
Review
J. Radiat. Res., 51, 503– 509 (2010)
Functions and Regulation of Artemis: A Goddess in the
Maintenance of Genome Integrity
Aya KUROSAWA
1
* and Noritaka ADACHI
1,2†
Artemis/DNA double-strand break/Non-homologous end-joining.
Artemis is a structure-specific endonuclease when associated with and phosphorylated by DNA-
dependent protein kinase catalytic subunit. This structure-specific endonuclease is responsible for the
resolution of hairpin coding ends in V(D)J recombination. In DNA double-strand break repair, Artemis is
implicated in the end-processing step of the non-homologous end-joining (NHEJ) pathway. Recently, we
have demonstrated that the involvement of Artemis in NHEJ depends on the type of DNA damage. Inter-
estingly, recent evidence suggests that the end-processing activity is not the only function of Artemis.
Indeed, Artemis is rapidly phosphorylated by ataxia telangiectasia mutated in response to DNA damage,
and such phosphorylation of Artemis appears to be involved in the regulation of cell cycle checkpoints.
These findings suggest that Artemis is a multifunctional protein participating in the maintenance of
genome integrity at two distinct levels; one at the end processing step of NHEJ, and the other at the
signaling pathway of cell cycle regulation. Therefore, understanding Artemis function may give us
profound insights into the DNA repair network. In this review, we summarize the functions and regulation
of Artemis.
DNA DOUBLE-STRAND BREAK REPAIR
DNA double-strand breaks (DSBs), which can be caused
by a variety of exogenous and endogenous agents, pose a
major threat to genome integrity and may cause cell death if
left unrepaired.
1,2)
Eukaryotic cells have evolved two major
pathways for repairing DSBs: homologous recombination
(HR) and non-homologous DNA end-joining (NHEJ).
2–5)
DNA repair by the HR pathway is restricted to the late S and
G2 phases of the cell cycle, while the NHEJ pathway is not
restricted to a particular phase in the cell cycle and hence
DSBs can be repaired via NHEJ throughout the cell cycle.
6)
Consistent with the roles of the HR and NHEJ pathways for
DSB repair, cells deficient in HR or NHEJ proteins show
increased sensitivity to DSB-causing agents.
6–13)
In higher
eukaryotes, DSBs are mainly repaired by NHEJ,
14)
and thus
NHEJ-deficient cells are in general more sensitive to ioniz-
ing radiation (IR) than are HR-deficient cells. Instead, as HR
plays a major role at the replication fork, HR-deficient cells
exhibit increased sensitivity to replication-associated, one-
ended DSBs that arise from replication fork collapse. It
should also be noted that NHEJ is also important for V(D)J
recombination, which generates the diversity of antibody
and T cell receptor molecules.
14)
The NHEJ reaction can be
divided into three steps; (1) end binding, (2) end processing,
and (3) ligation. In the end-binding step, the Ku complex
(the heterodimer of Ku70 and Ku80) immediately binds to
DSB ends. After the Ku complex binding to DSB ends,
DNA-dependent protein kinase catalytic subunit (DNA-
PKcs) is recruited to DSB ends. The Ku/DNA-PKcs com-
plex is thought to participate in end bridging during NHEJ
to protect the ends from nucleases as well as to facilitate end
processing reactions.
15,16)
The end processing step is partic-
ularly important when DSBs contain unligatable ends, such
as incompatible ends and chemically modified ends, because
all DNA ligases, including DNA ligase IV, catalyze the for-
mation of a phosphodiester bond between 5’-phosphate and
3’-hydroxyl termini.
17)
The end processing step relies on
several enzymes, including nuclease and polynucleotide
kinase, to generate DNA ends suitable for ligation reac-
tion.
18,19)
Finally, the ligatable ends are rejoined by DNA
ligase IV. Although higher eukaryotes have three different
genes that code for DNA ligase (LIG1, LIG3, and LIG4), the
LIG4 gene product DNA ligase IV is the only DNA ligase
that can ligate DSB ends in the NHEJ reaction, and other
*Corresponding author: Phone: +81-45-787-2228,
Fax: +81-45-787-2228,
E-mail: kurosawa@yokohama-cu.ac.jp
†Corresponding author: Phone: +81-45-787-2228,
Fax: +81-45-787-2228,
E-mail: nadachi@yokohama-cu.ac.jp
1
Graduate School of Nanobioscience, Yokohama City University,
Yokohama, Japan;
2
Advanced Medical Research Center, Yokohama City
University, Yokohama, Japan.
doi:10.1269/jrr.10017
A. Kurosawa and N. Adachi
504
DNA ligases cannot substitute for the DNA ligase IV func-
tion.
7)
ARTEMIS (named after the Hellenic goddess of the hunt
who aids in childbirth) was identified as the gene responsible
for radiosensitive-severe combined immunodeficiency (RS-
SCID) or Athabascan SCID (SCIDA).
20–23)
In vitro and in
vivo studies have revealed that Artemis is the nuclease
required for the resolution of hairpin coding ends during
V(D)J recombination.
24,25)
As mentioned above, Artemis-
deficient cells display increased IR sensitivity, suggesting
that Artemis is also required for the NHEJ pathway of DSB
repair.
20)
Interestingly, recent work suggests that Artemis
may be involved in the regulation of the cell cycle check-
points as a downstream factor of ataxia telangiectasia
mutated (ATM) and/or the ATM- and Rad3-related kinase
(ATR).
26–30)
It is therefore possible that Artemis is a multi-
functional protein in the maintenance of genome integrity
and an important protein for understanding the mechanism
of DSB repair. In this review, we summarize the recent
progress on biological functions of Artemis in DSB repair
and DNA damage response.
BIOCHEMICAL AND STRUCTURAL
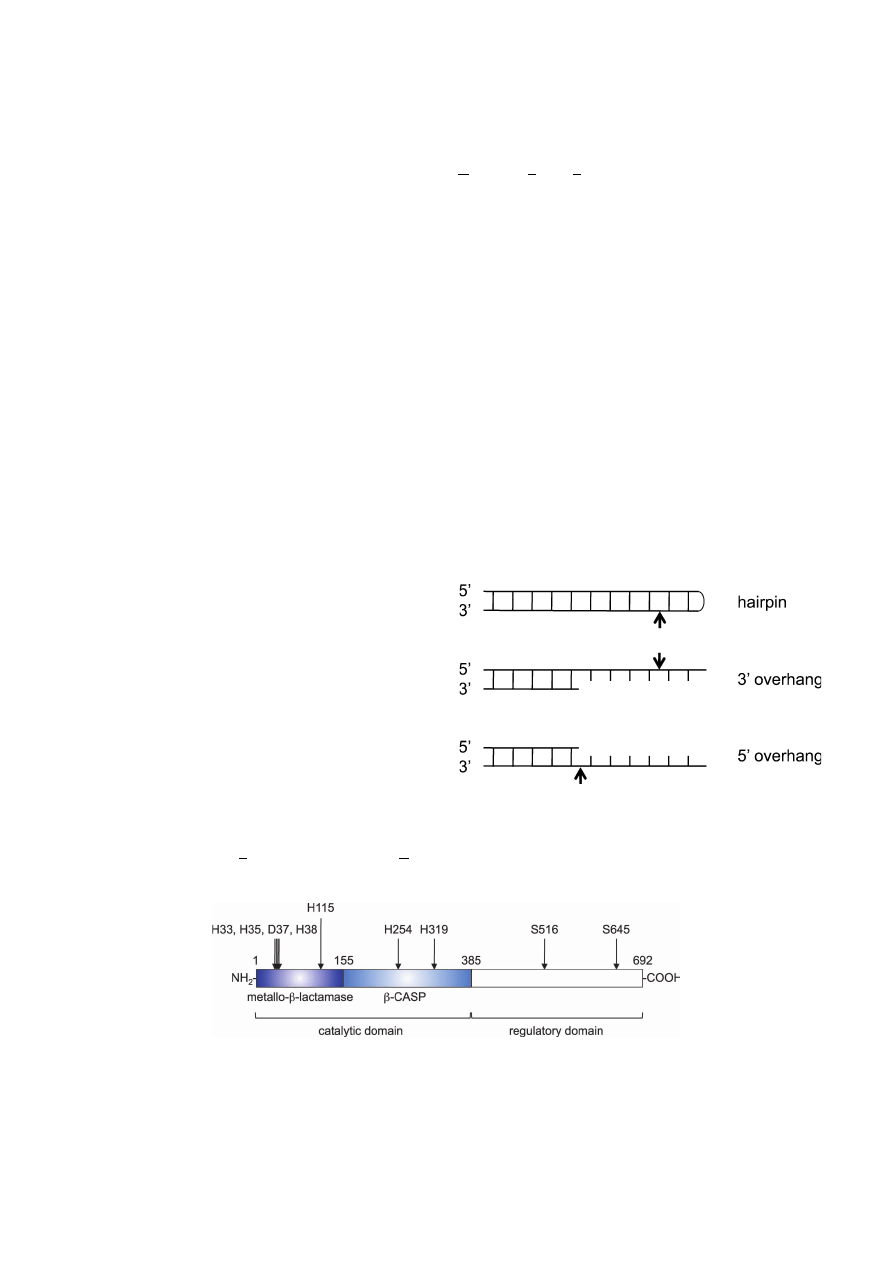
PROPERTIES OF ARTEMIS
Artemis has a ssDNA-specific 5’ to 3’ exonuclease act-
ivity and acquires an endonuclease activity when associated
with and phosphorylated by DNA-PKcs.
25)
The Artemis/
DNA-PKcs complex specifically cleaves boundary of
ssDNA and dsDNA and hairpin DNA, generating blunt or 3’
overhang DNA ends (Fig. 1).
25)
Although the three-dimen-
sional structure has not been determined yet, two domains
in the N-terminus of Artemis are shown to be important for
enzymatic activity.
31)
One of the domains is called the
metallo-
β-lactamase domain, amino acids 1–155 of human
Artemis, which is commonly observed in members of the
metallo-
β-lactamase superfamily (Fig. 2).
31)
Another domain,
amino acids 156–385 of human Artemis, is called the
β-
CASP domain (metallo-
β-lactamases-associated CPSF
ARTEMIS SNM1 PSO2). This domain is highly conserved
in other metallo-
β-lactamases that specifically act on nucleic
acids (Fig. 2).
31)
Recently, de Villartay et al. have reported
that a histidine residue within the
β-CASP domain (His254)
is critical for full activation of Artemis (Fig. 2).
32)
Although
the precise role of His254 is currently unclear, it is suggested
that His254 is involved in zinc binding.
32)
Pannicke et al.
have reported that aspartic acid residue 37 and histidine res-
idues 33, 35, 38, 115, and 319 directly coordinate two pro-
posed sites of metal (most likely Mg
2+
) binding (Fig. 2).
33)
While the N-terminus of Artemis is important for an enzy-
matic role, the C-terminus of Artemis appears to be a region
involved in the interaction with DNA-PKcs.
34,35)
Indeed,
Artemis is phosphorylated by DNA-PKcs only in the C-
terminal domain.
35)
Because the C-terminal domain is
dispensable for hairpin opening activity in V(D)J recombi-
nation in vivo,
35,36)
the phosphorylation of the C-terminus
may cause a conformational change, resulting in an activated
form of Artemis.
35)
On the other hand, Goodarzi et al.
reported that autophosphorylated DNA-PKcs recruits
Artemis to the sites of DSBs.
37)
As autophosphorylation of
Fig. 1.
Endonucleolytic properties of the Artemis/DNA-PKcs
complex. Shown are chematic structures of a hairpin end and DNA
ends with a 3’- or 5’-overhang. Arrows mark the major cleavage
sites.
Fig. 2.
Schematic representation of human Artemis. Human Artemis consists of 692 amino acids. The
metallo-
β-lactamase/β-CASP domain contains the active site of the enzyme. The C-terminus of this protein is
thought to be a regulatory domain. The positions of amino acid residues directly involved in metal ion-
binding (Asp37, His33, His35, His38, His115, and His319), playing a key role in the Artemis activity
(His254), and phosphorylated in response to DNA damage (Ser516 and Ser645) are indicated.
Functions and Regulation of Artemis
505
DNA-PKcs is also suggested to cause a conformational
change in DNA-PKcs, the conformational change of DNA-
PKcs rather than Artemis itself may be important for
Artemis activity. Taken together, Artemis protein is divided
into two functional domains, the N-terminal catalytic
domain and the C-terminal regulatory domain, and the reg-
ulation of endonuclease activity of Artemis may contribute
to preventing unnecessary DNA degradation (Fig. 3).
35)
ARTEMIS AND ITS RELATED PROTEINS
Artemis is a member of the SNM1 family, which is con-
stituted by gene products homologous to yeast SNM1, and
thus Artemis is often referred to as SNM1C. Yeast SNM1
possesses a 5’ to 3’ exonuclease activity, depending on its
catalytic domain.
38)
Genetic analysis has shown that SNM1
participates in the repair of interstrand cross-links (ICLs)
and that exonuclease activity of SNM1 is important for the
ICL repair.
38)
Interestingly, however, Artemis exonuclease
activity is independent of its catalytic domain,
33)
implying
that exonuclease activities of Artemis and SNM1 are func-
tionally unrelated. Consistent with this, fibroblast cell lines
established from RS-SCID/SCIDA patients do not exhibit
increased sensitivity to ICL-causing agents, though these
cells are hypersensitive to IR.
39,40)
Similarly, in chicken
DT40 cells, SNM1 is involved in ICL repair and not in DSB
repair, while Snm1c-deficient cells are sensitive to IR but not
to the ICL-causing agent cisplatin.
41)
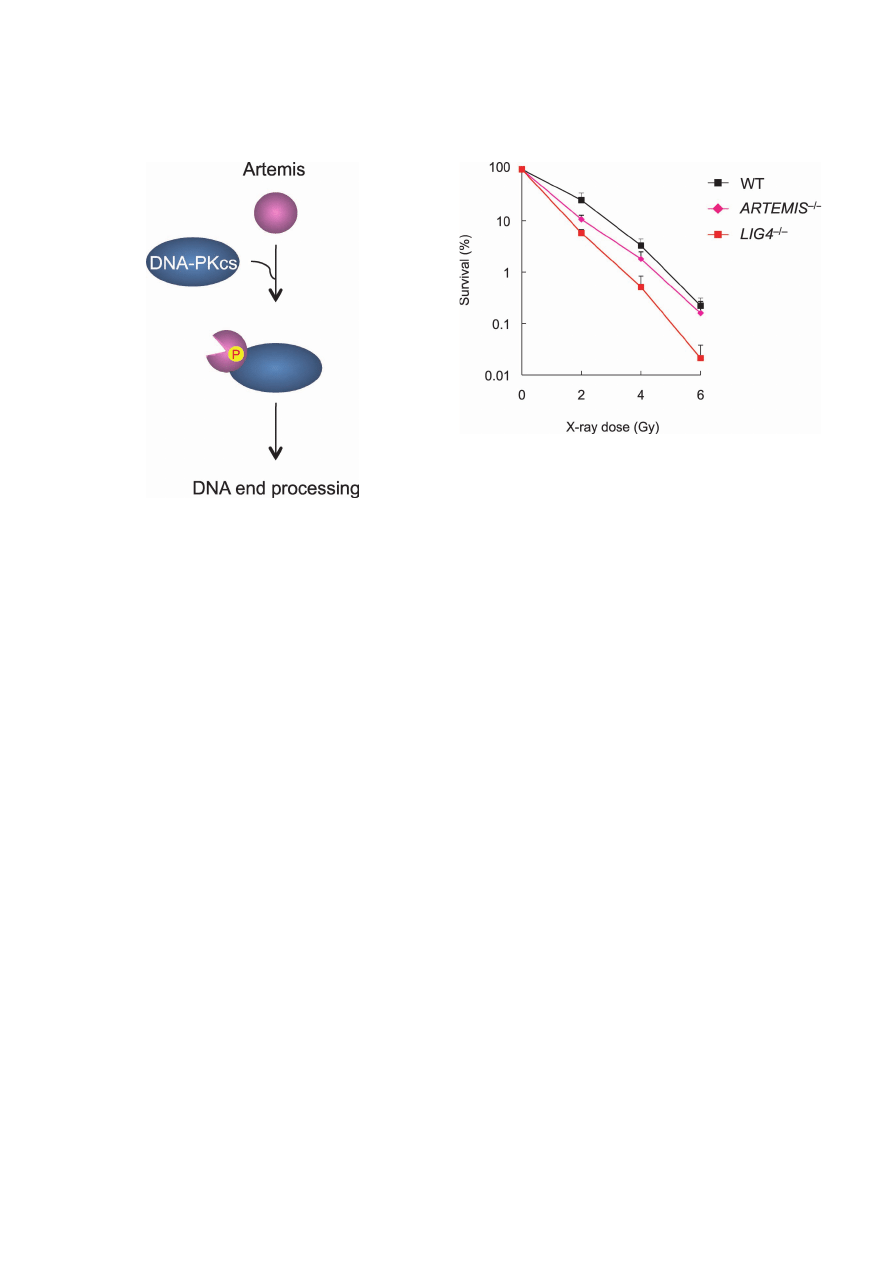
Recently, we per-
formed targeted disruption of the ARTEMIS gene in the
human pre-B cell line Nalm-6, and found that ARTEMIS
–/–
cells showed increased sensitivity to low-dose IR (Fig. 4),
but not to cisplatin.
42)
These findings are consistent with the
notion that Artemis is not involved in ICL repair, but is
involved in DSB repair by the NHEJ pathway. Indeed,
human ARTEMIS
–/–
cells displayed increased sensitivity to
etoposide, a potent topoisomerase II inhibitor that causes
DSBs. Importantly, however, the extent of etoposide sensi-
tivity of Artemis-deficient cells was much smaller than that
of cells lacking DNA ligase IV,
42,43)
suggesting a limited role
for Artemis in DSB repair by NHEJ. Intriguingly, Riballo et
al. have reported that Artemis is only required for the repair
of a subset (15%) of IR-induced DSBs, specifically those
repaired with slow kinetics and those located at regions of
heterochromatin.
27,44)
These findings provide important clues
to Artemis function, as it is suggested that the Artemis-
dependent DSB repair is ATM dependent (see below).
Another member of the SNM1 family, SNM1B, also pos-
sess a 5’ to 3’ exonuclease activity.
45,46)
SNM1B is referred
to as Apollo, as SNM1B is closely related to Artemis; Apollo
is the twin brother of Artemis in Hellenic mythology.
45,46)
Despite structural similarities between Apollo and Artemis,
it has been reported that Snm1b-deficient DT40 cells, unlike
Snm1c-deficient cells, display increased sensitivity to ICL-
generating agents, but not to IR, suggesting that Apollo is
involved in ICL repair and not in DSB repair.
41)
It is shown
in HEK293 cells that shRNA-mediated knockdown of
Apollo results in increased sensitivity to ICL-generating
agents.
47)
Interestingly, however, exonuclease activity of
Apollo appears to be dispensable for repairing ICLs. Since
Fig. 3.
Regulation of Artemis in NHEJ. When Artemis is associ-
ated with and phosphorylated by DNA-PKcs, a conformational
change occurs in Artemis, resulting in an activated form of
Artemis. The activated form of Artemis can create DNA ends suit-
able for ligation by DNA ligase IV.
Fig. 4.
Artemis is important for repairing low-dose X-ray-
induced DSBs in human lymphocytes. Sensitivities of Nalm-6
wild-type, ARTEMIS
–/–
cells and LIG4
–/–
cells to X-rays were deter-
mined by clonogenic survival assays. Shown are the mean
± SD of
three independent experiments. Where absent, error bars fall within
symbols.
A. Kurosawa and N. Adachi
506
Apollo interacts with the human telomeric protein TRF2 via
its N-terminal domain, Apollo may act to protect telomeres
from unwanted DNA repair.
45,46)
Thus, unlike Artemis,
SNM1 and Apollo are likely to be mainly involved in ICL
repair.
BIOCHEMICAL ROLE OF ARTEMIS IN NHEJ
As mentioned above, genetic analysis indicates the
involvement of Artemis in NHEJ.
41,42)
In vitro studies
showed that the Artemis/DNA-PKcs complex can generate
either blunt ends or 3’ overhangs of 2–4 bases.
25)
Therefore,
Artemis is believed to be involved in the end processing step
of NHEJ. As DNA ligase IV can ligate incompatible DNA
ends with 3’ overhangs in the presence of Ku,
48)
Artemis
may have a role in NHEJ only when end trimming is
necessary prior to ligation. For example, the Artemis/DNA-
PKcs complex may remove chemically modified termini.
Consistent with this idea, biochemical analysis revealed that
the Artemis/DNA-PKcs complex can convert such
chemically modified ends to a form suitable for ligation with
minimal loss of terminal sequence.
49,50)
Such chemically
modified DSBs are often induced by IR and other
radiomimetic agents.
51)
It is known that IR and other free
radicals induce DSBs with either 3’-phosphate or 3’-
phosphoglycolate termini,
52–55)
while radiomimetic enediyne
antibiotics, such as neocarzinostatin, induce 5’-aldehyde
termini.
54,55)
Thus, the end processing step mediated by an
endonuclease activity of the Artemis/DNA-PKcs complex
may be required for removing chemically modified termini.
Recently, Ma et al. have constructed a biochemically defined
system for mammalian NHEJ, and carefully examined the
joining of incompatible DNA ends.
56)
In that system, the
core NHEJ components, Ku70, Ku80, Artemis, DNA-PKcs,
DNA ligase IV, and XRCC4, were able to join incompatible
DNA ends.
56)
Interestingly, the length of the reaction prod-
ucts was affected by the presence of Artemis/DNA-PKcs,
consistent with a nucleolytic role of this complex in end
processing. Furthermore, recent studies have suggested that
the C-terminus of Ku80 is important not only for repairing
IR-induced DNA damage but also for Artemis-mediated
processing of DNA ends.
30)
Although it is possible that other
nucleases also participate in the processing reaction,
57–59)
the
Artemis/DNA-PKcs complex is likely to be a central
enzyme for the end processing step of NHEJ in higher
eukaryotes.
INVOLVEMENT OF ARTEMIS IN DNA
DAMAGE RESPONSE
It has been shown that ATM and ATR as well as DNA-PKcs
phosphorylate Artemis in response to DNA damage,
26–30,60)
and the phosphorylated Artemis physically associates with
the Mre11/Rad50/Nijmegen breakage syndrome 1 (Nbs1)
complex in an ATM-dependent manner.
28,29)
Remarkably,
Chen et al. revealed that serine residue 645 (Ser645) of
Artemis is phosphorylated in response to IR irradiation (Fig.
2).
29)
Several groups have investigated the relationship
between Artemis phosphorylation and cell cycle progres-
sion. Jeggo and coworkers reported that Artemis-deficient
cells had normal G2/M checkpoint and thus exhibited a pro-
longed G2/M arrest after IR irradiation; by contrast, Leger-
ski and coworkers presented data showing that Artemis was
required for normal G2/M arrest after IR irradiation.
28,60,61)
A possible explanation for this discrepancy could be that
those studies employed different cell lines (primary fibro-
blasts derived from SCIDA patients versus transformed 293
kidney cells depleted for Artemis). Alternatively, the dis-
crepancy may simply be due to the difference in cell cycle
phases when cells were irradiated in those studies (asynchro-
nous cells versus S phase-enriched cells).
The Legerski group also showed that Artemis is phospho-
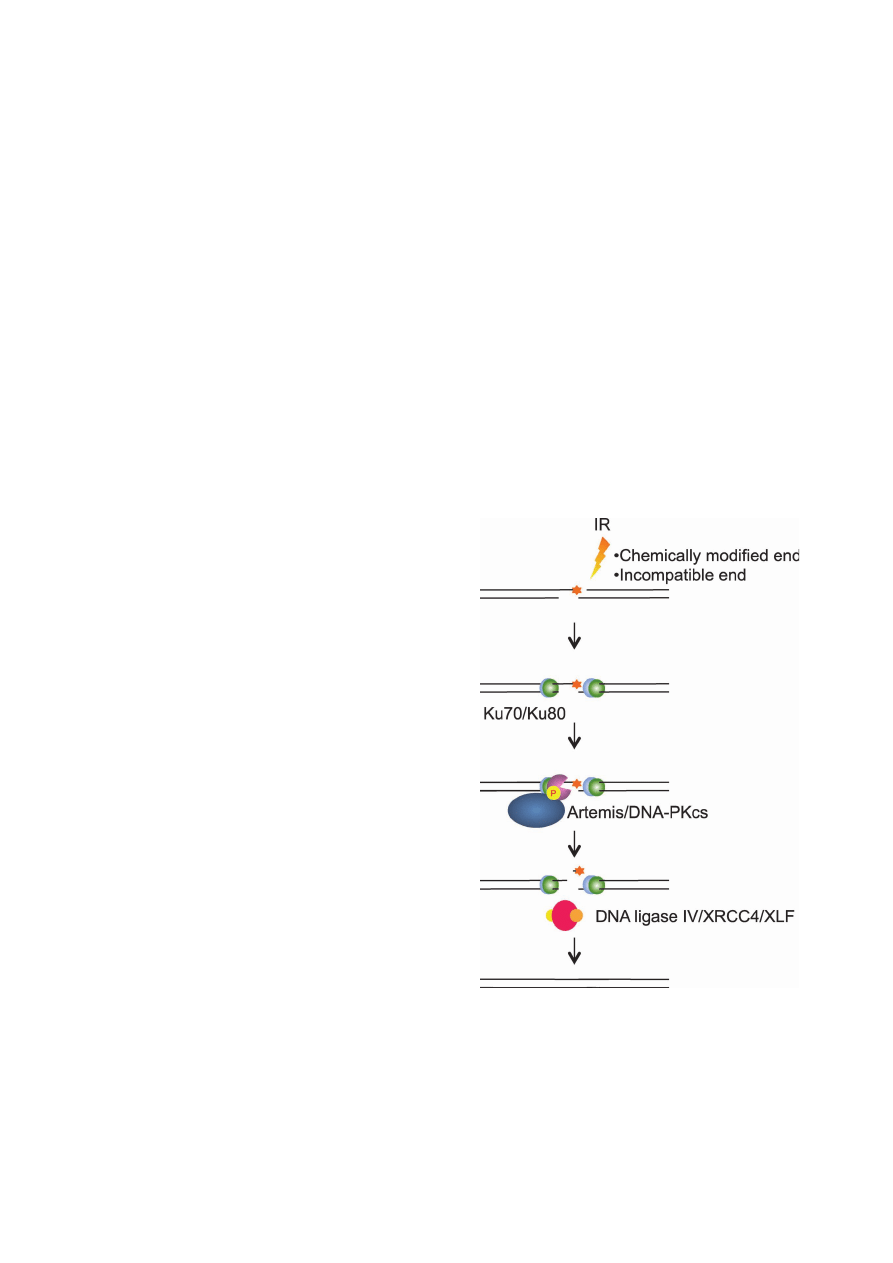
Fig. 5.
The Artemis/DNA-PKcs complex is required for generat-
ing ligatable DNA ends. IR and radiomimetic agents induce DSBs
with unligatable ends, such as 3’ phosphoglycolate termini. After
the Ku70/Ku80 complex immediately binds to DSB ends, the
Artemis/DNA-PKcs complex generates ligatable DNA ends with
minimal loss of nucleotides. Finally, the DNA ligase IV/XRCC4/
XLF complex ligates the DSB ends. When NHEJ proteins leave the
ligated DNA, NHEJ is completed.
Functions and Regulation of Artemis
507
rylated at Ser516 and Ser645 by ATR in response to UV
light (Fig. 2), and this phosphorylation is involved in the
recovery from S-phase arrest.
62)
In the ATM/ATR signaling
pathways, Chk1 and Chk2 kinases act as key downstream
regulators that phosphorylate various proteins involved in
cell cycle checkpoints after DNA damage.
63)
Intriguingly,
neither Chk1 nor Chk2 is involved in IR- and UV-induced
hyperphosphorylation of Artemis.
28,29)
These observations
suggest that Artemis is a direct downstream factor of the
ATM (and presumably ATR) signaling pathway. Consistent
with this notion, epistasis analysis using human fibroblasts
derived from ataxia telangiectasia patients and from
Artemis-deficient RS-SCID patients showed that ATM and
Artemis function in a common DSB repair pathway.
27,64)
Additionally, this pathway requires H2AX, 53BP1 and
DNA-PKcs, as well as Mre11 and Nbs1.
27)
Therefore,
Ser645 phosphorylation may trigger a conformational
change in Artemis that enables its physical interaction with
the Mre11/Rad50/Nbs1 complex. A genetic interaction
between 53BP1 and Artemis has been shown in mammalian
cells, consistent with the observation that Artemis physically
interacts with 53BP1,
27,65)
though in chicken DT40 cells
53BP1 reported to play a role in a pathway distinct from the
Artemis-dependent ATM pathway.
64)
Together, these obser-
vations strongly suggest that Artemis acts as a genome care-
taker to suppress genome instability, by regulating cell cycle
progression in concert with ATM and its downstream factors
(Fig. 6). As ATM triggers apoptosis via phosphorylation of
p53 at Ser15,
66)
it is possible that Artemis may be involved
in the p53-dependent apoptosis signaling pathway. Interest-
ingly, however, it was recently reported that Artemis may act
as a negative regulator of p53.
67)
Specifically, after oxidative
stress, Artemis knockdown led to a spontaneous activation
of p53 via phosphorylation at Ser15 and Ser37, resulting in
G1 arrest and subsequent apoptosis.
67)
Although the bio-
logical significance of such Artemis function is currently
unclear, this finding suggests the possibility that Artemis
may signal to p53 under certain conditions. Further analysis
will clarify the exact relationship between Artemis and the
p53 pathway.
In this review, we have described the biochemical proper-
ties of Artemis and its biological functions in light of the
maintenance of genome integrity. It is conceivable that
Artemis acts at two distinct levels; one at the end processing
step of NHEJ and the other at the signaling pathway of cell
cycle progression. In either case, such Artemis functions are
likely to be regulated through phosphorylation.
27,28,35,60,66)
Since Artemis does not exist in yeast, it is reasonable to
speculate that Artemis contributes to efficient DSB repair in
higher eukaryotes. For instance, the Artemis/DNA-PKcs
complex may serve to create DNA ends that are preferentially
rejoined by DNA ligase IV, resulting in prompt DSB repair
with minimal loss of nucleotides. Further analysis of
Artemis function will provide detailed information about the
mechanism that ensures the integrity of the genome in higher
eukaryotes.
ACKNOWLEDGEMENTS
We thank the Editor-In-Chief of the Journal of Radiation
Research, Dr. Yoshiya Furusawa, for giving us the oppor-
tunity to write this review article.
REFERENCES
1. Chu G (1997) Double strand break repair. J Biol Chem 272:
24097–24100.
2. Kanaar R, Hoeijmakers JH and van Gent DC (1998) Mole-
cular mechanisms of DNA double strand break repair. Trends
Cell Biol 8: 483–489.
3. Critchlow SE and Jackson SP (1998) DNA end-joining: from
yeast to man. Trends Biochem Sci 23: 394–398.
4. Liang F, et al (1998) Homology-directed repair is a major
double-strand break repair pathway in mammalian cells. Proc
Natl Acad Sci USA 95: 5172–5177.
5. Lieber MR (1999) The biochemistry and biological signifi-
cance of nonhomologous DNA end joining: an essential repair
process in multicellular eukaryotes. Genes Cells 4: 77–85.
6. Takata M, et al (1998) Homologous recombination and non-
homologous end-joining pathways of DNA double-strand
break repair have overlapping roles in the maintenance of
chromosomal integrity in vertebrate cells. EMBO J 17: 5497–
5508.
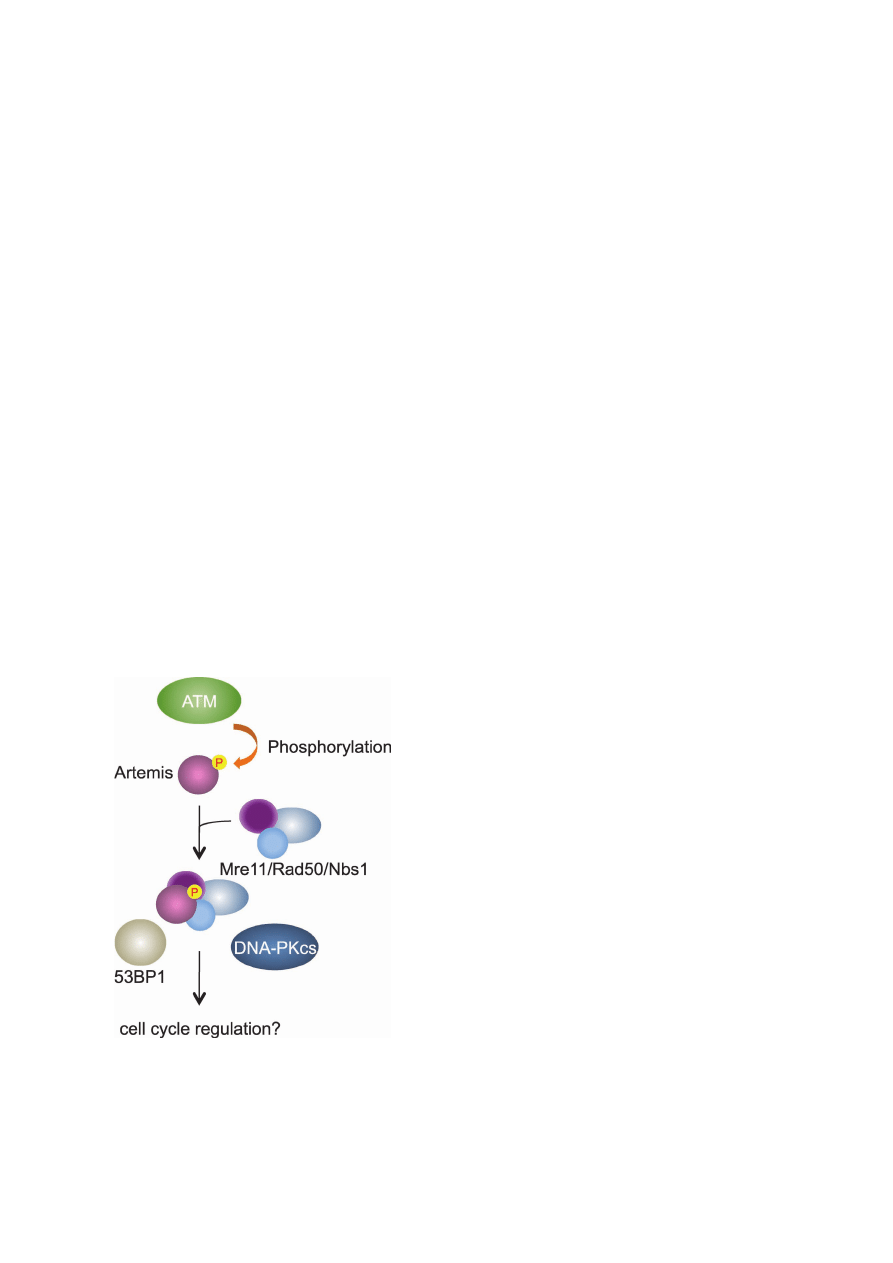
Fig. 6.
Artemis is a downstream factor of the ATM signaling
pathway. ATM phosphorylates Artemis at Ser645 in response to
DNA damage. The phosphorylated Artemis physically associates
with the Mre11/Rad50/Nbs1 complex to participate in the regula-
tion of cell cycle progression.

A. Kurosawa and N. Adachi
508
7. Grawunder U, et al (1998) DNA ligase IV is essential for
V(D)J recombination and DNA double-strand break repair in
human precursor lymphocytes. Mol Cell 2: 477–484.
8. Adachi N, et al (2001) DNA ligase IV-deficient cells are more
resistant to ionizing radiation in the absence of Ku70: Impli-
cations for DNA double-strand break repair. Proc Natl Acad
Sci USA 98: 12109–12113.
9. Frank KM, et al (1998) Late embryonic lethality and impaired
V(D)J recombination in mice lacking DNA ligase IV. Nature
396
: 173–177.
10. Riballo E, et al (1999) Identification of a defect in DNA ligase
IV in a radiosensitive leukaemia patient. Curr Biol 9: 699–
702.
11. Sado K, et al (2001) Identification of a mutated DNA ligase
IV gene in the X-ray-hypersensitive mutant SX10 of mouse
FM3A cells. J Biol Chem 276: 9742–9748.
12. Gu Y, et al (1997) Ku70-deficient embryonic stem cells have
increased ionizing radiosensitivity, defective DNA end-bind-
ing activity, and inability to support V(D)J recombination.
Proc Natl Acad Sci USA 94: 8076–8081.
13. Fukushima T, et al (2001) Genetic analysis of the DNA-
dependent protein kinase reveals an inhibitory role of Ku in
late S-G2 phase DNA double-strand break repair. J Biol Chem
276
: 44413–44418.
14. Lieber MR (2008) The mechanism of human nonhomologous
DNA end joining. J Biol Chem 283: 1–5.
15. DeFazio LG, et al (2002) Synapsis of DNA ends by DNA-
dependent protein kinase. EMBO J 21: 3192–3200.
16. Ramsden DA and Gellert M (1998) Ku protein stimulates
DNA end joining by mammalian DNA ligases: a direct role
for Ku in repair of DNA double-strand breaks. EMBO J 17:
609–614.
17. Ellenberger T and Tomkinson AE (2008) Eukaryotic DNA
ligases: structural and functional insights. Annu Rev Biochem
77
: 313–338.
18. Ma Y, et al (2005) Repair of double-strand DNA breaks by
the human nonhomologous DNA end joining pathway: the
iterative processing model. Cell Cycle 4: 1193–1200.
19. Chappell C, et al (2002) Involvement of human polynucle-
otide kinase in double-strand break repair by non-homologous
end joining. EMBO J 21: 2827–2832.
20. Moshous D, et al (2000) A new gene involved in DNA
double-strand break repair and V(D)J recombination is located
on human chromosome 10p. Hum Mol Genet 9: 583–588.
21. Moshous D, et al (2001) Artemis, a novel DNA double-strand
break repair/V(D)J recombination protein, is mutated in
human severe combined immune deficiency. Cell 105: 177–
186.
22. Jones JF, et al (1991) Severe combined immunodeficiency
among the Navajo. I. Characterization of phenotypes, epide-
miology, and population genetics. Hum Biol 63: 669–682.
23. Li L, et al (2002) A founder mutation in Artemis, an SNM1-
like protein, causes SCID in Athabascan-speaking Native
Americans. J Immunol 168: 6323–6329.
24. Li L, et al (2005) Targeted disruption of the Artemis murine
counterpart results in SCID and defective V(D)J recombina-
tion that is partially corrected with bone marrow transplanta-
tion. J Immunol 174: 2420–2428.
25. Ma Y, et al (2002) Hairpin opening and overhang processing
by an Artemis/DNA-dependent protein kinase complex in
nonhomologous end joining and V(D)J recombination. Cell
108
: 781–794.
26. Poinsignon C, et al (2004) Phosphorylation of Artemis fol-
lowing irradiation-induced DNA damage. Eur J Immunol 34:
3146–3155.
27. Riballo E, et al (2004) A pathway of double-strand break
rejoining dependent upon ATM, Artemis, and proteins locat-
ing to gamma-H2AX foci. Mol Cell 16: 715–724.
28. Zhang X, et al (2004) Artemis is a phosphorylation target of
ATM and ATR and is involved in the G2/M DNA damage
checkpoint response. Mol Cell Biol 24: 9207–9220.
29. Chen L, et al (2005) Ataxia-telangiectasia-mutated dependent
phosphorylation of Artemis in response to DNA damage.
Cancer Sci 96: 134 –141.
30. Weterings E, et al (2009) The Ku80 carboxy terminus stimu-
lates joining and Artemis-mediated processing of DNA ends.
Mol Cell Biol 29: 1134 –1142.
31. Callebaut I, et al (2002) Metallo-beta-lactamase fold within
nucleic acids processing enzymes: the beta-CASP family.
Nucleic Acids Res 30: 3592–3601.
32. de Villartay JP, et al (2009) A histidine in the beta-CASP
domain of Artemis is critical for its full in vitro and in vivo
functions. DNA Repair (Amst) 8: 202–208.
33. Pannicke U, et al (2004) Functional and biochemical dissec-
tion of the structure-specific nuclease ARTEMIS. EMBO J
23
: 1987–1997.
34. Niewolik D, et al (2006) DNA-PKcs dependence of Artemis
endonucleolytic activity, differences between hairpins and 5’
or 3’ overhangs. J Biol Chem 281: 33900–33909.
35. Ma Y, et al (2005) The DNA-dependent protein kinase cata-
lytic subunit phosphorylation sites in human Artemis. J Biol
Chem 280: 33839–33846.
36. Poinsignon C, et al (2004) The metallo-beta-lactamase/beta-
CASP domain of Artemis constitutes the catalytic core for
V(D)J recombination. J Exp Med 199: 315–321.
37. Goodarzi AA, et al (2006) DNA-PK autophosphorylation
facilitates Artemis endonuclease activity. EMBO J 25: 3880–
3889.
38. Li X, Hejna J and Moses RE (2005) The yeast Snm1 protein
is a DNA 5’-exonuclease. DNA Repair (Amst) 4: 163–170.
39. Nicolas N, et al (1998) A human severe combined immuno-
deficiency (SCID) condition with increased sensitivity to ion-
izing radiations and impaired V(D)J rearrangements defines a
new DNA recombination/repair deficiency. J Exp Med 188:
627–634.
40. Musio A, et al (2005) Damaging-agent sensitivity of Artemis-
deficient cell lines. Eur J Immunol 35: 1250–1256.
41. Ishiai M, et al (2004) DNA cross-link repair protein SNM1A
interacts with PIAS1 in nuclear focus formation. Mol Cell
Biol 24: 10733–10741.
42. Kurosawa A, et al (2008) The requirement of Artemis in
double-strand break repair depends on the type of DNA dam-
age. DNA Cell Biol 27: 55–61.
43. Adachi N, et al (2003) Hypersensitivity of nonhomologous
DNA end-joining mutants to VP-16 and ICRF-193: implica-
tions for the repair of topoisomerase II-mediated DNA dam-

Functions and Regulation of Artemis
509
age. J Biol Chem 278: 35897–35902.
44. Goodarzi AA, Noon AT and Jeggo PA (2009) The impact of
heterochromatin on DSB repair. Biochem Soc Trans 37: 569–
576.
45. Lenain C, et al (2006) The Apollo 5’ exonuclease functions
together with TRF2 to protect telomeres from DNA repair.
Curr Biol 16: 1303–1310.
46. van Overbeek M and de Lange T (2006) Apollo, an Artemis-
related nuclease, interacts with TRF2 and protects human
telomeres in S phase. Curr Biol 16: 1295–1302.
47. Bae JB, et al (2008) Snm1B/Apollo mediates replication fork
collapse and S phase checkpoint activation in response to
DNA interstrand cross-links. Oncogene 27: 5045–5056.
48. Gu J, et al (2007) XRCC4:DNA ligase IV can ligate incom-
patible DNA ends and can ligate across gaps. EMBO J 26:
1010–1023.
49. Yannone SM, et al (2008) Coordinate 5’ and 3’ endonucle-
olytic trimming of terminally blocked blunt DNA double-
strand break ends by Artemis nuclease and DNA-dependent
protein kinase. Nucleic Acids Res 36: 3354 –3365.
50. Povirk LF, et al (2007) Processing of 3’-phosphoglycolate-
terminated DNA double strand breaks by Artemis nuclease. J
Biol Chem 282: 3547–3558.
51. Povirk LF (2006) Biochemical mechanisms of chromosomal
translocations resulting from DNA double-strand breaks.
DNA Repair (Amst) 5: 1199–1212.
52. Bertoncini CR and Meneghini R (1995) DNA strand breaks
produced by oxidative stress in mammalian cells exhibit 3’-
phosphoglycolate termini. Nucleic Acids Res 23: 2995–3002.
53. Henner WD, Grunberg SM and Haseltine WA (1983) Enzyme
action at 3’ termini of ionizing radiation-induced DNA strand
breaks. J Biol Chem 258: 15198–15205.
54. Dedon PC and Goldberg IH (1992) Free-radical mechanisms
involved in the formation of sequence-dependent bistranded
DNA lesions by the antitumor antibiotics bleomycin, neo-
carzinostatin, and calicheamicin. Chem Res Toxicol 5: 311–
332.
55. Povirk LF (1996) DNA damage and mutagenesis by radiomi-
metic DNA-cleaving agents: bleomycin, neocarzinostatin and
other enediynes. Mutat Res 355: 71–89.
56. Ma Y, et al (2004) A biochemically defined system for mam-
malian nonhomologous DNA end joining. Mol Cell 16: 701–
713.
57. Macrae CJ, et al (2008) APLF (C2orf13) facilitates nonho-
mologous end-joining and undergoes ATM-dependent hyper-
phosphorylation following ionizing radiation. DNA Repair
(Amst) 7: 292–302.
58. Mimitou EP and Symington LS (2009) DNA end resection:
many nucleases make light work. DNA Repair (Amst) 8: 983–
995.
59. Zhou T, et al (2009) Tyrosyl-DNA phosphodiesterase and the
repair of 3’-phosphoglycolate-terminated DNA double-strand
breaks. DNA Repair (Amst) 8: 901–911.
60. Krempler A, et al (2007) An imperfect G
2
/M checkpoint con-
tributes to chromosome instability following irradiation of S
and G
2
phase cells. Cell Cycle 6: 1682–1686.
61. Geng L, et al (2007) Artemis links ATM to G2/M checkpoint
recovery via regulation of Cdk1-cyclin B. Mol Cell Biol 27:
2625–2635.
62. Wang H, et al (2009) Artemis regulates cell cycle recovery
from the S phase checkpoint by promoting degradation of
cyclin E. J Biol Chem 284: 18236–18243.
63. Chen Y and Poon RY (2008) The multiple checkpoint func-
tions of CHK1 and CHK2 in maintenance of genome stability.
Front Biosci 13: 5016–5029.
64. Iwabuchi K, et al (2006) 53BP1 contributes to survival of cells
irradiated with X-ray during G1 without Ku70 or Artemis.
Genes Cells 11: 935–948.
65. Nakamura K, et al (2006) Genetic dissection of vertebrate
53BP1: a major role in non-homologous end joining of DNA
double strand breaks. DNA Repair (Amst) 5: 741–749.
66. Kurz EU and Lees-Miller SP (2004) DNA damage-induced
activation of ATM and ATM-dependent signaling pathways.
DNA Repair (Amst) 3: 889–900.
67. Zhang X, et al (2009) Artemis is a negative regulator of p53
in response to oxidative stress. Oncogene 28: 2196–2204.
Received on February 10, 2010
Revision received on March 19, 2010
Accepted on March 20, 2010
J-STAGE Advance Publication Date: June 11, 2010
Wyszukiwarka
Podobne podstrony:
spis lab I sem 2010
2010 ZMP studenci
W4 2010
wyklad 14 15 2010
W 8 Hormony 2010 2011
RI 12 2010 wspolczesne koncepcje
2009 2010 Autorytet
wyklad 2 2010
Wykład 3 powtórzenie 2010 studenci (1)
PD W1 Wprowadzenie do PD(2010 10 02) 1 1
BIOMATERIALY IV 2010
spis wykład I sem 2010
Wykład 5 2010 studenci
Wykład 5 2010 studenci ppt
BLS 2010 stom [konspekt]ppt
BUZA 2010 UFPakt
więcej podobnych podstron