
BEHAVIOR (HOWARD S. KIRSHNER, SECTION EDITOR)
Transient Global Amnesia: A Brief Review and Update
Howard S. Kirshner
Published online: 7 September 2011
# Springer Science+Business Media, LLC 2011
Abstract Transient global amnesia (TGA) is a transitory
syndrome of memory loss, lasting less than 24 h. Although
there are many known causes of transient amnesia, the
syndrome of TGA remains of unknown etiology. Known
causes of transient amnesia, theories of pathogenesis of
TGA, and recommended evaluation and treatment are
discussed.
Keywords Transient global amnesia . Transient amnesia .
Amnesia . Transient ischemic attack . TIA . Memory loss
Introduction
Transient global amnesia (TGA) is a syndrome of tempo-
rary memory loss, or amnesia. Although earlier descriptions
of transitory memory loss have been cited, especially that
of Benon in 1909 [
], the modern syndrome of TGA was
first described in medical literature in 1956 by Bender [
],
and in the same year by Guyotat and Courjon [
]. Fisher
and Adams [
] gave the syndrome its current name and
described its key features in a 1958 abstract, and in an
extended manuscript with 17 detailed case descriptions in
1964 [
]. Since then, there have been many reports of cases
and series of TGA, and many theories of pathogenesis, yet
to date the precise cause remains unknown.
TGA typically occurs in an elderly or middle-aged
patient, lasts from 1 to 24 h, and then resolves
spontaneously and completely. Precipitating events such
as cold temperature, sexual intercourse, and compromis-
ing situations (e.g., an elderly man in the midst of an
extramarital affair, or bathing in the cold waters of the
North Atlantic) have been reported in some cases. The
key features of the syndrome involve an abrupt onset of
anterograde amnesia, or inability to form new memories,
and retrograde amnesia, a loss of recall of memories for
a variable period of time prior to the onset of symptoms.
During the episode, the patient maintains personal
knowledge, usually knowledge of family members,
preserved remote memories and intellect. Some patients
are disoriented, not knowing the exact time, or place.
They typically ask repetitive questions about where they
are and why they are there. The companion answers
these questions, but the patient then asks them over
again, forgetting the answers. As the patient
’s anterog-
rade memory recovers, at a time less than 24 h, the
period of retrograde amnesia
“shrinks.” After recovery, a
permanent gap in memory remains, equal to the sum of
the brief period of retrograde amnesia before the event,
plus the period of anterograde amnesia after onset.
Hodges and Warlow [
] provided seven diagnostic
criteria for TGA: 1) attacks must be witnessed by a
capable observer; 2) there must be anterograde amnesia
during the attack; 3) clouding of consciousness and loss of
personal identity must be absent, and no other neuro-
behavioral deficits such as aphasia or apraxia can be
present; 4) there should be no other focal neurologic signs
present during or after the attack; 5) evidence of epileptic
seizures must be absent; 6) the attacks must resolve within
24 h; and 7) patients with recent head injury or an active
seizure disorder (or another cause of amnesia) are
excluded.
H. S. Kirshner (
*)
Department of Neurology, Vanderbilt University Medical Center,
Nashville, TN 37232, USA
e-mail: howard.kirshner@vanderbilt.edu
Curr Neurol Neurosci Rep (2011) 11:578
–582
DOI 10.1007/s11910-011-0224-9

TGA is common in clinical practice. Miller et al. [
reported an overall incidence in Rochester, Minnesota of
about 5 cases per 100,000 of population, although in
patients above the age of 50 years the incidence was more
than 20 per 100,000. Others have estimated an incidence
of about 30 cases/100,000 of population/year [
Recurrent episodes occur in only a minority of cases,
roughly 15% to 30% [
–
]; Miller et al. [
] reported a
recurrence rate of 23.8%.
Identification of TGA requires the exclusion of other
causes of amnesia. The etiologies of transient amnesia are
numerous. All of these syndromes of transient amnesia
have in common a temporary disruption of the memory
system secondary to either generalized brain dysfunction or
focal disruption of the Papez
’s circuit, a pathway in each
hemisphere with connections from the hippocampus, to the
mammillary body, to the dorsomedial thalamus, to the
cingulate gyrus, and then back to the hippocampus. Papez
’s
circuit is also called the
“medial temporal-diencephalic
memory circuit
” [
••]. Head trauma or concussion is a
common cause of transient amnesia [
]. Epileptic
seizures, especially of the complex partial type involving
the medial temporal structures, frequently disrupt memory
[
–
]. If the seizure itself is not witnessed, the patient
may present with the clinical problem of an unexplained
episode of amnesia. Usually recurrent seizure episodes
make the diagnosis clear. Electroconvulsive therapy produ-
ces temporary amnesia by a similar mechanism. Transient
ischemia of the posterior cerebral artery territory can
produce a temporary amnesia [
]. Gorelick et al. [
reported a patient with three episodes of amnesia, the first
two transient, and the third persistent. The patient then had
an infarction in the left thalamus. Bogousslavsky and Regli
[
] also reported four cases of TGA associated with
strokes, two in the medial temporal lobe, one in the left
lentiform nucleus, and one in the left thalamus. Cases of
transient amnesia during coronary arteriography may result
from either unilateral or bilateral ischemia of the hippo-
campal complex [
].
Transient amnestic states in patients with migraine,
usually followed by severe headache, also likely reflect an
ischemic mechanism [
–
], although cortical spreading
depression has also been cited as a possible mechanism
[
]. The syndrome of confusional migraine in children
resembles TGA [
]. Drug intoxications, especially those
with benzodiazepines [
,
], are among the more
common causes of transient amnesia, presumably by
temporarily depressing the activity of the memory system.
Of course, physicians use this property of benzodiazepines
deliberately in pre-anesthesia with midazolam (Versed®;
Roche Laboratories, Basel, Switzerland) before esophago-
gastroscopies and similar procedures. Three neuroscientists
reported their own transient amnesia following airplane
travel in which they took triazolam (Halcion®; Pfizer, New
York, NY) for sleep and also consumed alcoholic beverages
[
]. Another more contemporary cause of transient
amnesia is the hypnotic zolpidem (Ambien®; Sanofi-
Aventis, Bridgewater, NJ) [
“Alcoholic blackouts”
have a similar mechanism [
]. Inebriated individuals may
appear awake and engage in activities for which they have
no recollection the following day. Occasionally, transient
amnesia occurs in patients with pituitary or brain tumors,
perhaps on the basis of seizures [
]. Finally, transient
amnesia can be of psychogenic cause, as in hysterical
fugue states.
Once the known causes of transient amnesia are
excluded, most cases of the idiopathic syndrome of
TGA remain of unknown etiology. Fisher and Adams
[
] initially favored an epileptic cause, but seizure
activity is not witnessed during or after the episode.
The low recurrence rate of TGA also argues against an
epileptic etiology. Electroencephalogram (EEG) record-
ings have been normal even during the episode [
Unwitnessed epileptic seizures would be a possible cause
of transient amnesia, however, and such cases are more
likely than typical TGA patients to have recurrent
attacks.
A second theory of TGA is vascular, or focal
ischemia [
]. As mentioned above, transient amnesia
can occur in migraine [
] and with presumed
transient ischemic attacks [
,
]. Migraine-associated
transient amnesia, like epilepsy, is more likely to be
recurrent than idiopathic TGA.
Typical TGA tends to occur in middle-aged and
elderly patients, who frequently have risk factors for
cerebrovascular disease, but follow-up studies have not
indicated a high incidence of transient ischemic attacks
or stroke [
,
,
]. Kushner and Hauser [
] used a
case
–control method and confirmed a higher incidence of
vascular risk factors in patients with TGA versus neuro-
logic controls, but most of the association went away
when patients with known ischemic attacks were exclud-
ed. Also contrary to the vascular hypothesis is the absence
of focal neurologic signs, such as visual field defects,
during the TGA episode.
Proponents of the ischemic theory of TGA have been
supported by reports of diffusion bright lesions in the
hippocampus on MRI. Recently, ultrasensitive brain imag-
ing modalities such as diffusion-weighted MRI and positron
emission tomography (PET) have been performed in
patients with TGA. Strupp et al. [
] reported that 7 of 10
patients imaged during TGA episodes showed abnormal
diffusion MRI signal in the left hippocampus; of these
seven cases, three had bilateral hippocampal abnormalities.
Curr Neurol Neurosci Rep (2011) 11:578
–582
579
The authors postulated that reduced extracellular space
caused by edema or spreading depression might be the
cause; permanent infarctions were not found. Other inves-
tigators have found frontal lobe abnormalities by diffusion-
weighted MRI [
] or PET imaging [
]. Several reports
have described diffusion-weighted imaging (DWI) lesions
in a high proportion of TGA cases, especially hippocampal
abnormalities [
]. The last article cited reported a
higher detection rate, 11/32 compared with 0/32 with 3 T
MRI, compared with 1.5 T. The experience in our hospital
has been less positive, in that most patients have had
negative DWI MRI scans. However, some investigators
have discovered such lesions several days after onset, and
our practice has been to obtain the scan immediately upon
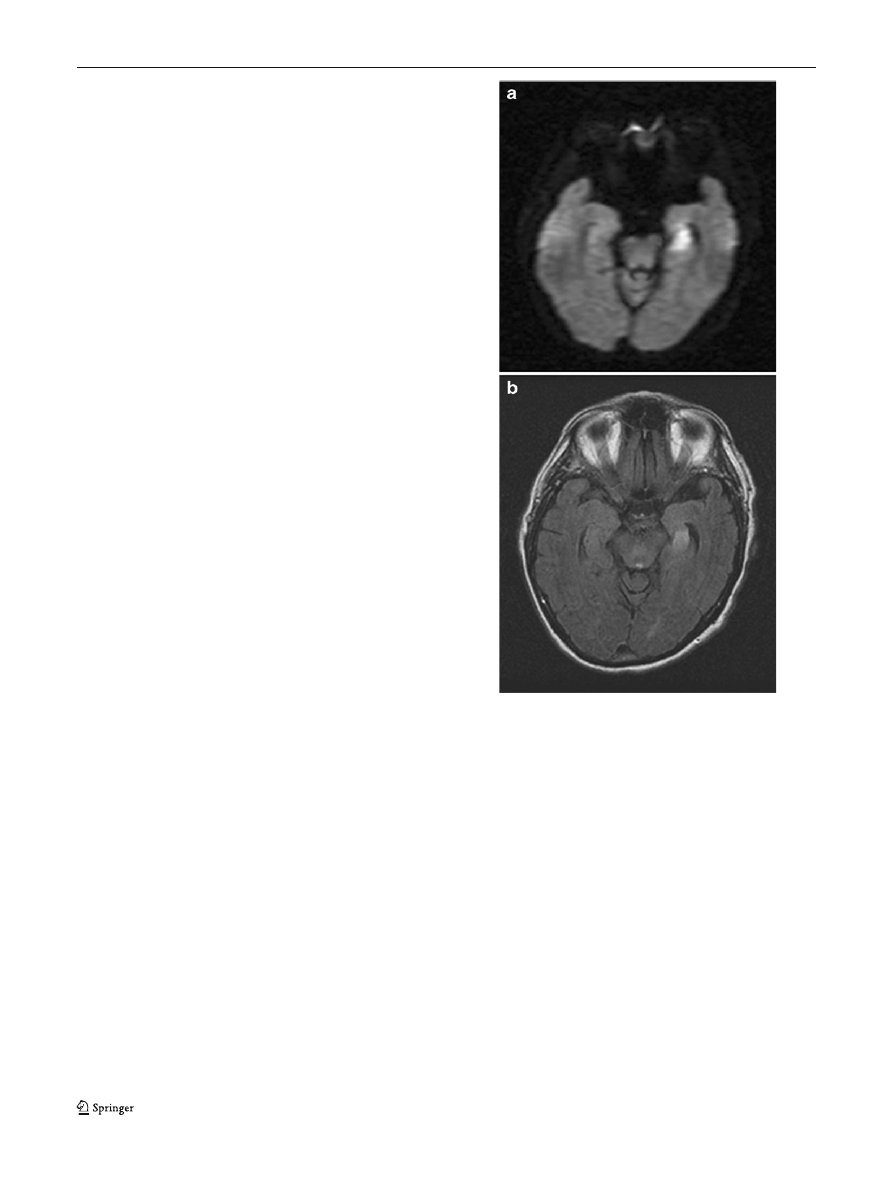
hospitalization for the event. Two cases in whom we saw
left medial temporal DWI lesions have had lasting, and not
transient amnesia; an example is shown in Fig.
. In
addition to the lack of other stroke signs in these patients,
and their low incidence of stroke in follow-up, diffusion
bright lesions may occur in association with seizures,
spreading depression, and other non-stroke causes. These
studies do not prove an ischemic etiology for TGA; rather,
they indicate transient dysfunction in either the hippocam-
pal system or its connections (Papez
’s circuit), as would be
expected.
A recent, vascular theory of TGA holds that venous
stasis may cause transitory ischemia in the hippocampus.
Several reports have emerged of incompetent valves in the
jugular vein, with back pressure [
–
•]. Proponents
of this theory point out that many cases of TGA appear to
be precipitated by events associated with the Valsalva
maneuver, such as severe stressors, sexual intercourse, and
so forth. Cejas et al. [
•] found valvular insufficiency by
Doppler ultrasound on at least one jugular vein in 113/142
patients with TGA. These accounts fail to explain the very
low recurrence rate of TGA, given that the individuals
always have venous incompetence and frequently do
Valsalva maneuvers.
Despite all of the theories, case series, and studies, the
etiology of TGA remains elusive. As stated by Altamura
and Vernieri [
],
“Investigating the case of TGA, we have
definitely identified the suspects, but we still have to prove
them guilty.
”
The evaluation of a patient with transient amnesia should
include observation of the patient until the symptoms have
passed. Brain imaging with CT or MRI is usually performed
to exclude other causes of memory loss. Ultrasound
evaluation of the jugular veins could be considered, if this
theory of TGA gains traction in the years ahead. EEG and
drug screening should also be performed if there is clinical
suspicion of seizures or drug abuse. There is no clear
evidence regarding treatment, and reassurance is the most
appropriate stance. Treatment of vascular risk factors, and
perhaps a baby aspirin daily, constitutes usual therapeutic
maneuvers.
Conclusions
TGA is a syndrome of unknown cause, or perhaps multiple
causes, although it is closely mimicked by transient
amnesia caused by migraine, ischemia, seizures, or trauma.
Fig. 1 Shows a 69-year-old man with history of hypertension and
hyperlipidemia, presented with sudden onset of memory difficulty,
repeatedly asking his wife questions about where they were and why
they were there. MRI with diffusion-weighted imaging (DWI) shows
acute infarction of the left hippocampus. The memory loss did not
resolve completely. a DWI sequence MRI in patient with amnesia,
which persisted. b Fluid-attenuated inversion recovery (FLAIR)
sequence, same patient with amnesia
580
Curr Neurol Neurosci Rep (2011) 11:578
–582

A thorough evaluation is appropriate, to exclude more
serious and potentially recurrent causes of transient amne-
sia. Reassurance is the usual treatment for the idiopathic
syndrome of TGA.
Disclosure
No potential conflict of interest relevant to this article
was reported.
References
Papers of particular interest, published recently, have been
highlighted as:
• Of importance
•• Of major importance
1. Benon R. Les ictus amnesiques dans les demences
‘organiques’.
Ann Med Psychol. 1909;67:2007
–19.
2. Bender MB. Syndrome of isolated episode of confusion with
amnesia. J Hillside Hosp. 1956;5:212
–5.
3. Guyotat MM, Courjon J. Les ictus amnesiques. J Med Lyon.
1956;37:697
–701.
4. Fisher CM, Adams RD. Transient global amnesia. Trans Am
Neurol Assoc. 1958;83:143
–6.
5. Fisher CM, Adams RD. Transient global amnesia. Acta Neurol
Scand. 1964;40 Suppl 9:1
–83.
6. Hodges JR, Warlow CP. The aetiology of transient global amnesia:
a case control study of 114 cases with prospective follow-up.
Brain. 1990;113:639
–57.
7. Miller JW, Peterson RC, Metter EJ. Transient global amnesia:
clinical characteristics and prognosis. Neurology. 1987;37:733
–7.
8. Zorzon M, Antonutti L, Mase G, et al. Transient global
amnesia and transient ischemic attack. Natural history, vascu-
lar risk factors, and associated conditions. Stroke.
1995;26:1536
–42.
9. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards
a classification. A study of 153 cases. J Neurol Neurosurg
Psychiatr. 1990;53:834
–43.
10. Nausieda PA, Sherman IC. Long-term prognosis in transient
global amnesia. JAMA. 1979;241:392
–3.
11. Olesen J, Jorgensen MB. Leao
’s spreading depression in the
hippocampus explains transient global amnesia: a hypothesis. Acta
Neurol Scand. 1986;73:219
–20.
12. Shuping JR, Rollinson RD, Toole JF. Transient global amnesia.
Ann Neurol. 1980;7:281
–5.
13.
•• Bartsch T, Deuschl G. Transient global amnesia: functional
anatomy and clinical implications. Lancet Neurol. 2010;9:205
–14.
This is a good review of the syndrome, with discussion of the
neuroanatomy of memory pathways.
14. Lynch S, Yarnell PR. Retrograde amnesia: delayed forgetting after
concussion. Am Psychol. 1973;86:643
–50.
15. Haas DC, Ross GS. Transient global amnesia triggered by mild
head trauma. Brain. 1986;109:251
–7.
16. Gilbert GJ. Transient global amnesia: manifestation of medial
temporal lobe epilepsy. Clin Electroencephalogr. 1978;9:147
–
52.
17. Dugan TM, Nordgren RD, O
’Leary P. Transient global amnesia
associated with bradycardia and temporal lobe spikes. Cortex.
1981;17:633
–8.
18. Bilo L, Meo R, Ruosi P, et al. Transient epileptic amnesia: an emerging
late-onset epileptic syndrome. Epilepsia. 2009;50 Suppl 5:58
–61.
19. Poser CM, Ziegler DK. Temporary amnesia as a manifestation of
cerebrovascular insufficiency. Trans Am Neurol Assoc.
1960;85:221
–3.
20. Gorelick PB, Amico LL, Ganellen R, Benevento LA. Transient
global amnesia and thalamic infarction. Neurology. 1988;38:496
–9.
21. Bogousslavsky J, Regli F. Transient global amnesia and stroke.
Eur Neurol. 1988;28:106
–10.
22. Shuttleworth EC, Wise GR. Transient global amnesia due to
arterial embolism. Arch Neurol. 1973;29:340
–2.
23. Gilbert JJ, Benson DF. Transient global amnesia: report of two
cases with definite etiologies. J Nerv Ment Dis. 1972;154:461
–4.
24. Olivarius B, Jensen TS. Transient global amnesia in migraine.
Headache. 1979;19:335
–8.
25. Caplan L, Chedru F, Lhermitte F, Mayman C. Transient global
amnesia and migraine. Neurology. 1981;31:1167
–70.
26. Olesen J, Jørgensen MB. Leao
’s spreading depression in the
hippocampus explains transient global amnesia. A hypothesis.
Acta Neurol Scand. 1986;73:219
–20.
27. Ehyai A, Fenichel GM. The natural history of acute confusional
migraine. Arch Neurol. 1978;35:368
–9.
28. Schmidtke K, Ehmsen L. Transient global amnesia and migraine.
A case control study. Eur Neurol. 1998;40:9
–14.
29. Morris HH, Estes ML. Traveler
’s amnesia. Transient global
amnesia secondary to Triazolam. JAMA. 1987;258:945
–6.
30. Tsai MY, Tsai MH, Yang SC, et al. Transient global amnesia-like
episode due to mistaken intake of zolpidem: drug safety concern
in the elderly. J Patient Saf. 2009;5:32
–4.
31. Goodwin DW, Crane JB, Guze SB. Alcoholic
“blackouts”: a
review and clinical study of 100 alcoholics. Am J Psychiatr.
1969;126:191
–8.
32. Hartley TC, Heilman KM, Garcia-Bengochia F. A case of a
transient global amnesia due to a pituitary tumor. Neurology.
1974;24:998
–1000.
33. Lisak RP, Zimmerman RA. Transient global amnesia due to a
dominant hemisphere tumor. Arch Neurol. 1977;34:317
–8.
34. Jaffe R, Bender MB. E.E.G. studies in the syndrome of isolated
episodes of confusion with amnesia
“transient global amnesia”. J
Neurol Neurosurg Psychiatr. 1966;29:472
–4.
35. Mathew NT, Meyer JS. Pathogenesis and natural history of
transient global amnesia. Stroke. 1974;5:303
–11.
36. Kushner M, Hauser W. Transient global amnesia: a case-control
study. Ann Neurol. 1985;18:684
–91.
37. Strupp M, Bruning R, Wu RHH, et al. Diffusion-weighted MRI in
transient global amnesia: elevated signal intensity in the left mesial
temporal lobe in 7 of 10 patients. Ann Neurol. 1998;43:164
–70.
38. Ay H, Furie KL, Yamada K. Diffusion-weighted MRI characterizes
the ischemic lesion in transient global amnesia. Neurology.
1998;51:901
–3.
39. Eustache F, Desgranges B, Petit-Taboue MC. Transient global
amnesia: implicit/explicit memory dissociation and PET assess-
ment of brain perfusion and oxygen metabolism in the acute stage.
J Neurol Neurosurg Psychiatr. 1997;63:357
–67.
40. Yang Y, Kim S, Kim JH. Ischemic evidence of transient global
amnesia: location of the lesion in the hippocampus. J Clin Neurol.
2008;4:59
–66.
41. Alberici E, Pichiecchio AE, Caverzasi E, et al. Transient global
amnesia: hippocampal magnetic resonance imaging abnormalities.
Funct Neurol. 2008;23:149
–52.
42. Lee SY, Kim WJ, Suh SH, et al. Higher lesion detection by 3.0 T
MRI in patients with transient global amnesia. Yonsei Med J.
2009;50:211
–4.
43. Lewis SL. Aetiology of transient global amnesia. Lancet.
1998;352:397
–9.
44. Sander D, Winbeck K, Etgen T, et al. Disturbance of venous flow
patterns in patients with transient global amnesia. Lancet.
2000;356:1982
–4.
Curr Neurol Neurosci Rep (2011) 11:578
–582
581

45. Schrieber SJ, Doepp F, Klingebiel R, Valdueza JM. Internal
jugular vein valve incompetence and intracranial venous anatomy
in transient global amnesia. J Neurol Neurosurg Psychiatr.
2005;76:509
–13.
46. Chung C, Hsu H, Chao A, et al. Detection of intracranial venous
reflux in patients with transient global amnesia. Neurology.
2006;66:1873
–7.
47.
• Cejas C, Cisneros LF, Lagos R, et al. Internal jugular vein valve
incompetence is highly prevalent in transient global amnesia. Stroke.
2010;41:67
–71. This article contains an up-to-date summary on the
evidence for jugular vein incompetence as a cause of TGA.
48. Altamura C, Vernieri F. Internal jugular vein valve incompetence
in transient global amnesia. More circumstantial evidence or the
proof solving the mystery? Stroke. 2010;41:1
–2.
582
Curr Neurol Neurosci Rep (2011) 11:578
–582
Document Outline
Wyszukiwarka
Podobne podstrony:
grupa 1 pamiec Zakieta
grupa 1 pamiec Schudy
grupa 1 pamiec Zochowska
KOLOSY, Kolos-pamięć,6, grupa A
03 Odświeżanie pamięci DRAMid 4244 ppt
wykład 12 pamięć
8 Dzięki za Pamięć
06 pamięć proceduralna schematy, skrypty, ramyid 6150 ppt
test poprawkowy grupa 1
19 183 Samobójstwo Grupa EE1 Pedagogikaid 18250 ppt
Pamięć
PAMIĘĆ 3
Architektura i organizacja komuterów W5 Pamięć wewnętrzna
Grupa 171, Podstawy zarządzania
więcej podobnych podstron