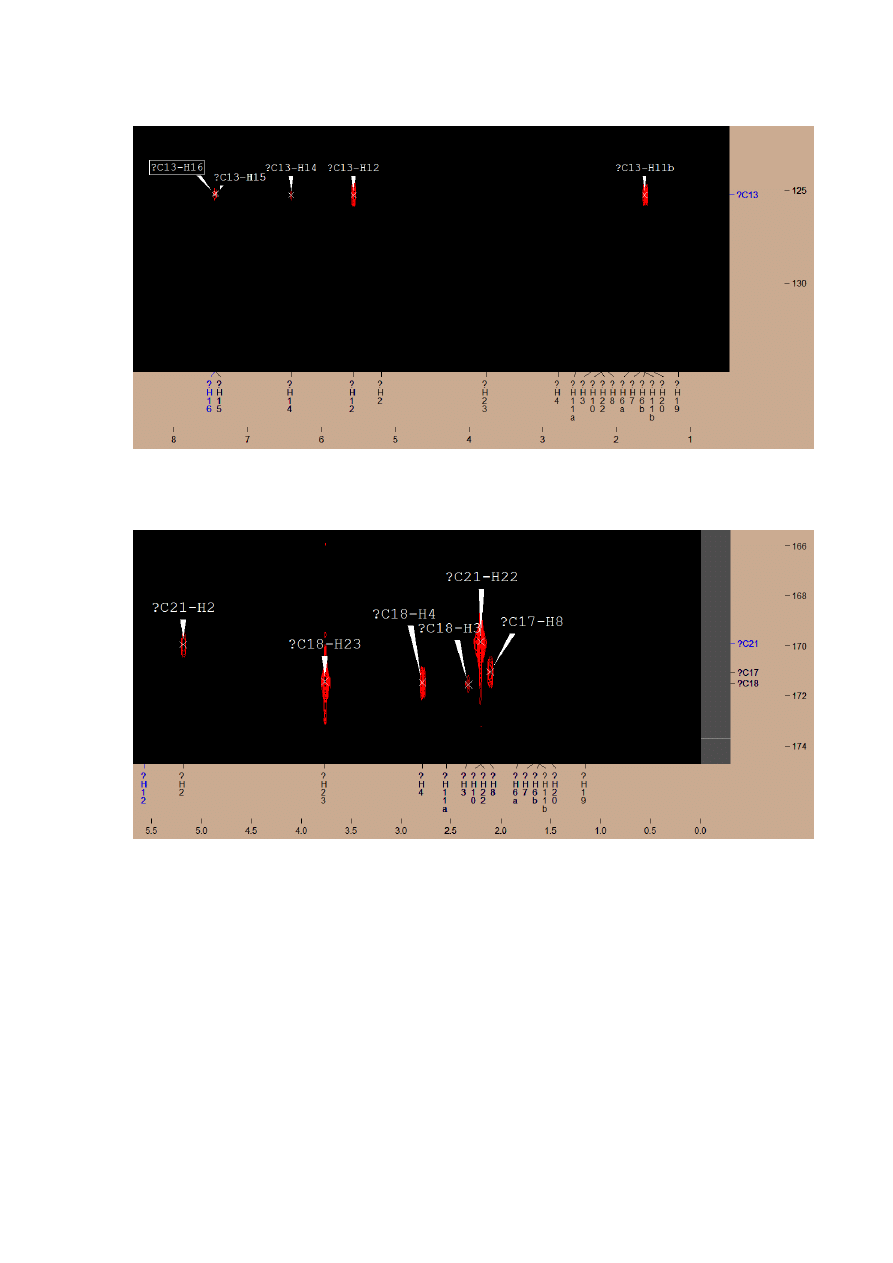
Connected with H16, H15, H14, H12, H11 -> C13
Couplings for C17, C18, C21
4.
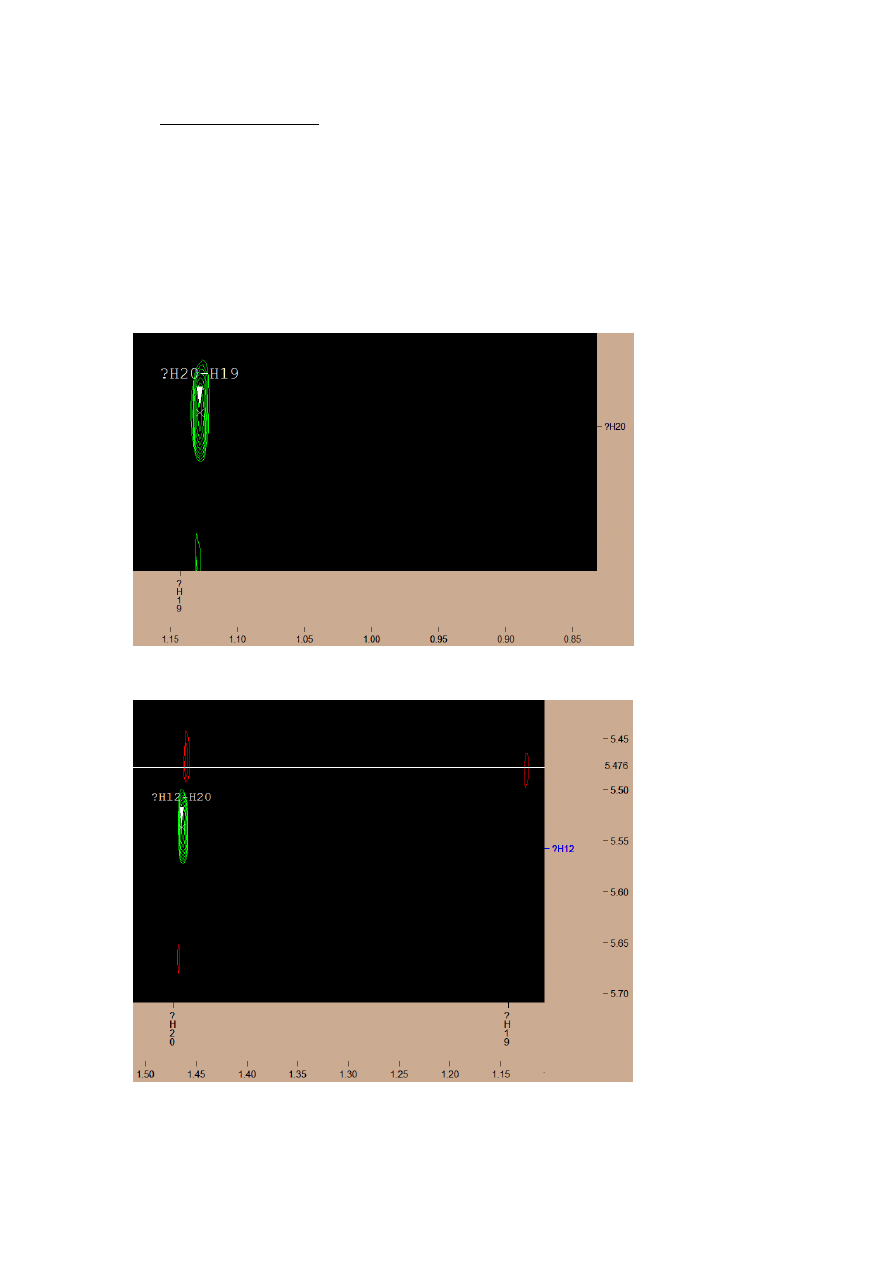
ROESY SPECTRUM
The spectrum shows the coupling of hydrogens contained close to each other in the space,
even if they are distant from each other by a lot of carbon bonds.
Most signals are from adjacent or closely spaced hydrogen atoms, just few of them can
give us information about the exact spatial structure of the molecule.
Coupling between group H19 and H20 probably shows, that both groups are on the same
side of planar carbon skeleton.
The same situation is with coupling between H12 and H20 groups.

The level of my knowledge does not allow me to fully, correct analysis of this spectrum.
However I can assume that after hearing the basics of two-dimensional spectral analysis and
using the program SPARKY it is possible to determine the exact structure of the compound
and solut one-dimensional spectra.
Wyszukiwarka
Podobne podstrony:
projekt o narkomanii(1)
!!! ETAPY CYKLU PROJEKTU !!!id 455 ppt
Wykład 3 Dokumentacja projektowa i STWiOR
Spektroskopia NMR
Projekt nr 1piątek
Projet metoda projektu
34 Zasady projektowania strefy wjazdowej do wsi
PROJEKTOWANIE ERGONOMICZNE
Wykorzystanie modelu procesow w projektowaniu systemow informatycznych
Narzedzia wspomagajace zarzadzanie projektem
Zarządzanie projektami 3
Metody Projektowania 2
BYT 109 D faza projektowania
p 43 ZASADY PROJEKTOWANIA I KSZTAŁTOWANIA FUNDAMENTÓW POD MASZYNY
więcej podobnych podstron