R
t Hi hli ht f
th
Recent Highlights from the
Danishefsky Laboratory
Danishefsky Laboratory
H
HO
O
O
OH
H
HO
N
O
Me
OMe
O
HO
11-0-Debenzoyltashironin
N
H
NH
11 0 Debenzoyltashironin
NMe
2
Phalarine
Anne-Marie Dechert
N
b
7 2007
November 7, 2007
11-0-Debenzoyltashironin
Background and Structural Features
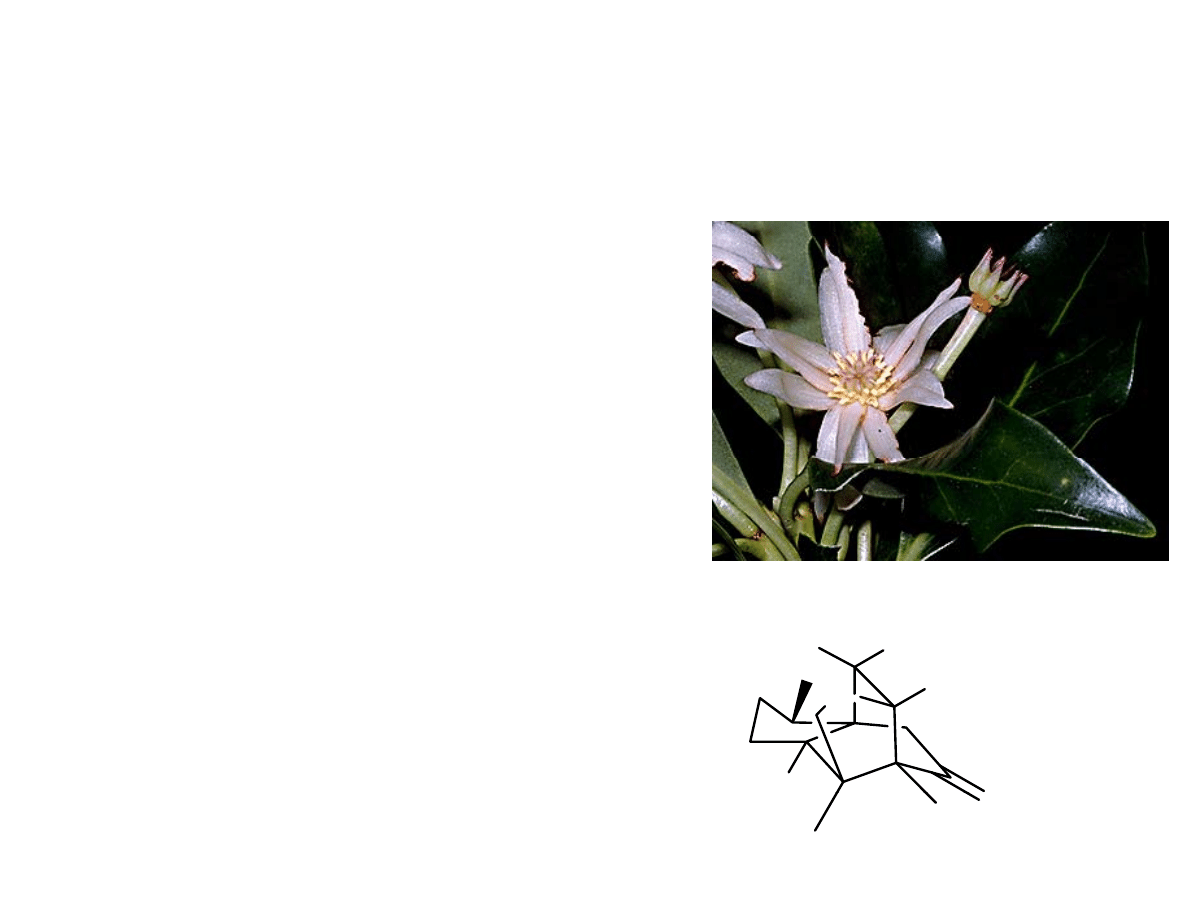
• Isolated from the pericaps of
Illicium merrillianum
• Shown to induce neurite
outgrowth in fetal rat cortical
g
neurons at low
concentrations
• 7 contiguous stereocenters,
3 all carbon quarternary
OH
H
HO
3 all carbon quarternary
centers
O
HO
OH
O
HO
11-0-Debenzoyltashironin
Synthetic Strategy
Synthetic Strategy
OH
Oxidative
O
OMe
OH
OH
MsO
Oxidative
Dearomatization
O
OMe
O
MsO
OTs
OTs
Transannular
H
HO
Transannular
Diels-Alder
O
MsO
OH
H
HO
OTs
MsO
O
OH
H
HO
O
HO
11-0-Debenzoyltashironin
Danishefsy, et al. J. Am. Chem. Soc. 2006, 128, 16440-16441
Synthetic Strategy
Synthetic Strategy
OH
Oxidative
D
ti
ti
O
OMe
OMe
OH
MsO
Dearomatization
OMe
O
MsO
OTs
OTs
Transannular
Di l
Ald
OH
H
HO
Diels-Alder
O
OTs
MsO
OH
X
OTs
Believed that the cyclization didn’t occur because of the electronic
O
OH
H
HO
and steric constraints that the mesyl enol ether imposed.
O
HO
11-0-Debenzoyltashironin
Danishefsy, et al. J. Am. Chem. Soc. 2006, 128, 16440-16441
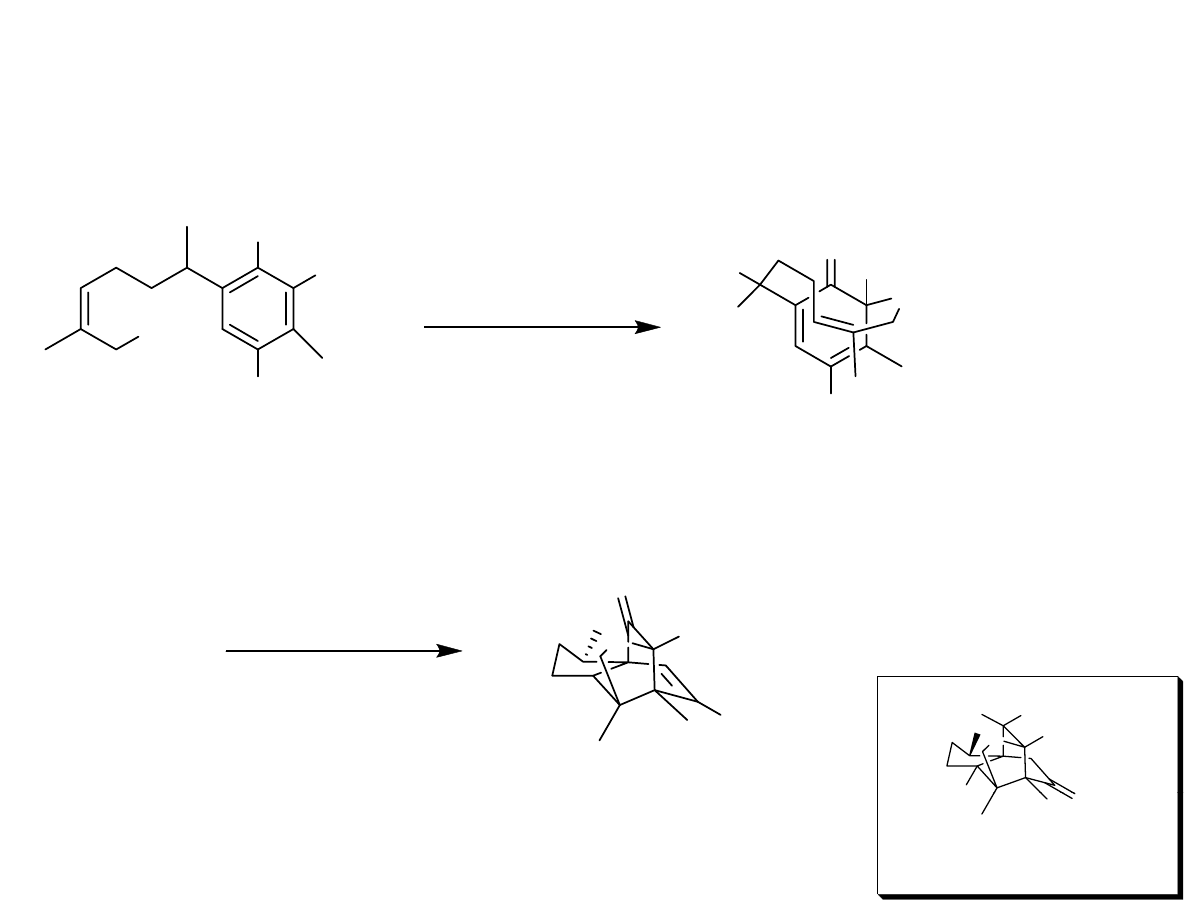
Synthetic Strategy
Synthetic Strategy
OH
OMe
OH
OH
Oxidative
Dearomatization
O
OMe
O
Me
H
OTs
OTs
O
OMe
O
Transannular
Diels-Alder
OTs
X
W
i
!
O
HO
OH
H
HO
Wrong epimer!
O
HO
11-0-Debenzoyltashironin
Danishefsy, et al. J. Am. Chem. Soc. 2006, 128, 16440-16441
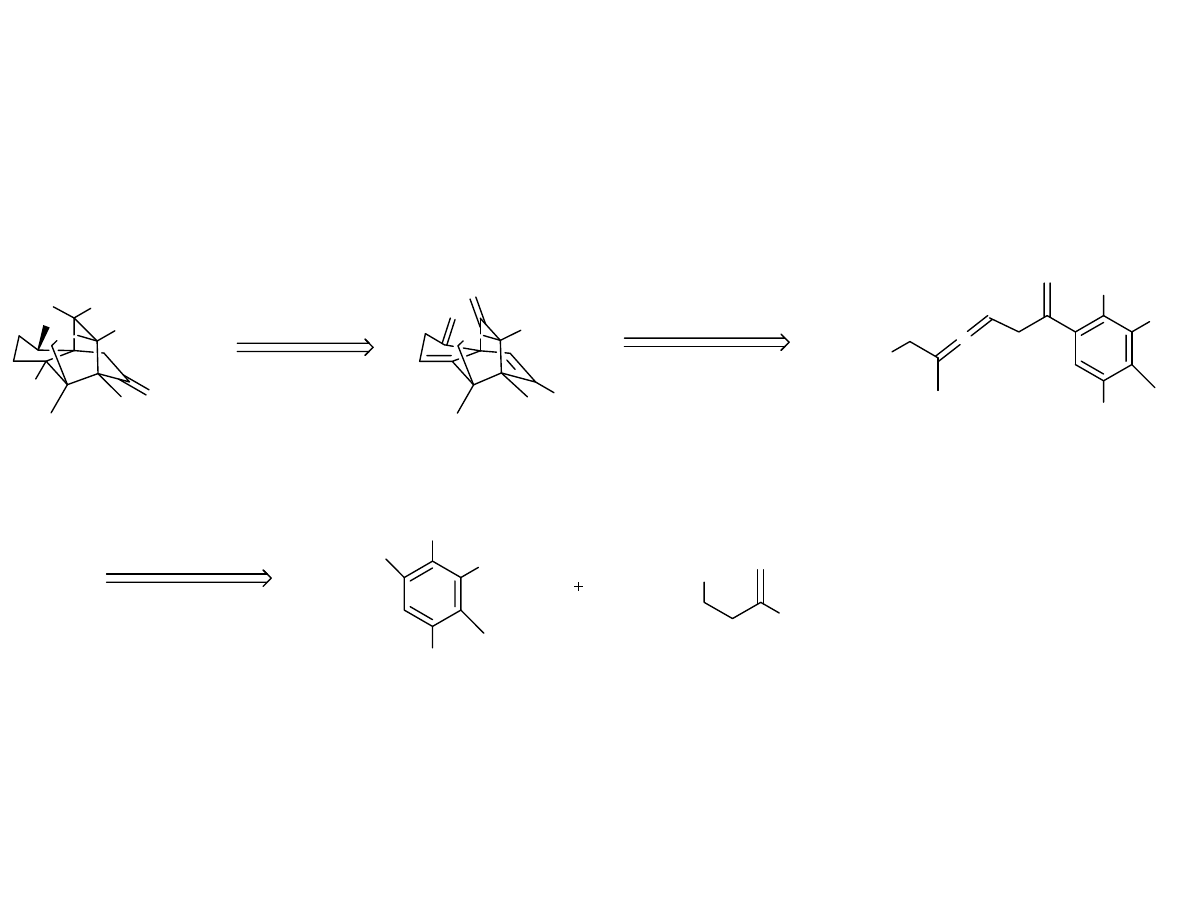
Synthetic Strategy
Synthetic Strategy
OR
OH
•
O
OR
O
O
OH
H
HO
OTs
HO
OTs
O
HO
OTBS
Br
OBn
OH
OTs
OBn
OH
SnBu
3
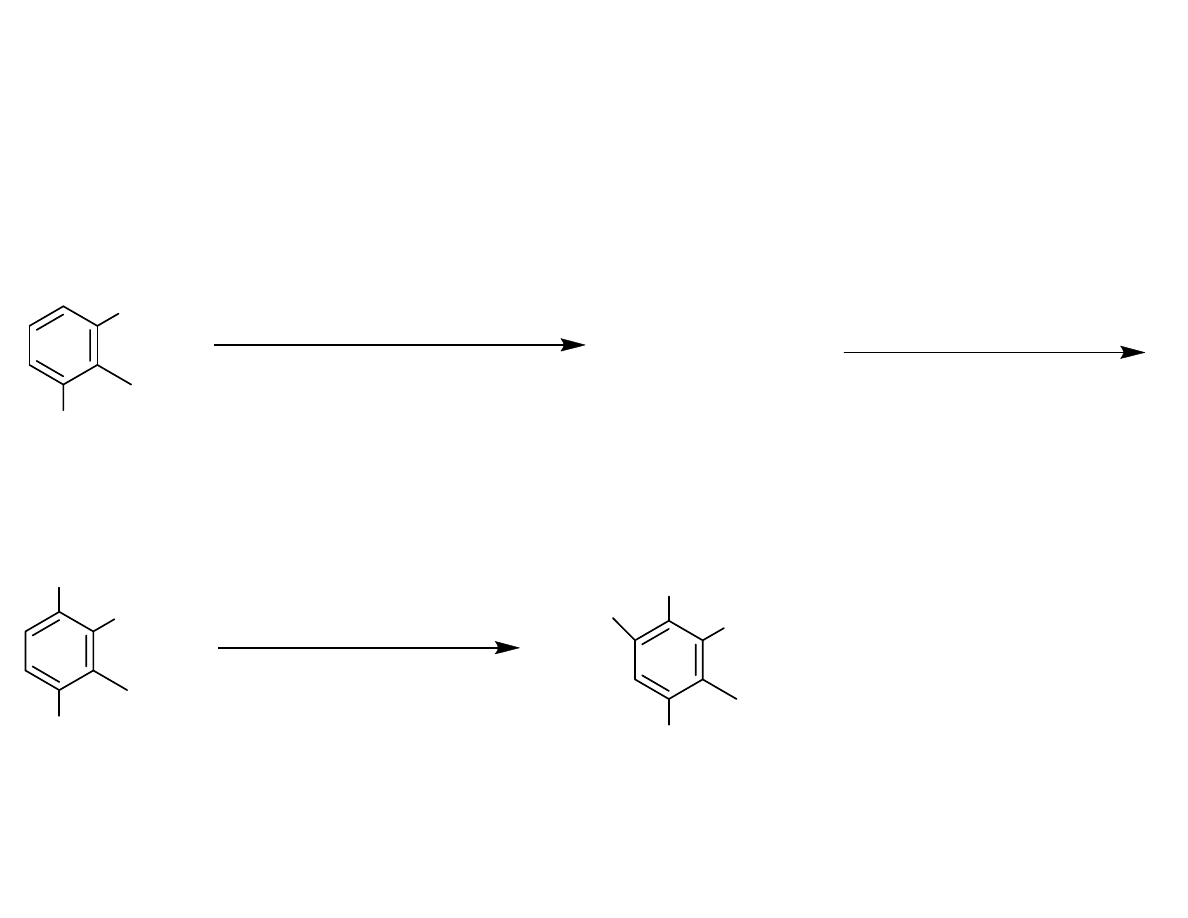
Synthesis of the Aryl Bromide
Synthesis of the Aryl Bromide
OH
1. Zn(CN)
2
, HCl (99 %)
2. TsCl (57 %)
3. BnBr, K
2
CO
3
, TBAI (97 %)
1. mCPBA
2. TEA
OH
98 % for 2 steps
OH
OBn
OH
1. NBS (85 %)
2. TBSCl, TEA (87%)
OBn
OTBS
Br
OTs
OTs
Danishefsy, et al. J. Am. Chem. Soc. 2006, 128, 16440-16441
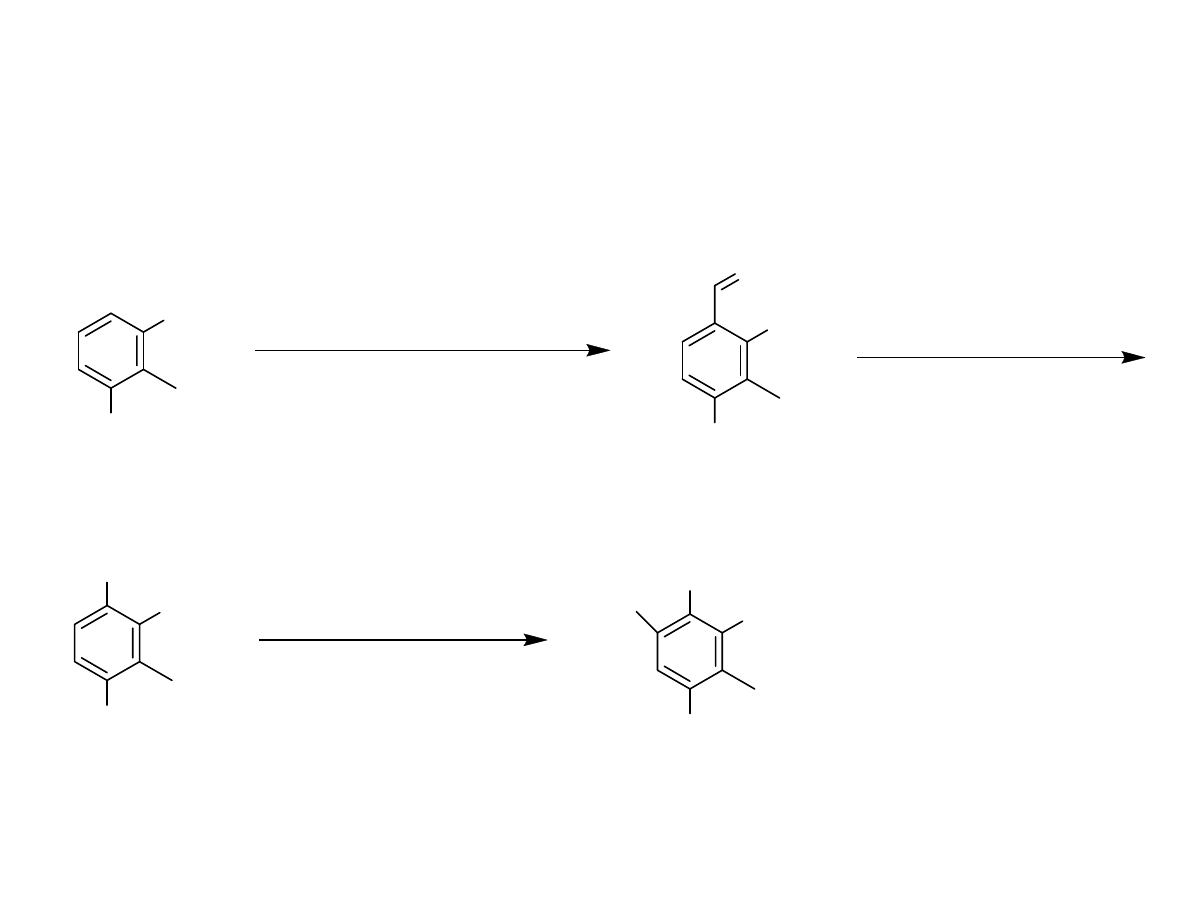
Synthesis of the Aryl Bromide
Synthesis of the Aryl Bromide
OH
1. Zn(CN)
2
, HCl (99 %)
2. TsCl (57 %)
3. BnBr, K
2
CO
3
, TBAI (97 %)
OBn
O
1. mCPBA
2. TEA
OH
OTs
2. TEA
98 % for 2 steps
OH
OTBS
OBn
OH
1. NBS (85 %)
2. TBSCl, TEA (87%)
OBn
OTBS
Br
OTs
OTs
Danishefsy, et al. J. Am. Chem. Soc. 2006, 128, 16440-16441
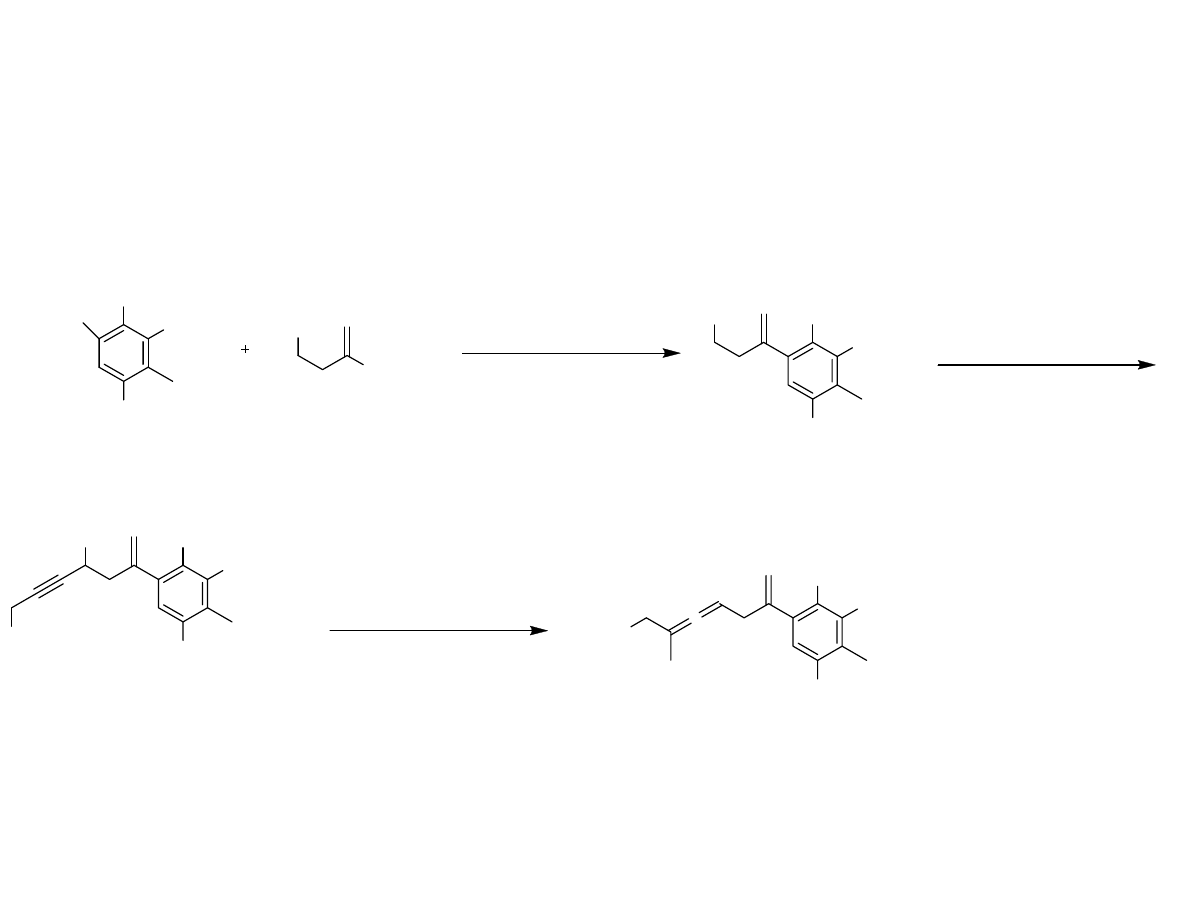
Synthesis of the Oxidative
Dearomatization Precursor
OBn
OTBS
Br
OH
Pd
2
(dba)
3
, tBu
3
P
OBn
OTBS
OH
1. DMP (98 %)
2. 2-propargyloxyTBS,
Et
2
Zn, Ti(OiPr)
4
(91 %)
OTs
SnBu
3
OBn
OTs
Et
2
Zn, Ti(OiPr)
4
(91 %)
77 %
OBn
OTBS
OH
1. MsCl, TEA
O
OBn
OTs
OTBS
,
2. Me
2
Cu(CN)Li
2
(88 %)
3.Bu
4
NF, AcOH (95 %)
OR
OH
•
HO
OTs
Danishefsy, et al. J. Am. Chem. Soc. 2006, 128, 16440-16441
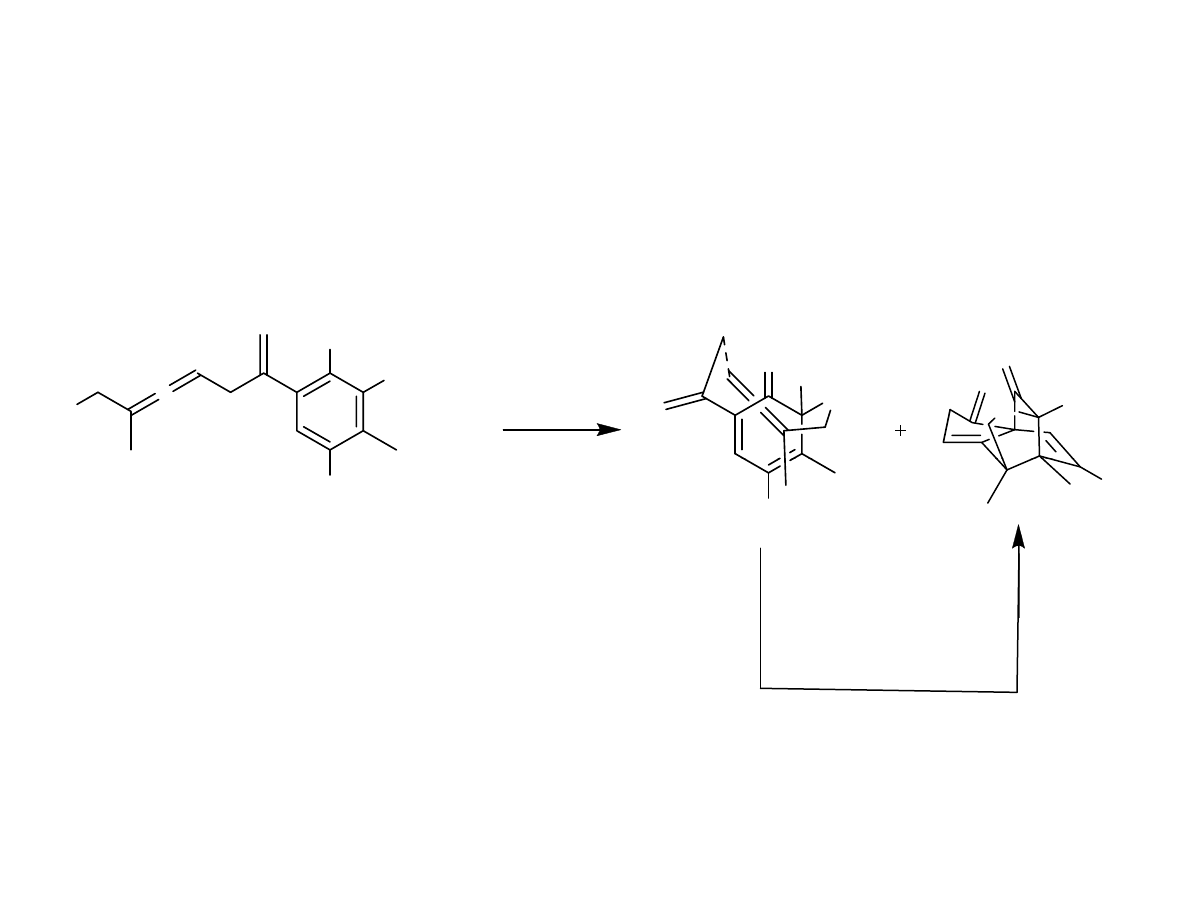
Oxidative Dearomitization,
Transannular Diels-Alder
OH
O
OBn
•
HO
PIDA
O
OBn
O
•
O
OBn
O
OTs
OTs
OTs
μW
μ
65 %
Danishefsy, et al. J. Am. Chem. Soc. 2006, 128, 16440-16441
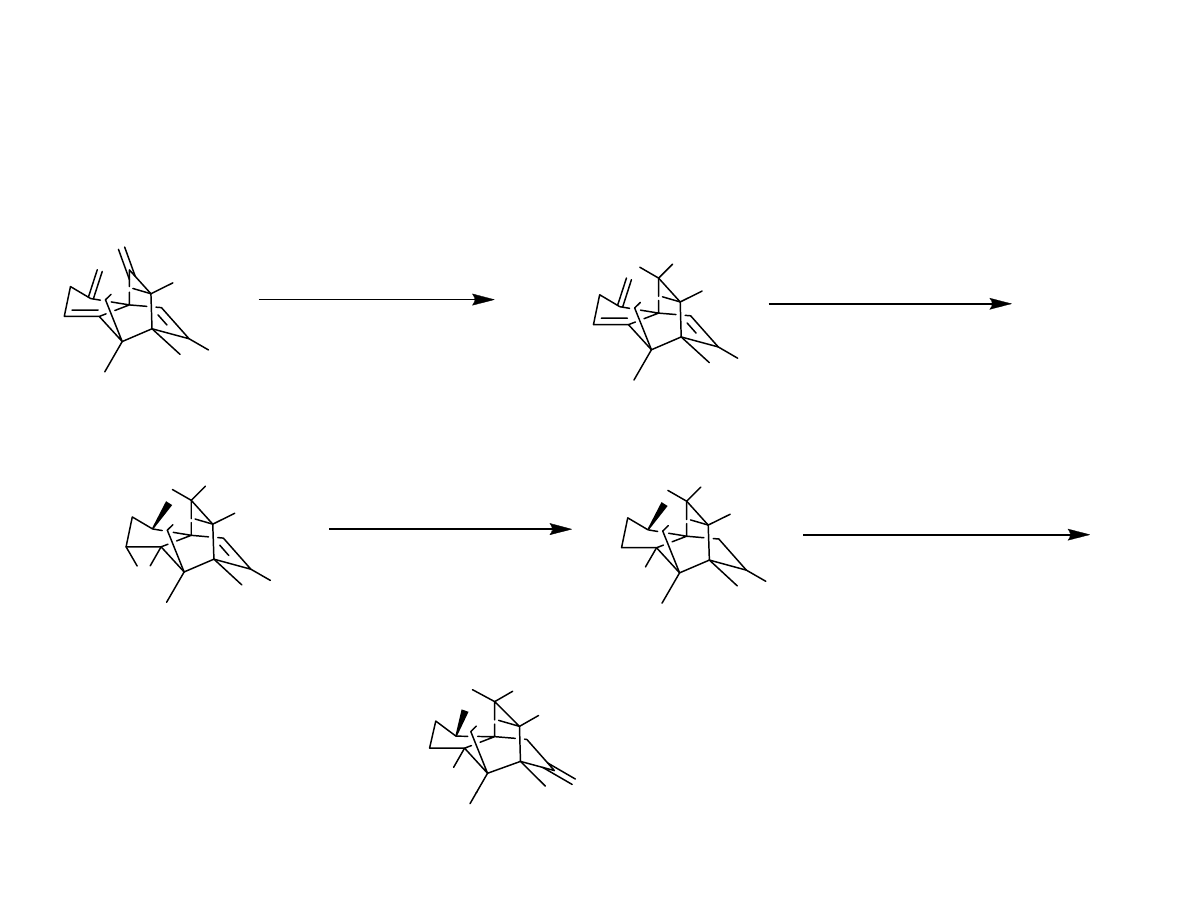
End Game
End Game
O
1 mCPBA (71 %)
O
OBn
O
1. NaBH
4
(83 %)
2. TMS-imidazole (99 %)
O
OBn
H
TMSO
1. mCPBA (71 %)
2. Wilkinson's Catalyst,
H
2
(74 %)
OTs
OTs
1 DMP (96 %)
O
OBn
H
TMSO
LiEt
3
BH
O
OBn
H
TMSO
32 %
1. DMP (96 %)
2. HF- pyr, Bu
4
NF (87 %)
3. H
2
, Pd/C (91 %)
OTs
O
OH
HO
H
HO
O
O
HO
OH
H
HO
O
Danishefsy, et al. J. Am. Chem. Soc. 2006, 128, 16440-16441
Phalarine
Phalarine
O
Me
•Isolated from the phalris coerulescens,
h
t i
d i 1999
N
H
N
NH
Me
OMe
characterized in 1999
NH
NMe
2
•Furanobisindole alkaloid
Phalarine
•First synthesis reported by Danishefsky
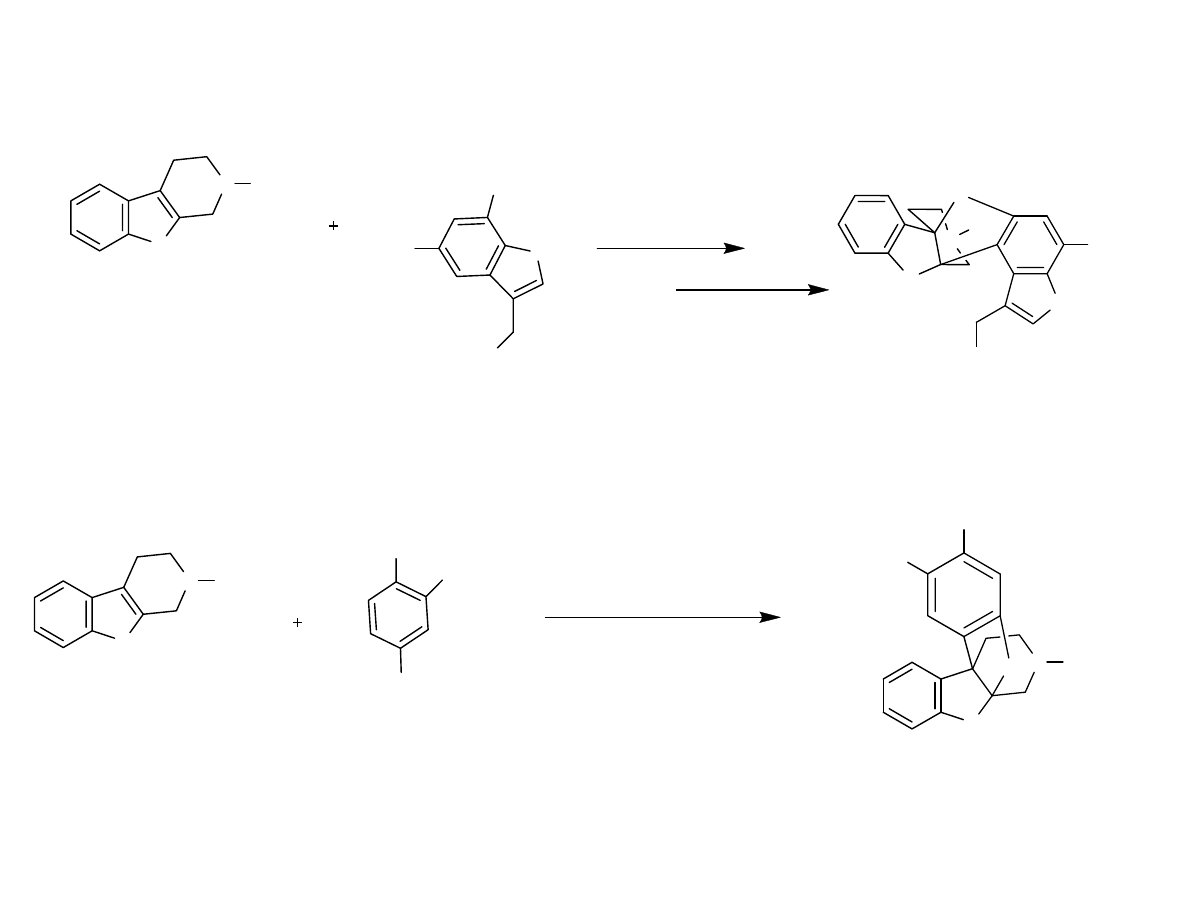
Synthetic Stratagy
N
Me
OMe
1
1'
2
N
O
Me
N
H
NH
HO
1
2
2'
N
H
N
NH
OMe
Me
2
N
P halarine
NMe
2
O Me
OM
OMe
MeO
N
H
N
CO
2
Me
O H
OMe
N
CO
2
Me
O
The model system gave the opposite of what was desired!
O H
N
H
The model system gave the opposite of what was desired!
Danishefsky, et al. Angew. Chem. Int. Ed. 2007, 46, 1444-1447.
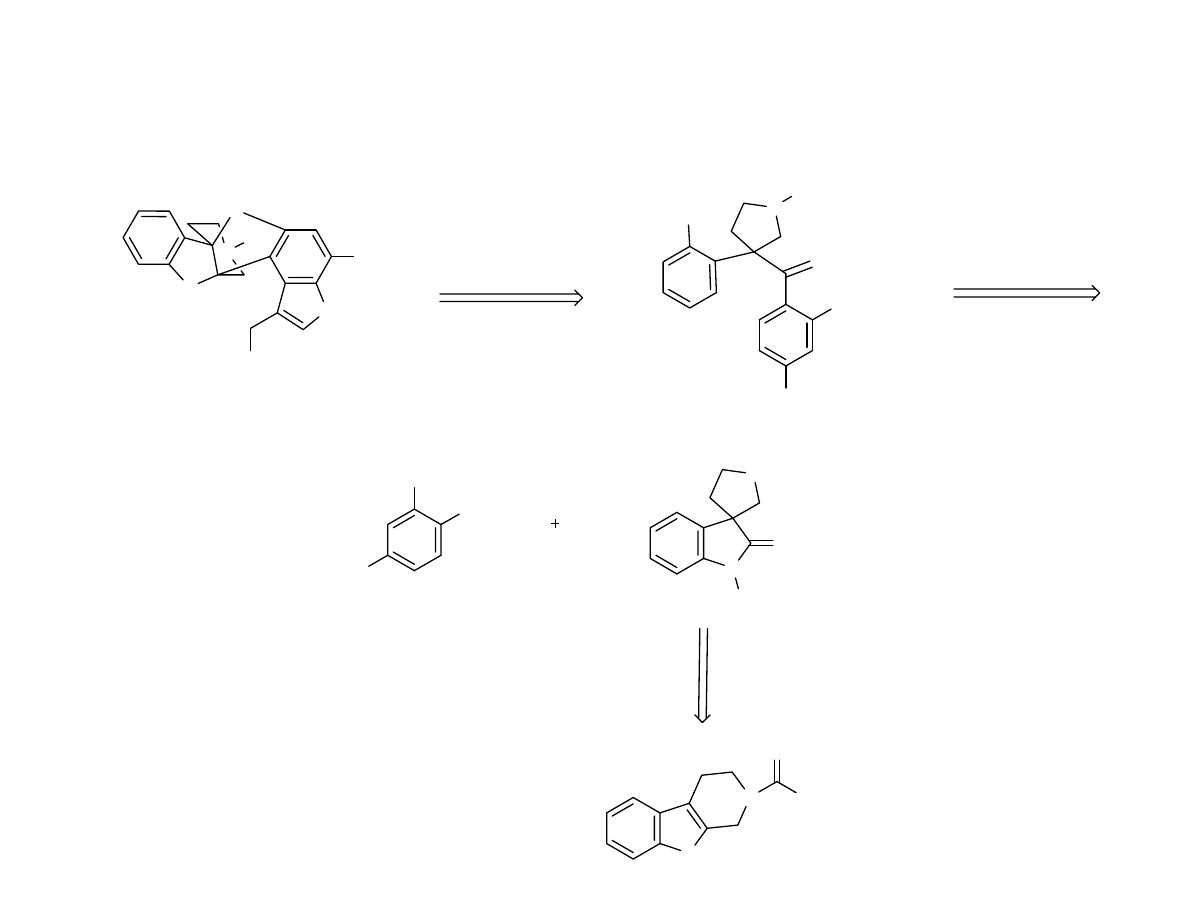
Retrosynthesis
Retrosynthesis
TsHN
N
Me
O
M
O
OMOM
N
H
N
NH
Me
OMe
O Me
NMe
2
Pha larine
N
O
N-Me
O MOM
Li
M O
N
Ts
MeO
O
N
H
N
O Me
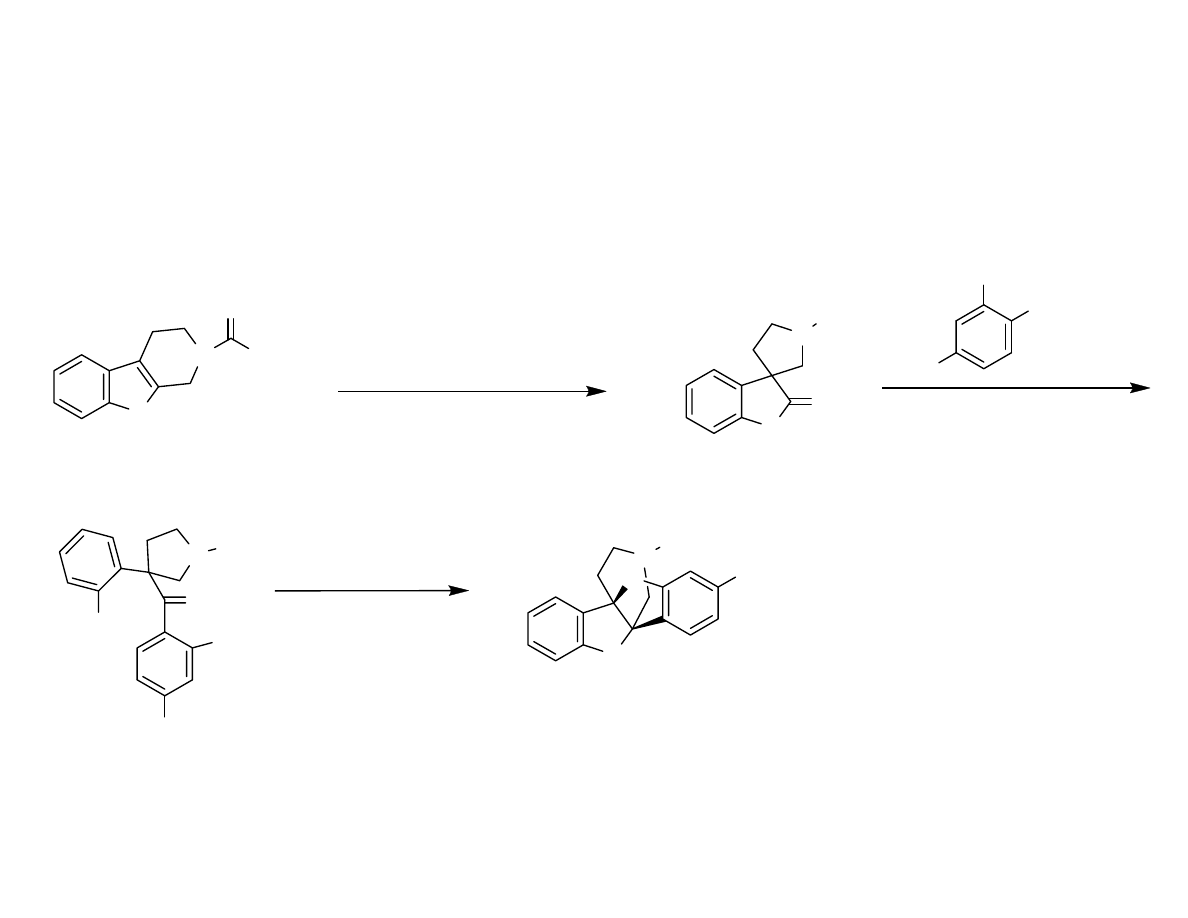
Azaspiroindolenine synthesis
Azaspiroindolenine synthesis
OMOM
Li
O
1. LiAlH
4
(99%)
2 NBS H O AcOH (79%)
N
Me
MeO
96 %
N
H
N
OMe
2. NBS, H
2
O, AcOH (79%)
3. LiHMDS, TsCl (89 %)
N
Ts
O
N Me
1. TFA (98 %)
OMe
N
Me
O
OMOM
2. CSA (72 %)
NHTs
N
Ts
O
OMe
OMe
Ts
Danishefsky, S.; et al. Angew. Chem. Int. Ed. 2007, 46, 1444-1447.
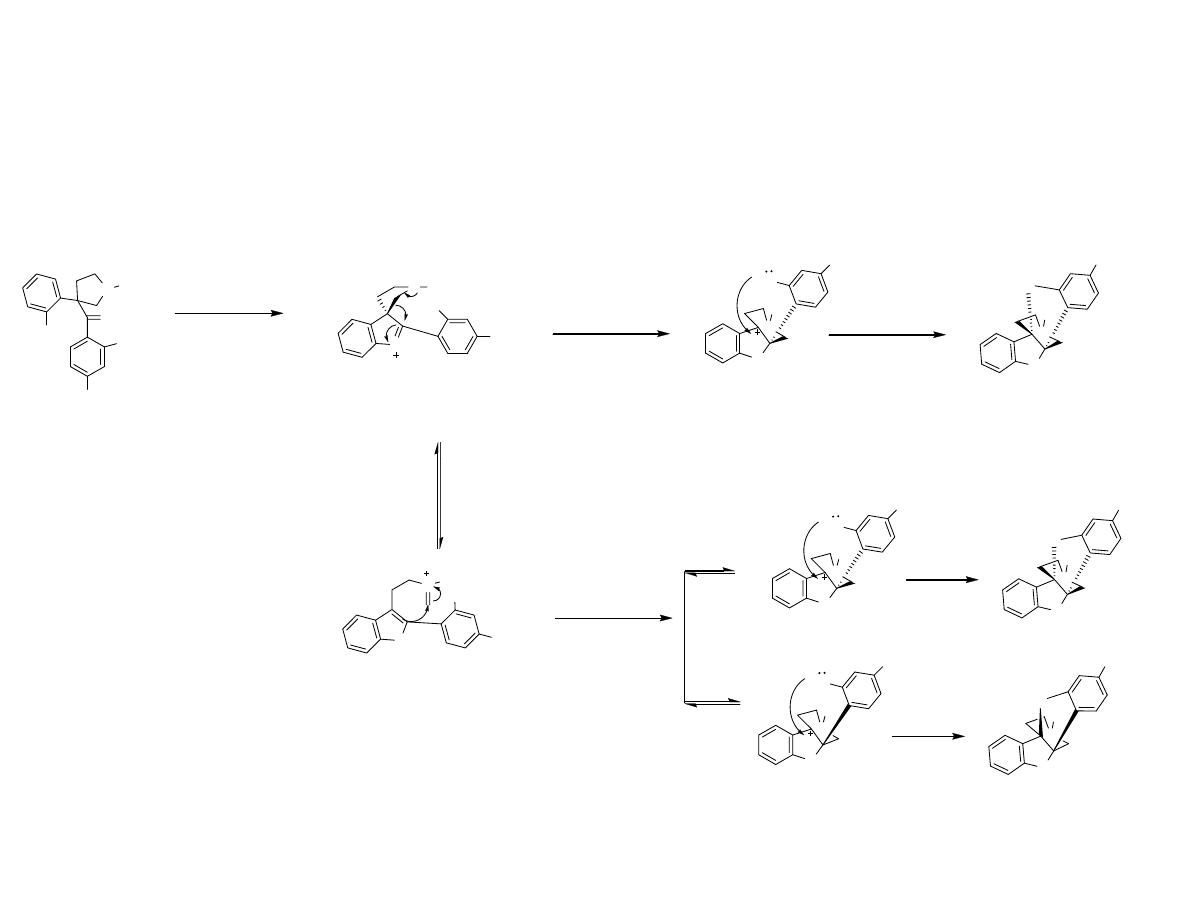
Two Mechanistic Possibilities
Two Mechanistic Possibilities
N Me
O
OH
NHTs
-H
2
O
NTs
N Me
HO
Path A
1, 2 Wagner-
Meerwein Shift
N
HO
N
O
M e
Me
OMe
OMe
OMe
OH
OMe
NTs
Path B
N
Ts
NTs
Retro-Mannich
N
HO
Me
O
Me
OMe
OMe
NTs
N Me
Pictet-Spengler
OH
N
Ts
N
NTs
N
HO
OMe
OMe
OM e
N
Ts
N
HO
Me
NTs
N
O
Me
Danishefsky, S.; et al. Angew. Chem. Int. Ed. 2007, 46, 1444-1447.
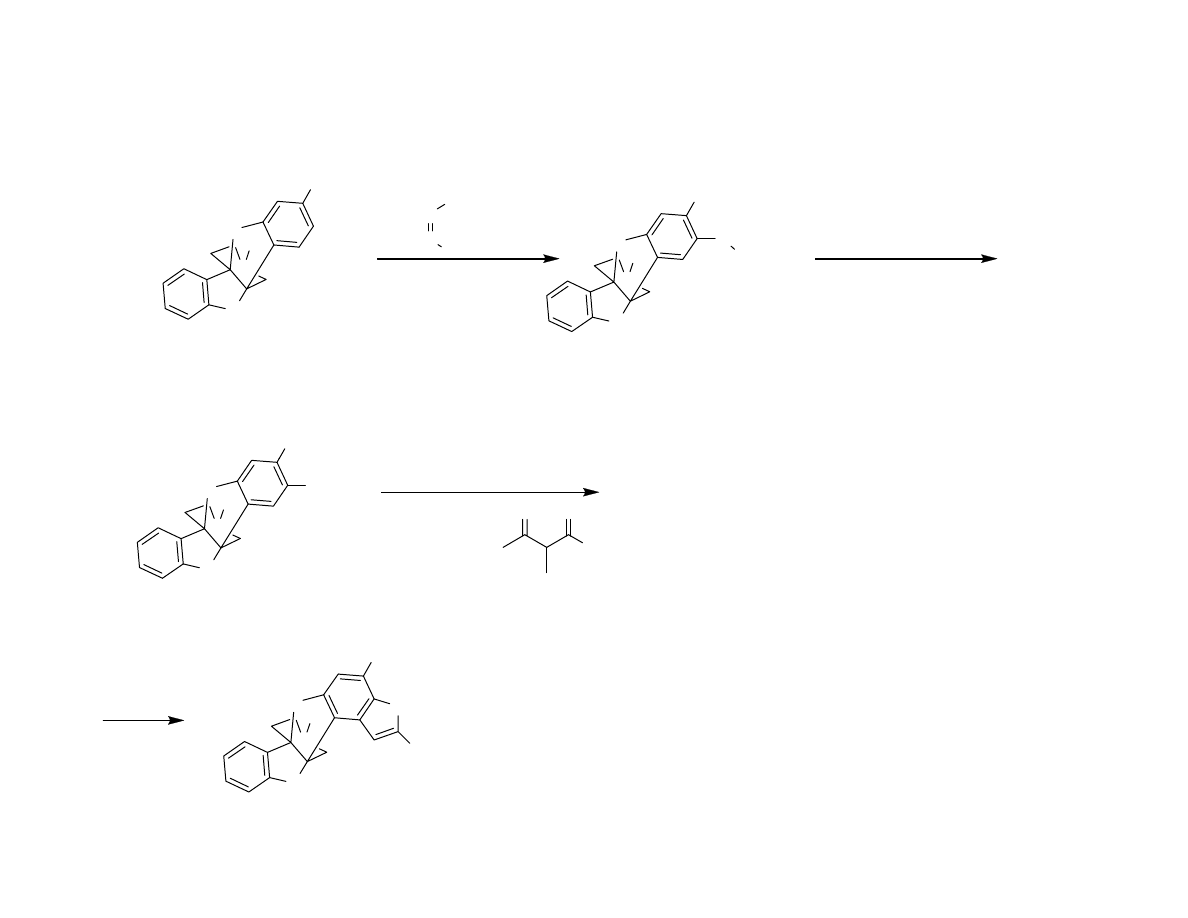
Attempted Completion
Attempted Completion
O
M
OMe
N
N
Troc
OMe
Troc
N
NTs
N
O
Me
N
Troc
TFA,
95 %
NTs
N
O
Me
N
NHTroc
Zn dust, AcOH
88 %
OMe
NTs
N
O
Me
NH
2
1. NaNO
2
, HCl (aq)
2. KOH (aq),
88 %
O
O
OEt
NTs
88 %
OMe
TsOH
NTs
N
O
Me
NH
CO
2
Et
Danishefsky, S.; et al. Angew. Chem. Int. Ed. 2007, 46, 1448-1450.
< 5% desired product
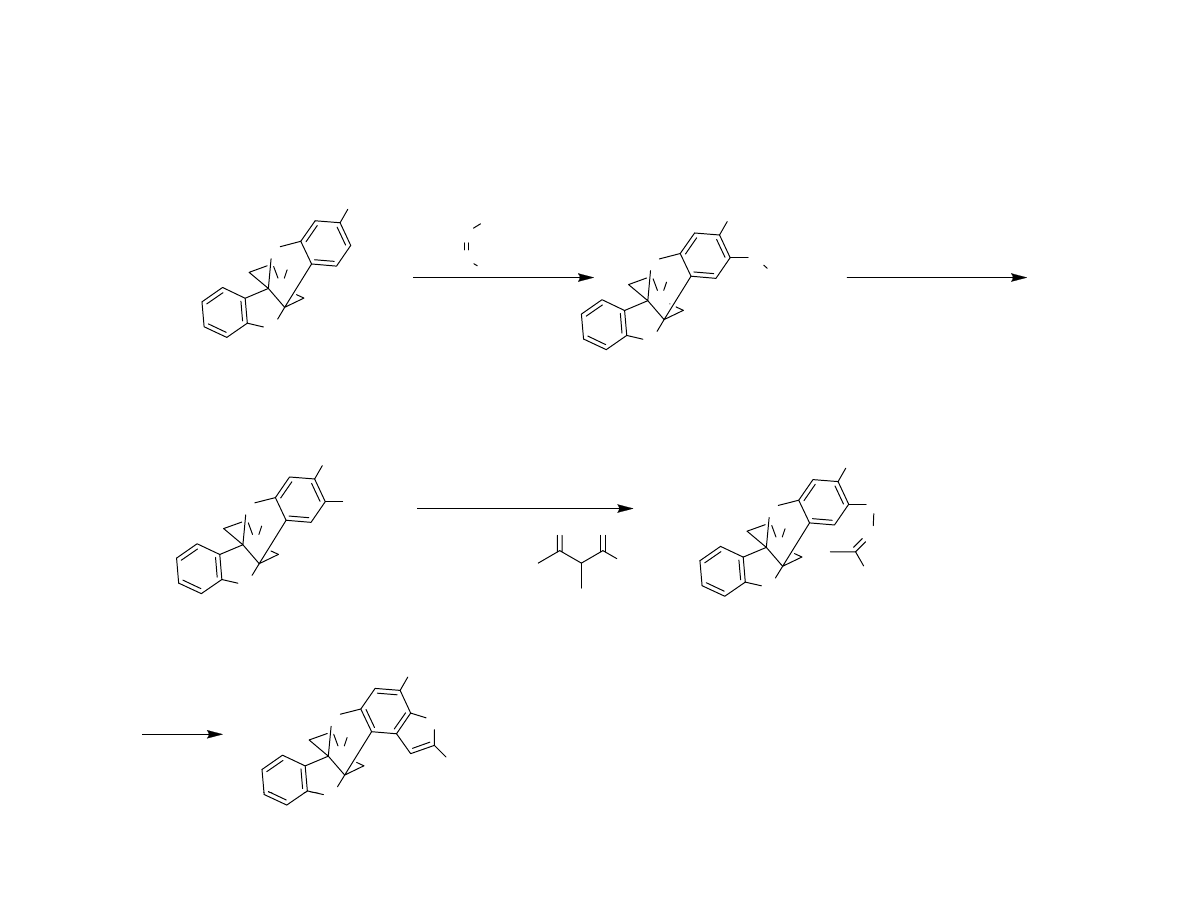
Attempted Completion
Attempted Completion
OMe
N
Troc
OMe
NTs
N
O
Me
N
N
Troc
TFA,
95 %
NTs
N
O
Me
Troc
N
NHTroc
Zn dust, AcOH
88 %
NTs
OMe
OM
N
O
Me
OMe
NH
2
1. NaNO
2
, HCl (aq)
2. KOH (aq),
O
O
OEt
N
O
Me
OMe
NH
N
CO Et
NTs
88 %
OEt
NTs
CO
2
Et
OMe
TsOH
NT
N
O
Me
NH
CO
2
Et
NTs
< 5% desired product
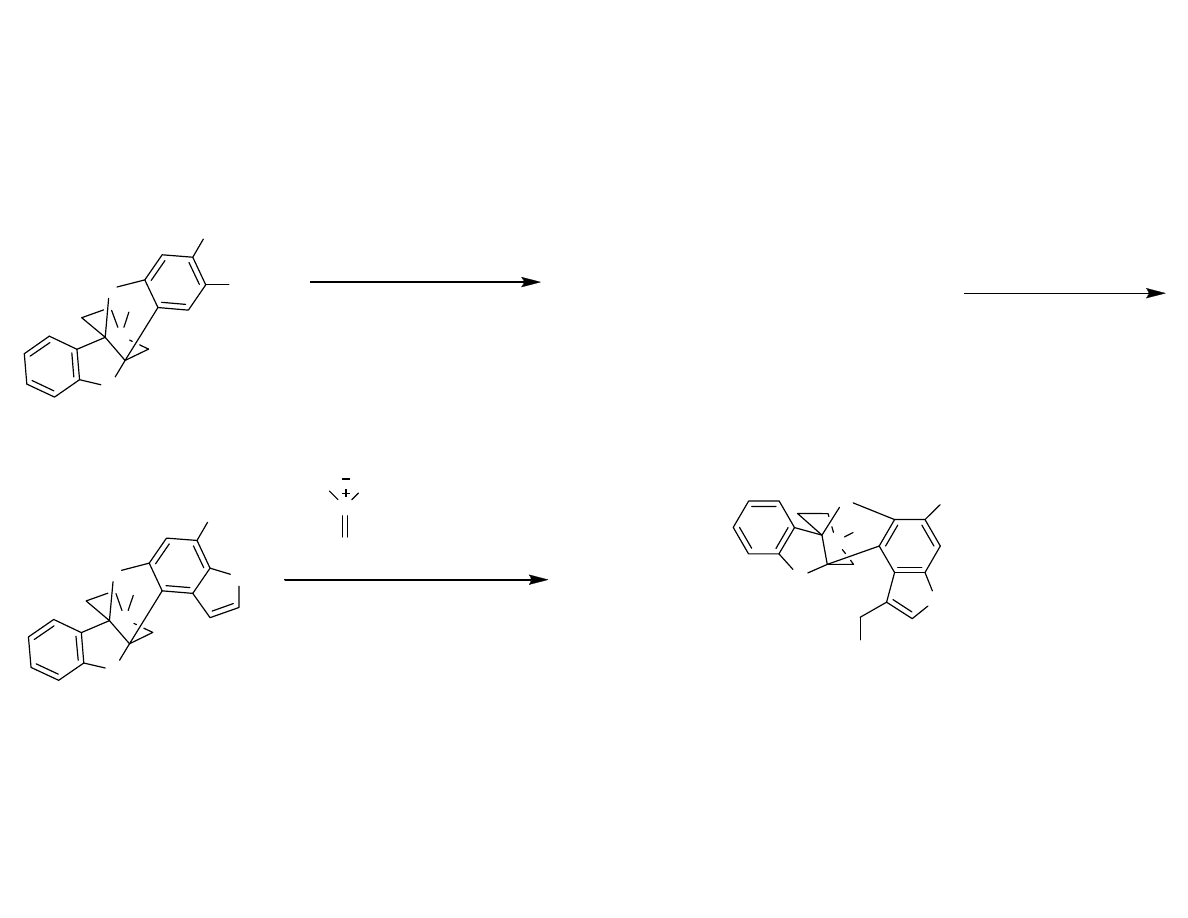
End Game
End Game
O Me
a Me SCH
2
CO
2
Et SO
2
Cl
2
N
O
Me
O Me
NH
2
a.Me SCH
2
CO
2
Et, SO
2
Cl
2
b. proton sponge
c. Et
3
N, AcOH
66 %
1. BH
3
2. RaNi
(90 % ove r 2 steps)
NTs
Cl
O
OMe
N
Me
Me
AcOH (74 % )
1.
2. Na(Hg), Na
2
HPO
4
(90 %)
,
N
O
O Me
Me
Cl
NTs
N
O
Me
NH
( g),
(
)
N
H
NH
NMe
2
P halarine
Danishefsky, S.; et al. Angew. Chem. Int. Ed. 2007, 46, 1448-1450.
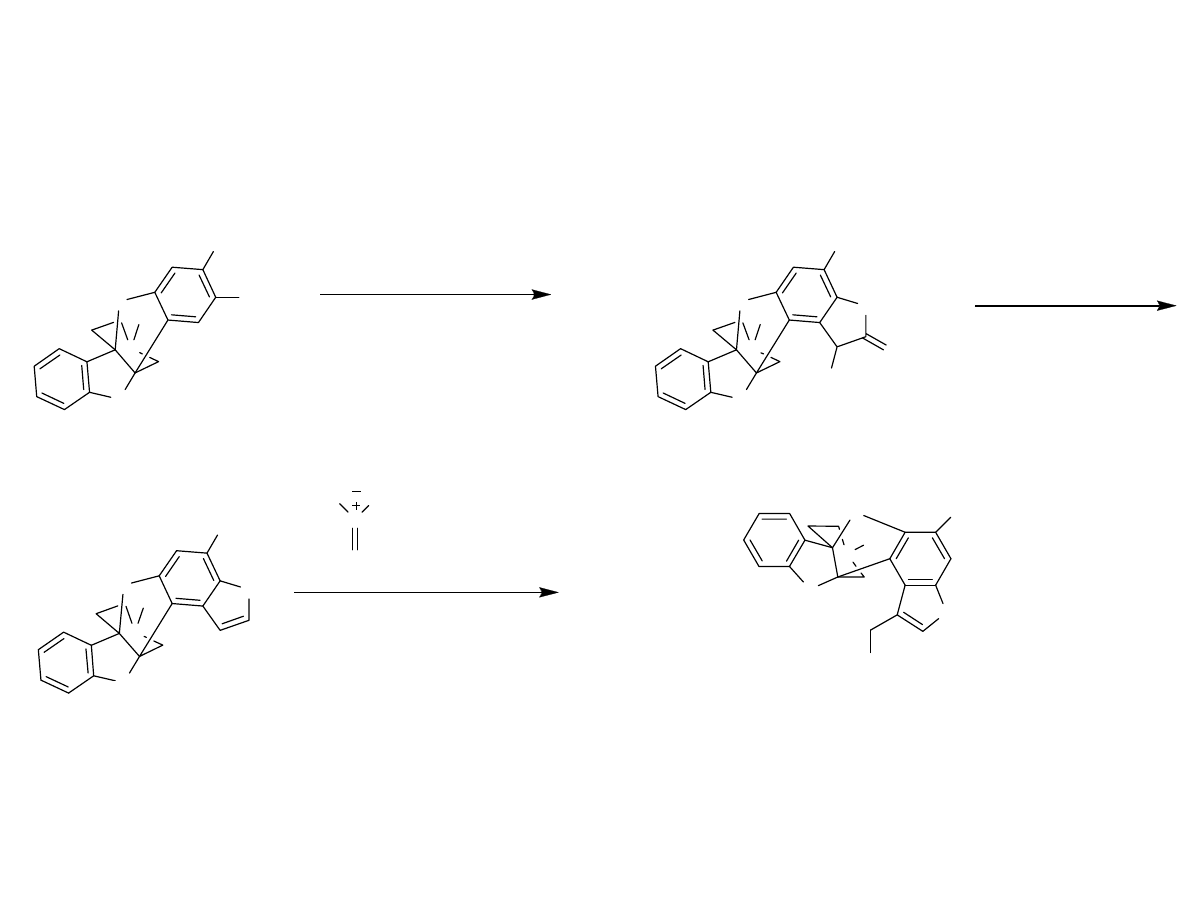
End Game
End Game
N
O
Me
O Me
NH
2
a.Me SCH
2
CO
2
Et, SO
2
Cl
2
b. proton sponge
c. Et
3
N, AcOH
N
O
Me
O Me
NH
66 %
1. BH
3
2. RaNi
(90 % ove r 2 steps)
NTs
N
NTs
N
O
MeS
OMe
N
Me
Me
AcOH (74 % )
1.
,
N
O
O Me
Me
Cl
NT
N
O
Me
NH
2. Na(Hg), Na
2
HPO
4
(90 %)
N
H
NH
NMe
2
NTs
P halarine
Danishefsky, S.; et al. Angew. Chem. Int. Ed. 2007, 46, 1448-1450.
Wyszukiwarka
Podobne podstrony:
Stewart Hamilton, Alicia Micklethwait Greed and Corporate Failure; The Lessons from Recent Disaster
the illict preparation of morphine and heroin from pharmaceutical products containing codeine homeba
Kontrola badań laboratoryjnych
badania laboratoryjne 6
ROZRÓD Badanie terenowe i laboratoryjne mleka
Diagnostyka laboratoryjna chorób serca i mięśni poprzecz (2)
Diagnostyka laboratoryjna zaburzen gospodarki lek 2010
medycyna laboratoryjna
Medycyna laboratoryjna 12 13
7) Laboratoria EMG i MMG na pziomach sily i ko
3 1 5 CCNA1 Laboratorium pl
laboratorium2
Laboratorium 7
Laboratorium jezyk c4 2013
więcej podobnych podstron