
© 2013 Wichtig Editore - ISSN 0391-3988
Int J Artif Organs
(
2013;
:
7 ) 506-517
36
506
Immobilization of BMP-2 on a nano-hydroxyapatite-
coated titanium surface using a chitosan calcium
chelating agent
Sung-Hyun Kim
1
, Jung-Keug Park
1
, Kug-Sun Hong
2
, Hyun-Suk Jung
3
, Young-Kwon Seo
1
1
Department of Medical Biotechnology, Dongguk University, Seoul - Korea
2
Department of Materials Science and Engineering, Seoul National University, Shillim-dong, Kwanak-gu, Seoul - Korea
3
School of Advanced Materials Science and Engineering, Sungkyunkwan University, Suwon - Korea
Department of Medical Biotechnology, Dongguk University, Seoul - Korea
Department of Medical Biotechnology, Dongguk University, Seoul - Korea
Department of Materials Science and Engineering, Seoul National University, Shillim-dong, Kwanak-gu, Seoul - Korea
School of Advanced Materials Science and Engineering, Sungkyunkwan University, Suwon - Korea
Department of Medical Biotechnology, Dongguk University, Seoul - Korea
We conducted experiments to determine the most effective calcium chelating agents for use in en-
hancing adhesion of human bone marrow mesenchymal stem cells (BM-MSCs) on nano-hydroxyap-
atite (nHAp)-coated titanium substrates by covalently immobilizing bone morphogenetic protein-2
(BMP-2). The quantity of amine groups on the chitosan chelated surface was 7 µg/surface area, and
it was 1.4 µg/surface area on the alendronate chelated surface. The quantity of BMP-2 on the BMP-2
immobilized surface chelated with chitosan (4 ng/surface area) was higher than that on BMP-2 immo-
bilized surface chelated with alendronate (2.2 ng/surface area). Contact angles of the nHAp-coated
titanium, alendronate chelated, chitosan chelated, and BMP-2 immobilized surfaces chelated with
alendronate were 68.8 ± 3.6°, 78.2 ± 1.9°, 74.8 ± 5.2°, and 76.0 ± 2.5°, respectively. The contact
angle of the BMP-2 immobilized surface chelated with chitosan was significantly lower (56.2 ± 2.0°)
than that of any of the other groups. BM-MSCs on the chitosan surface and BMP-2 immobilized on
the surface chelated with chitosan appeared to be healthy and showed a spindle-like fibroblastic
morphology. In addition, BM-MSCs on these surfaces appeared to have the ability to differentiate
into bone-forming cells. We suggest that chitosan can be used as an effective calcium chelating
agent for implants.
Keywords: Titanium, Nano-hydroxyapatite, Bone morphogenetic protein-2, Chitosan, Osseointegration
Accepted: February 20, 2013
original article
DOI: 10.5301/ijao.5000215
INTRODUCTION
It is essential to maintain a stable bone-biomaterial inter-
face to ensure long-term success of an endosseous den-
tal implant. Early osseointegration and biocompatibility in
the location of the implant are associated with long-term
success (1). Metal prostheses have excellent mechanical
properties (2); however, osseointegration and biocompat-
ibility are dependent on biomolecules that enhance bone
regeneration. In addition, it has been revealed that the
use of bone cement for permanent bone implantation
presents several problems (3).
Interest in surface modification methods to stimulate cell
function at the bone-implant interface has increased (4).
The key part of a clinical implant application is to immo-
bilize biomolecules on biomaterials, and this has been a
widely researched approach to modify metal surfaces to
control cell and tissue responses (5). Hence, delivering
growth factors to the bone-implant interface using implant
coating techniques has recently become a popular method
to control healing and fixation of implants (6). Adsorption
of bone morphogenetic protein-2 (BMP-2) on the surface
of titanium or hydroxyapatite (HAp) ceramics results in
intense acceleration of implant osseointegration (7-9).

© 2013 Wichtig Editore - ISSN 0391-3988
507
Kim et al
human or mouse fibroblasts in tissue cell culture (20).
Chitosan (C
6
H
13
NO
5
) has chelating ability toward metal
and calcium ions, since the calcium chelating effect oc-
curs with the amine group of the chitosan molecule (21).
In our study, we evaluated the possibility of using chito-
san as a novel calcium chelating agent for immobilizing
BMP-2 by culturing bone marrow mesenchymal stem
cells (BM-MSCs) and Jurkat cells on a modified titanium
surface to test biocompatibility.
MATERIALS AND METHODS
Materials
Grade 4 titanium discs were used. The triethyl phosphate
(P(OC
2
H
5
)
3
) solution, propionic acid, and calcium ethox-
ide (Ca(OC
2
H
5
)
2
) powder were obtained from the Elec-
tronic Functional Materials Laboratory of Seoul National
University. 4-amino-1-hydroxy-1-phosphonobutyl phos-
phonic acid monosodium (alendronate sodium trihydrate)
was purchased from Sigma-Aldrich Chemical (St Louis,
MO, USA). Chitosan and Protosan UP CL 213 were pur-
chased from Novametrix (Brakeroya, Drammen, Norway).
Human BMP-2 was a gift from Daewoong Research and
Development, Daewoong Pharmaceutical Co., Ltd (Seoul,
Korea).
Preparation of titanium discs
The diameters of the titanium discs used in this experiment
were 8 and 14 mm (Fig. 1), and the size of titanium foil
was 5 cm × 5 cm. The titanium discs and foil were coated
with nHAp using a spin-coater. The nHAp solution was
Osteoconductive calcium phosphate, which is mainly com-
posed of HAp, is an attractive and typical biocompatible
ceramic material used in prosthetic devices and has re-
ceived much attention for its application as an endosse-
ous dental implant (10-12). HAp, Ca
10
(PO
4
)
6
(OH)
2
, was first
established as the mineral component of bone in 1926 by
DeJong, and synthetic HAp was approved as a biomate-
rial for use in orthopedics, bone grafts, and dentistry about
35 years ago (13). HAp can enhance osseointegration,
but it is brittle, which restricts its use in endosseous den-
tal applications (14-15). The technique of HAp coating by
methods such as plasma spray, sputtering, electrolysis,
and sol-gel have been studied to overcome this defect.
Effective coating of a load-bearing substrate with HAp can
overcome the physical weakness of HAp (13).
However, despite the enormous benefit of biomimetic
coatings on a titanium surface, proteins such as BMP-2,
which is pre-adsorbed onto the material surface, may be
insufficiently immobilized and release may be uncontrolled
due to a lack of functional groups. Covalent immobiliza-
tion on the material surface achieves prolonged retention
of BMP-2 and growth factors at their site of action. A two-
step, zero length, cross-linking strategy can be applied
to covalently immobilize BMP-2 by exposing the amino-
functionalized ceramic discs to a cross-linker (16). A solu-
tion to this problem may be the use of calcium chelating
techniques that provide amine groups needed for covalent
attachment of proteins with the cross-linker (17).
Many calcium chelating agents have been researched for
calcium chelating technique applications. Pamidronate
(C
3
H
11
NO
7
P
2
) and alendronate (C
4
H
18
NNaO
10
P
2
) have been
used widely by researchers to evaluate the suitability of
this approach (16). In addition, EDTA (C
10
H
16
N
2
O
8
) is an ef-
fective calcium chelating agent as it is a single hexadentate
chelon. Due to the lone pairs of electrons, the four oxygen
atoms in the four carboxyl groups and two nitrogen atoms
chelate with the metal and calcium ions (18). However,
due to their cytotoxic properties, bisphosphonates such
as pamidronate and alendronate can cause apoptosis in a
variety of cell types in vitro (19).
In this study, we investigated the effectiveness of calcium
chelating agents for providing amine groups to immobilize
protein on nano-hydroxyapatite (nHAp) surfaces. We ad-
opted natural chelating material that is non-cytotoxic, has
excellent biocompatibility, and immobilizes BMP-2. Chito-
san, which is widely used in various forms of biomaterials,
shows good biocompatibility and no cytotoxicity in either
Fig. 1 - 8 mm and 14 mm nano-hydroxyapatite (nHAp)-coated
titanium discs. Sample
(1) is the 14 mm cont nHAp group disc,
(2) is the same size as that of the alen group disc, and (3) is the same
size as that of the chito group discs, respectively. Sample
(4) is the
8 mm cont nHAp group disc,
(5) is the same size as that of the alen
group discs, and
(6) is the same size as that of the chito group discs,
respectively.

© 2013 Wichtig Editore - ISSN 0391-3988
508
Immobilization of BMP-2 using a chitosan calcium chelating agent
prepared inside a glove box that was purged with argon
gas (99.999%). P(OC
2
H
5
)
3
liquid was diluted with propionic
acid to produce the nHAp solution, then Ca(OC
2
H
5
)
2
pow-
der was dissolved with propionic acid as a solvent. Two
bottles of each solution were stored separately in the glove
box and stirred until absolutely dissolved. The two bottles
of each solution were then mixed in a 1:1 ratio in the glove
box for 10 min. Afterwards, the mixed solution was stirred
at a temperature of 60°C in a water bath for 6 h. The nHAp
solution was spin-coated onto the titanium discs with a
spinning velocity of 4000 rpm and a 20 s spin coating time.
nHAp spin-coated titanium discs were then sintered in a
sintering oven at a temperature of 500°C for 2 h.
Calcium chelating agent treatment
The nHAp coated 8 mm and 14 mm discs and the 5 cm ×
5 cm titanium foil were placed in 12-well plates containing
2 mL of a 1 mg/ml alendronate or chitosan aqueous solu-
tion. The titanium discs were then totally immersed in solu-
tion. The well plates were shaken at room temperature for
4 h on an orbital shaker set at 20 rpm in the dark. The tita-
nium samples were washed three times with distilled water,
dried, and stored under vacuum until further use.
Immobilization of BMP-2
The concentration of human BMP-2 stock solution was
1 μg/ml in phosphate-buffered saline (PBS). BMP-2 was
dispersed on the nHAp coated titanium surfaces, which
had been treated with each of the calcium chelating
agents. Before BMP-2 treatment, human BMP-2 stock
solution and cross-linking solution were mixed for 5 h
at a 1:1 ratio to achieve efficient covalent immobilization
of BMP-2 on the calcium chelated nHAp coating sur-
face. The cross-linking solution was composed of 20 mL
40% (v/v) ethanol and 50 mM MES (pH 5.5), 24 mM 1-eth-
yl-3-(3-dimethyl aminopropyl)carbodiimide (Fluka Chemic
AG, Milwaukee, WI, USA) and 5 mM N-hydroxysuccin-
imide (Fluka Chemic AG). The diluted human BMP-2
solution was 0.5 μg/ml. A 10 μL aliquot of the diluted hu-
man BMP-2 solution was loaded onto the 8 mm titanium
disc surfaces, 30 μL was loaded onto the 14 mm titanium
disc surfaces, and 500 μL was added to the 5 cm × 5 cm
titanium foil surfaces. All samples were placed overnight
at room temperature, washed five times with distilled water,
and dried at room temperature. We compared the nHAp-
coated titanium surface (cont nHAp group), the alendro-
nate chelated cont nHAp group (alen group), the BMP-2
immobilized alen group (alen + BMP group), the chitosan
chelated cont nHAp group (chito group), and the BMP-2
immobilized chito group (chito + BMP group).
Amine assay
The surface density of the amine groups introduced onto
the 8 mm calcium chelated titanium discs was quantified
by reaction with 2,4,6-trinitrobenzenesulfonic acid (TNBS),
which interacts with primary amine groups to form trinitro-
phenyl derivatives (22). The amine assay was performed ac-
cording to the method of Puleo (5). Titanium samples were
incubated with 0.1% TNBS in 3% sodium borate at 70°C for
5 min, followed by washing with triple distilled water, then
hydrolyzed with 1 N NaOH at 70°C for 10 min. These reac-
tions provided a yellow color that was proportional to the
number of trinitrophenyl groups, which was proportional to
the number of amine groups. Absorbance of hydrolysate
was detected at 410 nm. Standard curves were prepared
by adding L-DOPA (Sigma-Aldrich) in 0.1% TNBS in 3% so-
dium borate, followed by a serial dilution. The standard was
then hydrolyzed with 1 N NaOH at 70°C for 10 min.
BMP-2 assay
The BMP-2 assay was performed using the Human BMP-2
Super X-ELISA kit (Antigenix America, Huntington Station,
NY, USA). BMP-2 immobilized on the 8 mm nHAp-coated
titanium discs and other titanium discs were placed in
48-well plates. These titanium discs were then immersed
in 1 mL of 0.1 mg/ml bovine serum albumin (BSA) solu-
tion and incubated at room temperature for 2 h in the dark.
Then, the samples were washed four times with the wash
buffer provided in the kit. A 20 μL aliquot of the 0.5 μg/ml
biotin-labeled tracer (tracer antibody) stock solution was
loaded onto each titanium disc surface, and the titanium
discs were incubated at room temperature for 2 h in the
dark. Samples were then washed four times again using
the wash buffer. The streptavidin-HRP conjugate solu-
tion was diluted with diluent, 0.05% Tween-20 (Uniqema,
Wilmington, DE, USA), and 0.1% BSA in PBS, at a ratio of
1:500. A 20 μL aliquot of the diluted streptavidin-HRP con-
jugate solution was loaded onto each titanium disc surface,
and the titanium discs were incubated at room tempera-
ture for 30 min in the dark. The samples were then washed

© 2013 Wichtig Editore - ISSN 0391-3988
509
Kim et al
four times again using the wash buffer provided in the kit.
Titanium discs were placed upside down in another 48-well
plate. TMB substrate solutions A and B were mixed at a 1:1
ratio. A 100 μL aliquot of TMB substrate mixed solution was
added to the 48-well plates, followed by a 30-min incuba-
tion at room temperature in the dark. After completing the
incubation, 100 μL of stop solution (2 N sulphuric acid) was
added, and absorbance was measured at 450 nm.
Contact angle measurement
A contact angle measurement device (Tensiometer/Pen-
dant Drop, Model DSA100; KRUSS Advancing Surface
Science, Hamburg, Germany) was used to measure wa-
ter contact angles. The Drop Shape Analysis software was
used to estimate the contact angle of various nHAp-coat-
ed titanium surfaces. Water contact angles were measured
on dry surfaces.
BM-MSC culture on surface-modified titanium
discs
BM-MSCs were purchased from Lonza (Basel, Switzer-
land). Human BM-MSCs were cultured on 14 mm nHAp
coated titanium discs and 5 cm × 5 cm titanium foil surfaces
in high glucose DMEM medium (Invitrogen, Carlsbad, CA,
USA) containing 10% foetal bovine serum (FBS; Cambrex,
East Rutherford, NJ, USA), 1% penicillin streptomycin
(10,000 units/ml penicillin, 10,000 μg/ml streptomycin,
WelGENE Inc., Daejeon, Korea), and 25 μM ascorbic acid
(Sigma-Aldrich) at a density of 2.5 × 10
4
cells/disc and
1 × 10
5
cells/foil. The 14 mm nHAp-coated titanium discs
were placed in 24-well plates with the medium, and the
cells were incubated at 37°C in a humidified atmosphere
of 5% CO
2
for 3 days. The 5 cm × 5 cm titanium foil sheets
were placed in 100 mm diameter Petri dishes with medium,
and the cells were incubated at 37°C in a humidified atmo-
sphere of 5% CO
2
for 10 days. Then, the culture medium,
which was low glucose DMEM medium containing 10%
FBS, 1% penicillin streptomycin, 50 μM ascorbic acid,
10 mM β-glycerophosphate (TCI, Seoul, Korea), and 10
-7
M
dexamethasone (Sigma-Aldrich) was used during 2 weeks.
Scanning electron microscopy (SEM)
SEM samples were fixed overnight at room temperature
using a fixing reagent (2.5% glutaraldehyde in PBS); they
were fixed in 2% OsO
4
:0.2 M phosphate buffer (1:1) re-
agent for 2 h in the dark. The samples were then rinsed
with PBS twice for 10 min. The BM-MSC samples were
washed with an ethanol: water mixture (30%, 50%, 70%,
90%, and 100% ethanol) twice for 10 min, in sequence.
Samples were then treated with 98% 1,1,1,3,3,3-hexa-
methyldisilazane (HMDS):100% ethanol (1:1) solution for
5 min. They were then treated with 98% HMDS twice for
10 min and allowed to dry overnight.
The morphology of the BM-MSCs on nHAp-coated titanium
disc surfaces was examined under SEM (Model HITACHI
S-3000N; Hitachi Instruments, Tokyo, Japan).
MTT assay of BM-MSCs
Cell number was determined using a 3-(4,5-dimethylthiazol-
2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich)
assay (18). This assay identifies metabolically active cells
through the action of a mitochondrial dehydrogenase that
is changed into an insoluble formazan pigment. BM-MSCs
in 24-well plates were incubated for the designated times in
0.33 mg/ml MTT supplemented cell culture medium at 37°C
and 5% CO
2
for 2 h. The intense purple-colored formazan
derivative formed during active cell metabolism was eluted
and dissolved in 1 mL dimethyl sulfoxide and absorbance
was measured at 540 nm.
T-lymphocyte culture
T-cells were cultured for the biocompatibility analysis. The
Jurkat cell line (T-lymphocyte, human leukemia, suspen-
sion cell line) was purchased from the ATCC (Manassas, VA,
USA). Jurkat cells were cultured in 12-well plates containing
8 mm nHAp-coated titanium discs with 1 mL of RPMI1640
(Invitrogen) containing 10% FBS and 1% penicillin strepto-
mycin (10,000 units/ml of penicillin, 10,000 μg/ml streptomy-
cin) at a density of 5 × 10
5
cells/well. Cells were incubated
at 37°C in a humidified atmosphere of 5% CO
2
for 3 days.
The BrdU assay was performed using the Cell Prolifera-
tion ELISA Assay, BrdU (colorimetric) kit (Roche Diagnos-
tics, Mannheim, Germany) to evaluate proliferation of the
Jurkat cell line. All 8 mm nHAp-coated titanium discs were
removed during this assay. One microliter of the BrdU label-
ing solution provided in the kit was added to the Jurkat cell
line cultured in 12-well plates, followed by a 2-h incubation
at 37°C in the dark. The final concentration of BrdU label-
ing solution was 10 μM. The solution was centrifuged at

© 2013 Wichtig Editore - ISSN 0391-3988
510
Immobilization of BMP-2 using a chitosan calcium chelating agent
300 × g for 10 minutes using a 1.5 mL tube and the labeling
medium was removed. The 1.2 mL of FixDenat provided in
the kit was then added to the cells, followed by a 30-min
room temperature incubation. The solution was centrifuged
again at 300 × g for 10 minutes, followed by removal of the
FixDenat solution. Then, 600 μL of anti-BrdU-POD working
solution (antibody conjugate), which was diluted 1:100 with
antibody dilution solution, was added to the cells, followed
by a 90-min room temperature incubation. The antibody
conjugate was removed by aspiration of the centrifuged
samples, and the wells were rinsed three times with 1.2 mL
PBS. A 600 μL aliquot of substrate solution was added to
the cells, followed by 15-min room temperature incubation.
Absorbance was measured immediately at 370 nm.
Tumor necrosis factor-α (TNF-α ) assay
The quantity of secreted TNF-α, an inflammatory cytokine,
was estimated after the T-cell culture. The TNF-α assay was
performed using the TNF-α ELISA kit (Biosource, Nivelles,
Belgium). Two hundred microliters of standards and sam-
ples were added to anti-TNF-α-coated wells of the microti-
ter plate with 50 μL of incubation buffer provided in the kit.
The 96-well microtiter plates were incubated for 2 h at room
temperature on a horizontal shaker set at 700 ± 100 rpm.
The liquid was aspirated from each well, and the plates were
washed three times by dispensing 0.4 mL of wash solution
into each well, and aspirating the contents. One hundred mi-
croliters of standard 0 and 50 μL of anti-TNF-α conjugate,
which were diluted 1:10 with conjugate buffer, were added to
all wells. The 96-well plates were incubated for 2 h at room
temperature on a horizontal shaker set at 700 ± 100 rpm. The
liquid was then aspirated from each well. The plates were
washed three times by dispensing of 0.4 mL of wash so-
lution into each well and aspirating the contents. Following
the washing step, 200 μL of freshly prepared chromogenic
solution was added to each well within 15 min and the plates
were incubated for 30 min at room temperature on a hori-
zontal shaker set at 700 ± 100 rpm, avoiding direct sunlight.
Finally, 50 μL of Stop Solution was added to each well, and
absorbance was read at 450 nm within 3 h.
Reverse transcription polymerase chain reaction
(RT-PCR) of BM-MSCs
The RT-PCR analysis was performed to compare the ex-
pression of bone-inducing markers in BM-MSCs. Total
cellular RNA was isolated using 1 mL of Trizol reagent (In-
vitrogen). cDNA was synthesised by reverse transcription
using 1 μg of total RNA. PCR was conducted by subject-
ing the samples to 23 to 35 cycles (within the linear range
of amplification) of denaturation (94°C, 1 min), annealing
(53-57°C, 1 min), and extension (72°C, 1 min). The prod-
ucts were then analyzed on 2% agarose gels and visualized
by SYBR Safe DNA Gel Staining (Invitrogen). The relative
abundance of type I collagen, type III collagen, osteonec-
tin, osteopontin, vimentin, BMP-2, bone sialoprotein (BSP),
and GAPDH (an internal control) transcripts was measured
using RT-PCR. Primers used for RT-PCR were purchased
from Bioneer, and their sequences, reaction conditions,
and product size (bp) are listed in Table I. Image J software
(Wayne Rasband, National Institutes of Health, Bethesda,
MD, USA) was used to quantitatively analyze the RT-PCR
amplicons on digitized gel images.
Statistical analysis
Student’s t-test was used to evaluate the artificial titanium
sample data. Data are given as means ± standard devia-
tions. A p<0.05 was considered significant.
RESULTS
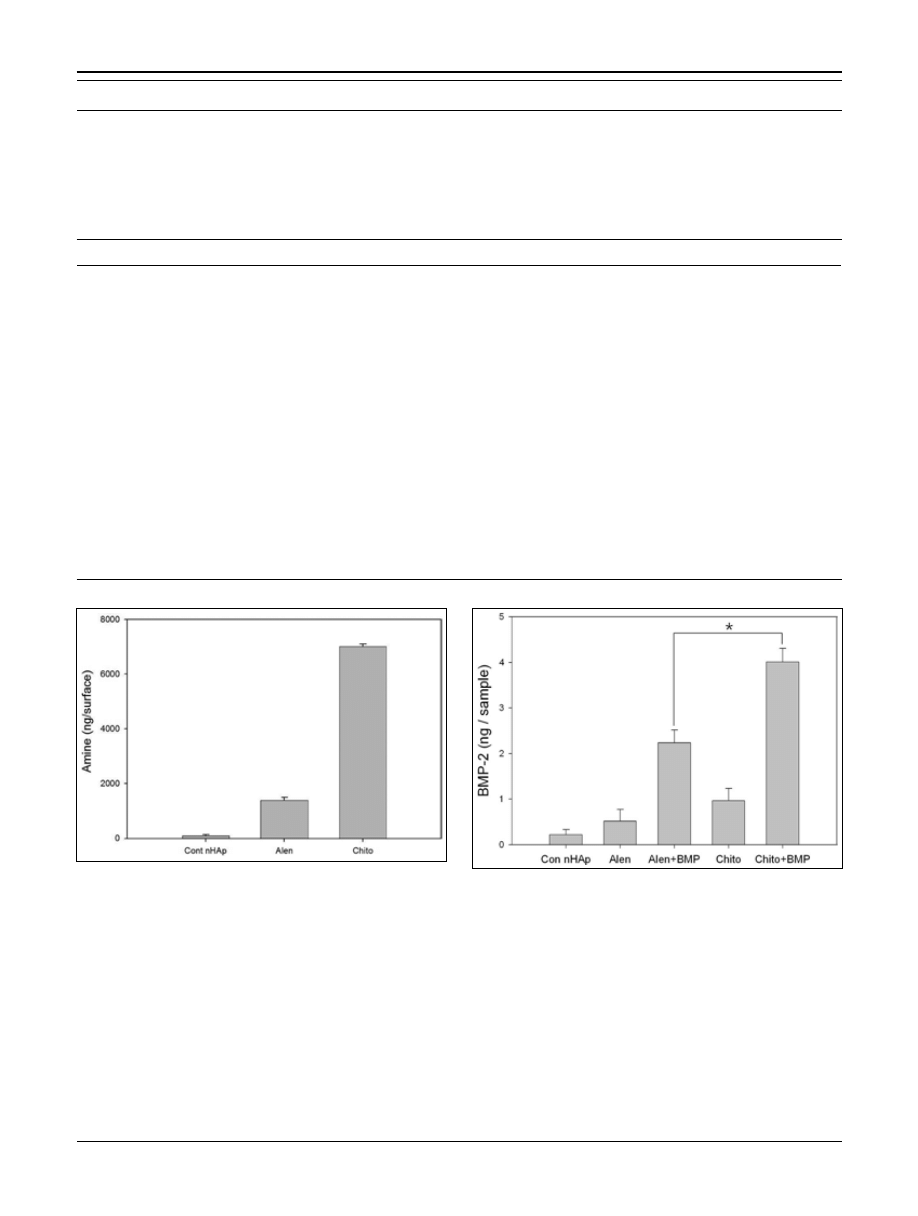
Quantification of amine groups
Amine groups were quantified using the amine assay with
the TNBS reaction. The quantity of amine groups on the
chito group surface was approximately 7 μg/surface area
(Fig. 2). However, the alen group surface contained ap-
proximately 1.4 μg/surface area of amine groups. Thus, the
chitosan chelated surface appeared to have a large num-
ber of amine groups, compared with the alendronate che-
lated surface. As expected, the cont nHAp group surface
had scarcely any amine groups on its surface.
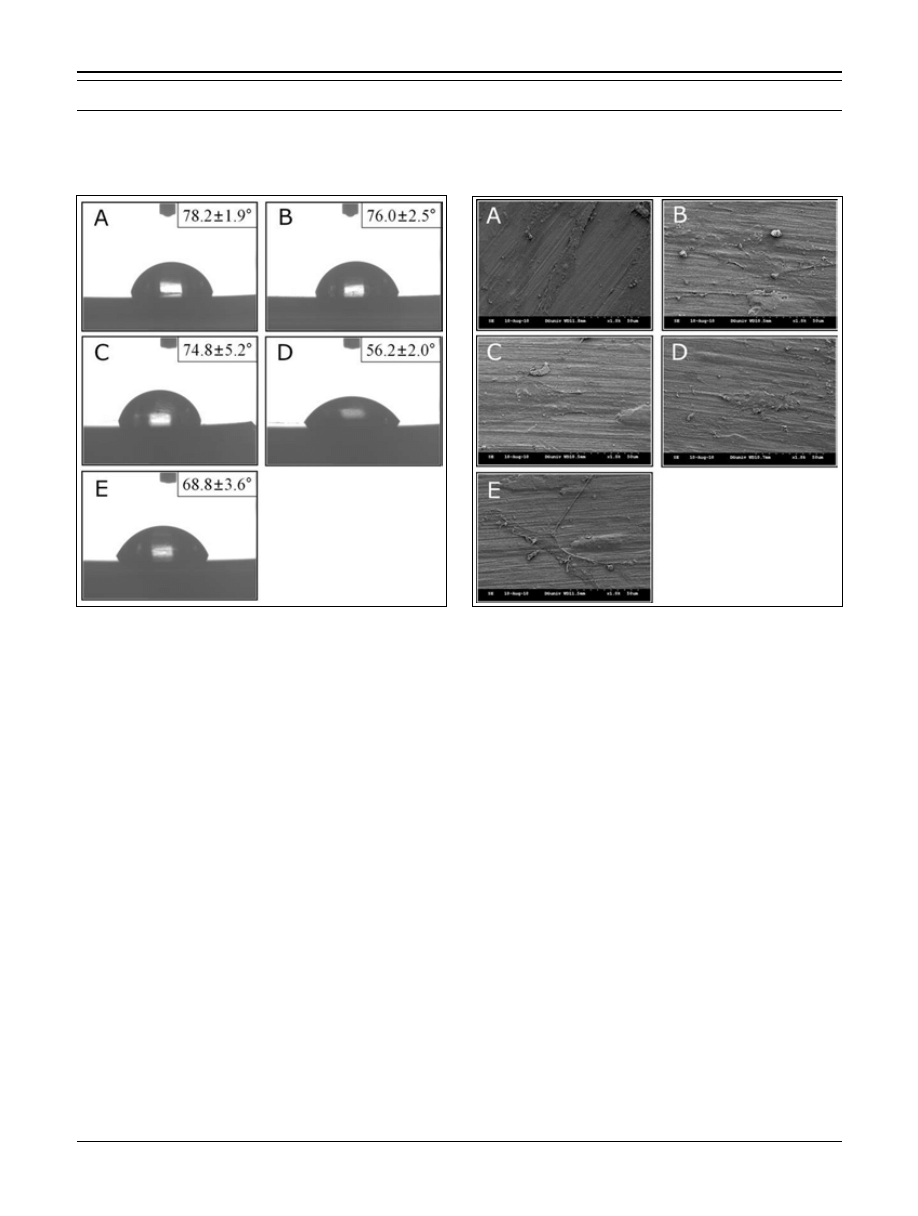
BMP-2 estimation
The BMP-2 assay was performed when all samples were
dried perfectly. We compared the quantity of BMP-2 im-
mobilized on the cont nHAp, alen, alen + BMP, chito, and
chito + BMP groups (Fig. 3). The quantity of BMP-2 on
the chito + BMP (about 4 ng/surface area) group was
higher than that on the alen + BMP (about 2.2 ng/surface
© 2013 Wichtig Editore - ISSN 0391-3988
511
Kim et al
area). The chitosan agent appeared to hold many BMP-2
growth factors due to the large quantity of amine functional
groups. We also detected some BMP-2 on the surface of
the sample groups that were not immobilized with BMP-2,
which may have been caused by a reaction with the cal-
cium chelating agent.
taBle i - PRIMER SEqUENCES, REACTION CONDITIONS, AND PRODUCT SIzE FOR THE REVERSE TRANSCRIPTION-
POLYMERASE CHAIN REACTION (RT-PCR) ANALYSIS
gene
Sequence
Product Size (bp)
annealing (°c)
cycles
GAPDH
F : ACC ACA GTC CAT GCC ATC AC
R : TTC ACC ACC CTG TTG CTG TA
450
55
25
Collagen 1
F : GAA AAC ATC CCA GCC AAG AA
R : CAG GTT GCC AGT CTC CTC AT
270
57
23
Collagen 3
F : CAG GTG AAC GTG GAG CTG C
R : TGC CAC ACG TGT TTC CGT GG
661
849
57
23
Osteonectin
F : CCA GAA CCA CCA CTG CAA AC
R : GGC AGG AAG AGT CGA AGG TC
161
57
23
Osteopontin
F : TCG CAG ACC TGA CAT CCA GT
R : TCG GAA TGC TCA TTG CTC TC
267
57
32
Vimentin
F : GGA ACA GCA TGT CCA AAT CG
R : TCA GTG GAC TCC TGC TTT GC
214
55
25
BMP-2
F : GTC CAG CTG TAA GAG ACA CC
R : GTA CTA GCG ACA CCC ACA AC
316
54
31
BSP
F : AAC CTA CAA CCC CAC CAC AA
R : GTT CCC CGT TCT CAC TTT CA
147
57
36
Fig. 2 - Quantification of amine groups on the calcium chelated
surface. The nano-hydroxyapatite (nHAp)-coated titanium surface
(cont nHAp) graph indicates quantification of amine groups on the
nHAp-coated titanium surface. The Alen graph indicates quantifi-
cation of amine groups on the alendronate chelated surface. The
Chito graph indicates quantification of amine groups on the chitosan
chelated surface.
Fig. 3 - Estimation of bone morphogenetic protein-2 (BMP-2) on the
calcium chelated surface. The Cont nHAp graph indicates quanti-
fication of BMP-2 on the nano-hydroxyapatite (nHAp) coated tita-
nium surface. The Alen graph indicates quantification of BMP-2 on
the alendronate chelated surface. The Alen + BMP graph indicates
quantification of BMP-2 on the BMP-2 immobilized surface chelated
with alendronate. The Chito graph indicates quantification of BMP-2
on the chitosan chelated surface. The Chito + BMP graph indicates
quantification of BMP-2 on the BMP-2 immobilized surface chelated
with chitosan. Results are expressed as means ± standard deviation
(n = 3) (*p<0.0001).
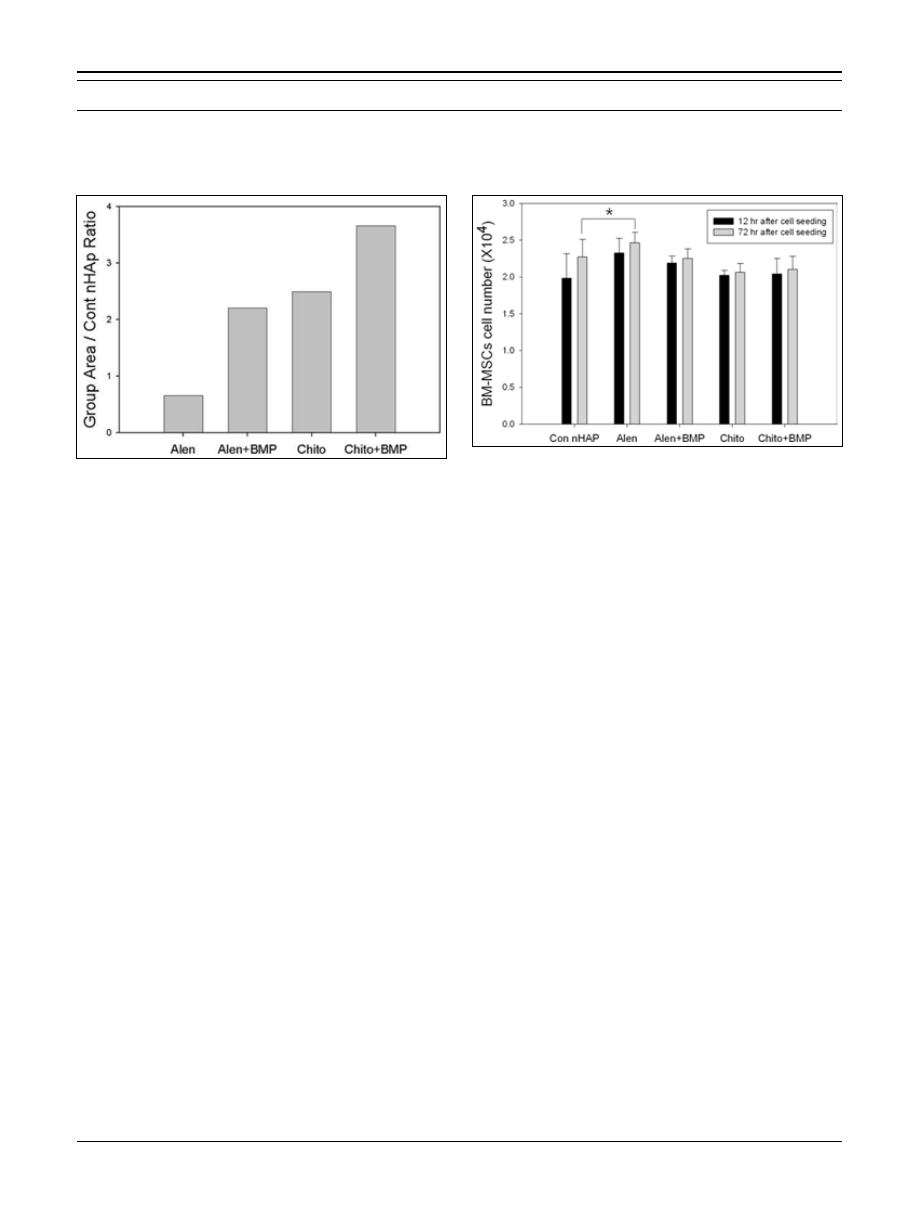
Contact angle goniometry
Contact angles of the alen and chito groups were signifi-
cantly higher (more hydrophobic) than those of the cont
© 2013 Wichtig Editore - ISSN 0391-3988
512
Immobilization of BMP-2 using a chitosan calcium chelating agent
were more adherent on the alen + BMP (Fig. 5B) group than
those on the cont nHAp and alen groups (Figs. 5A and E).
No differences were observed between the cont nHAp and
alen groups. BM-MSCs on the chito and chito + BMP (Figs.
5C and D) groups were more adherent than those on the
cont nHAp group.
In particular, adhesion of the chito + BMP group was higher
than that of the chito group, which may have occurred due
to the presence of abundant amine functional groups, sug-
gesting that chitosan has the ability to increase adhesion
of BM-MSCs. Thus, chitosan is an effective biomolecule
with an adhesion role in cells. In addition, BMP-2 on chito-
san increased cell adhesion.
Normally, cells attached on the surface, and the adhesion
area was not large. But if the cells spread their cytoplasm
on the ECM or growth factor-coated surface, then the
nHAp group. In addition, the contact angle of the alen +
BMP group was also significantly higher than that of the
Cont nHAp group. Angles of the cont nHAp, alen, chito,
and alen + BMP groups were 68.8 ± 3.6°, 78.2 ± 1.9°,
74.8 ± 5.2°, and 76.0 ± 2.5°, respectively. However, the
contact angle of the chito + BMP group was significantly
lower (56.2 ± 2.0°) than that of any other group (Fig. 4).
Consequently, the chitosan chelated surface was modified
by immobilizing BMP-2 to improve the hydrophilicity of its
surface.
BM-MSC morphology and spreading
The morphology of BM-MSCs on the nHAp-coated titanium
discs was assessed by SEM. High-magnification images
(
×
1.0 K) of these samples show cell adhesion. BM-MSCs
Fig. 4 - Results for the calcium chelated surface contact angles. (A):
The contact angle of the alendronate chelated surface (alen group)
was approximately 78.2 ± 1.9°.
(B): The contact angle of the bone
morphogenetic protein-2 (BMP-2) immobilized surface chelated
with alendronate (alen + BMP group) was approximately 76.0 ± 2.5°.
(C): The contact angle of the chitosan chelated surface (chito group)
was approximately 74.8 ± 5.2°.
(D): The contact angle of the BMP-2
immobilized surface chelated with chitosan (chito + BMP group) was
approximately 56.2 ± 2.0°.
(E): The contact angle of the nano-hy-
droxyapatite (nHAp)-coated titanium surface (cont nHAp group) was
approximately 68.8 ± 3.6°.
Fig. 5 - Scanning electron microscopy (SEM) images of bone mar-
row mesenchymal stem cells (BM-MSCs) on a calcium chelated
surface (magnification: ×2.0 k). (A): SEM of BM-MSCs on an alendro-
nate chelated surface (alen group).
(B): SEM of BM-MSCs on a bone
morphogenetic protein-2 (BMP-2) immobilized surface chelated
with alendronate (alen + BMP group).
(C): SEM of BM-MSCs on a
chitosan chelated surface (chito group).
(D): SEM of BM-MSCs on
a BMP-2 immobilized surface chelated with chitosan ( chito + BMP
group).
(E): SEM of BM-MSCs on a nano-hydroxyapatite (nHAp)-
coated titanium surface (cont nHAp group).
© 2013 Wichtig Editore - ISSN 0391-3988
513
Kim et al
surface increased adhesion and the spreading area in-
creased. In Figure 6, when the cells adhered on the HAp-
coated surface, the adhesion area of the cytoplasm was
not large. But, when MSCs attached to the BMP linked
surface, the spreading area increased because BMP in-
creased cytoplasmic spreading. Thus, even though the
cell number was similar, the spreading area was wider. The
spreading cell area was measured by Image J software
and is shown in Figure 6.
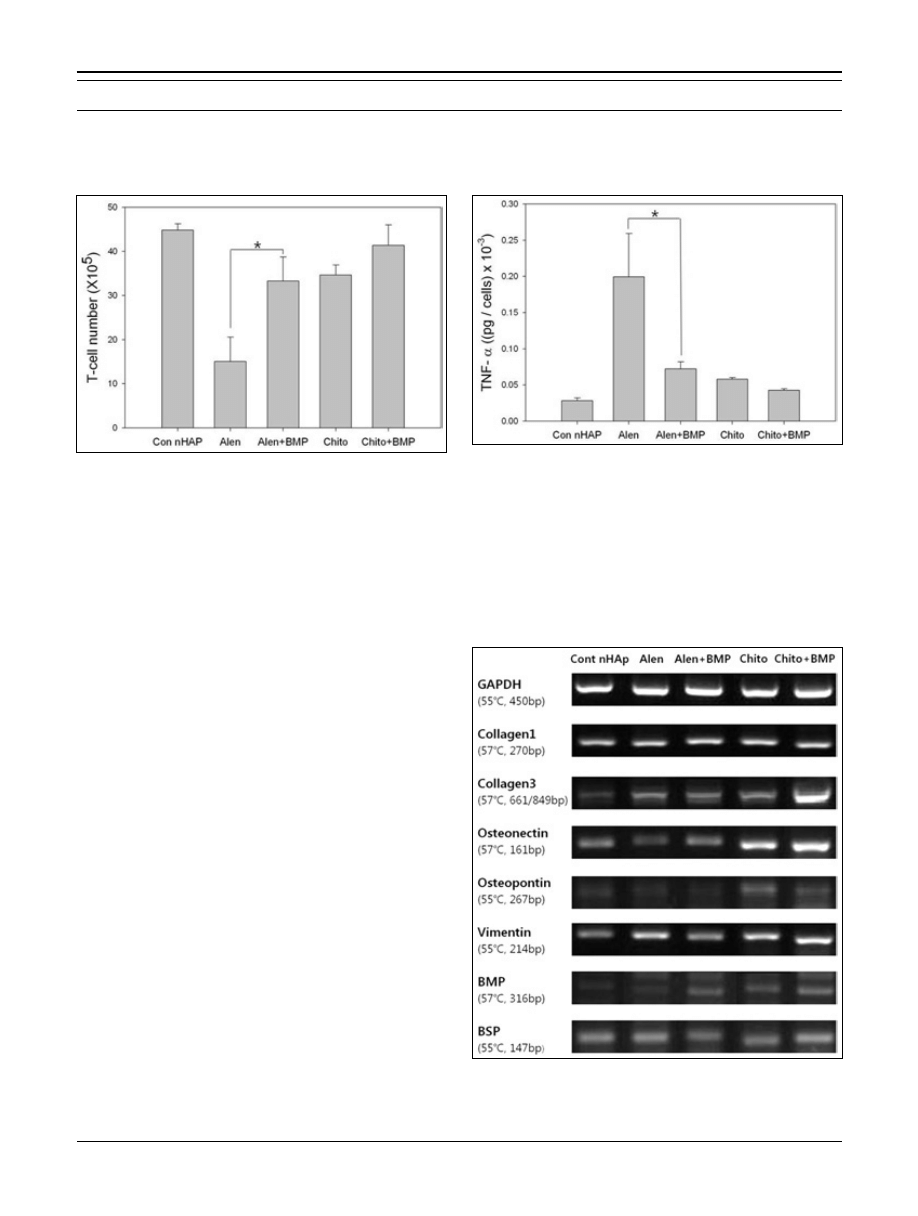
BM-MSC proliferation
BM-MSC proliferation on nHAp coated titanium discs
was evaluated by the MTT assay (Fig. 7) 3 days after cell
seeding. The number of BM-MSCs was measured with a
standard MTT assay.
The number of BM-MSCs on the cont nHAp group sur-
face after 12 h and 72 h was approximately 2.01 ± 0.33
×
10
4
cells/disc and 2.27 ± 0.23
×
10
3
cells/disc, respectively.
The cell number appeared to increase slightly over 3 days.
However, the other groups did not show an outstanding
growth effect. The numbers of BM-MSCs on the alen group
surface after 12 h and 72 h were approximately 2.32 ±
0.20
×
10
4
cells disc and 2.43 ± 0.54
×
10
3
cells/disc. The
numbers on the alen + BMP group surface after 12 h
and 72 h were approximately 2.18 ± 0.09
×
10
4
cells/disc
and 2.25 ± 0.13
×
10
3
cells/disc. The numbers on the chito
group surface after 12 h and 72 h were approximately 2.02
± 0.07
×
10
4
cells/disc and 2.06 ± 0.12
×
10
3
cells/disc.
The numbers on the chito + BMP group surface after 12 h
and 72 h were approximately 2.04 ± 0.21
×
10
4
cells/disc
and 2.10 ± 0.17
×
10
3
cells/disc, respectively. We found no
differences in cell proliferation. Despite what appeared to
be enhanced cell attachment on the alen group, no differ-
ences were observed among the samples.
Number of Jurkat cells
The BrdU assay was performed to measure the number of
Jurkat cells 3 days after cell seeding (Fig. 8). The number
of Jurkat cells on the alen + BMP group (approximately
33.3 ± 4.94
×
10
5
cells/well) was higher than that of cells
on the alen group (approximately 15 ± 5.06 × 10
5
cells/
well). We also confirmed that the number of Jurkat cells
on the chito + BMP group (approximately 41.4 ± 4.23 ×
10
5
cells/well) was higher than that of cells on the chito
group (approximately 34.7 ± 2.04 × 10
5
cells/well). In other
reported research, exposure to toxic molecules results in
a concentration-dependent decrease in Jurkat T-cell pro-
liferation (23). Our results suggest that alendronate is more
Fig. 6 - Analysis of spreading cell area measured by Image J software.
Group area was divided by the nHAp-coated titanium surface (cont
nHAp) area. (Group area = spreading cell area for each experiment
group; cont nHAp = spreading cell area of the control group).
Fig. 7 - Proliferation of bone marrow mesenchymal stem cells
(BM-MSCs) on the calcium chelated surface. The Cont nHAp
graph represents cell proliferation on the nano-hydroxyapatite
(nHAp) coated titanium surface. The Alen graph represents cell
proliferation on the alendronate chelated surface. The Alen + BMP
graph represents cell proliferation on the bone morphogenetic
protein-2 (BMP-2) immobilized surface chelated with alendronate.
The Chito graph represents cell proliferation on the chitosan che-
lated surface. The Chito + BMP graph represents cell proliferation
on the BMP-2 immobilized surface chelated with chitosan. Each
cell proliferation graph is divided into 12 and 72 h after cell seed-
ing. Results are expressed as means ± standard deviations (n = 3)
(*p<0.5).
© 2013 Wichtig Editore - ISSN 0391-3988
514
Immobilization of BMP-2 using a chitosan calcium chelating agent
cytotoxic than chitosan; however, BMP-2 had the ability to
reduce cytotoxicity of alendronate by increasing the num-
ber of Jurkat cells.
Quantification of TNF-α
The TNF-α assay was performed with RPMI1640 me-
dium from Jurkat cells, which was obtained 3 days after
cell seeding (Fig. 9). This result showed a large amount of
TNF-α secreted in the alen group. TNF-α decreased sig-
nificantly in the alen + BMP group, when compared with
that of the alen group due to immobilization of BMP-2 on
the surface. In addition, the chito and chito + BMP groups
showed decreased TNF-α. Thus, chitosan was less cyto-
toxic than alendronate in Jurkat cells.
RT-PCR analysis
We investigated whether preconditioning BM-MSCs re-
sults in an increase in their bone-inducing activity us-
ing RT-PCR analysis (Fig. 10). Although type I collagen
mRNA expression appeared to increase in the chito group
when compared with that in the control group, no signifi-
cant difference was observed among any of the groups.
Fig. 8 - The number of Jurkat cells was measured by the BrdU
assay. The Con nHAp graph represents Jurkat cell number on the
nano-hydroxyapatite (nHAp) coated titanium surface. The Alen graph
represents Jurkat cell number on the alendronate chelated surface.
The Alen + BMP graph represents Jurkat cell number on the bone
morphogenetic protein-2 (BMP-2) immobilized surface chelated with
alendronate. The Chito graph represents Jurkat cell number on the
chitosan chelated surface. The Chito + BMP graph represents Jurkat
cell number on the BMP-2 immobilized surface chelated with chi-
tosan. Results are means ± standard deviations (n = 3) (*p<0.0001).
Fig. 9 - Tumor necrosis factor-α (TNF-α) assay results for Jurkat
cells on the calcium chelated surface. The Con nHAp graph repre-
sents the quantity of TNF-α on the nHAp coated titanium surface.
The Alen graph represents the quantity of TNF-α on the alendronate
chelated surface. The Alen + BMP graph represents the quantity
of TNF-α on the bone morphogenetic protein-2 (BMP-2) immobi-
lized surface chelated with alendronate. The Chito graph represents
the quantity of TNF-α on the chitosan chelated surface. The Chito +
BMP graph represents the quantity of TNF-α on the BMP-2 immobi-
lized surface chelated with chitosan. Results are means ± standard
deviations (n = 3) (*p<0.005).
Fig. 10 - Reverse transcription polymerase chain reaction (RT-PCR)
analysis of GAPDH, collagen type I, collagen type III, osteonectin,
osteopontin, vimentin, bone morphogenetic protein (BMP), and
bone sialoprotein (BSP).
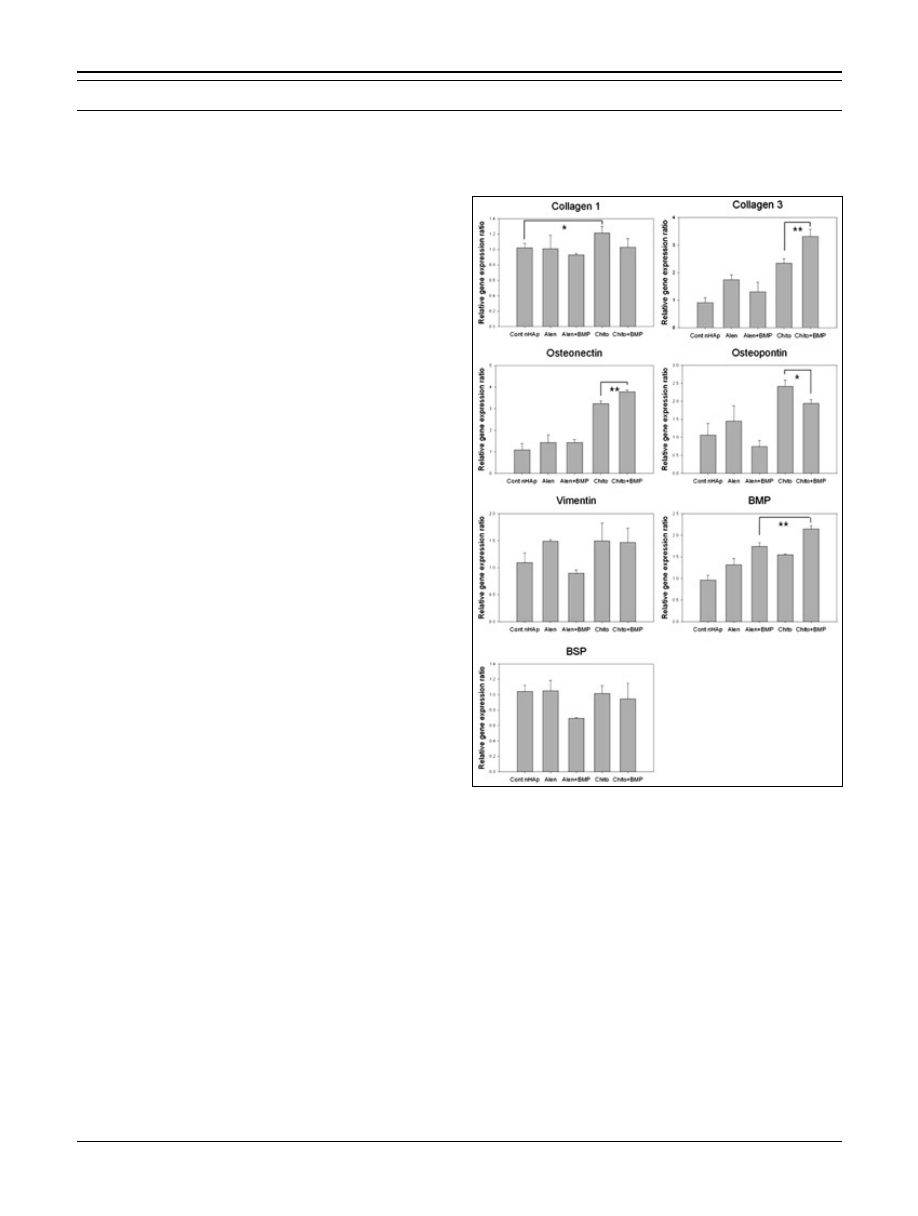
© 2013 Wichtig Editore - ISSN 0391-3988
515
Kim et al
In contrast, type III collagen expression increased in the
chito + BMP group when compared with that in the other
groups. Levels of osteonectin and osteopontin expression
were higher in the chito and chito + BMP groups, com-
pared with the other groups. Osteonectin expression in the
chito + BMP group increased more than that in the chito
group. In addition, the chito + BMP group showed great-
er BMP expression than that in any of the other groups.
These combined results suggest that chitosan may have
a significant autonomous, osteoconductive effect. Thus,
bone-inducing activity was more enhanced when BMP-2
was immobilized on the chitosan chelated surface. Ad-
ditionally, we analyzed BMP release from the BMP-2 im-
mobilized surface and found that 10% of the BMP was
released into the media every 24 h during the 4 days. As a
result, this osteogenic effect was due to immobilized BMP
and BMP released into the media. Alendronate may also
have an osteoconductive effect. However, the levels of
type III collagen, osteopontin, vimentin, and BSP mRNA
expression decreased when BMP-2 was immobilized on
the alendronate chelated surface. The quantitative analysis
of the RT-PCR amplicons on digitized gel images is shown
in Figure 11. We suggest that chitosan can be used as a
ligand to bind BMP-2 and can work as a bioactive agent
for biomaterials that promote osteogenic differentiation.
DISCUSSION
The key point of our study was immobilization of BMP bio-
molecules on biomaterials, which is a widely researched
approach to modify metal surfaces to control cell and
tissue responses (5). We used alendronate and chitosan
as calcium chelating agents to improve immobilization of
BMP-2 on an nHAp-coated surface. We hoped to find the
most effective calcium chelating agent to enhance adhe-
sion of BM-MSCs on nHAp-coated titanium substrates by
covalently immobilizing BMP-2. The results suggest that
chitosan, a biomolecule that contains amine functional
groups, increased the ability to retain BMP-2, increased
the adhesion of BM-MSCs, and reduced cytotoxicity. In
addition, BMP-2 immobilized on a chitosan chelated sur-
face was increasingly hydrophilic, which can enhance
differentiation to bone compared with a chitosan-only che-
lated surface. Chitosan is a non-toxic, non-immunogenic,
and biodegradable natural biopolymer that enhances bone
healing in various animal models (24).
We found that the chitosan chelated surface had a larger
number of amine groups compared to that of the alendro-
nate chelated surface. Alendronate has a primary amine on
its R2 side chain (25), whereas chitosan has many amine
groups that have a chelating effect (26). BMP-2 tends to
adhere to cross-linker activated surfaces. Other research-
ers have revealed that titanium surface hydroxyl groups
can be activated with carbonyldiimidazole and that the
carboxyl groups are activated with N-hydroxysuccinimide
to bind amine-containing molecules. In addition, BMP-2
covalently attaches to activated titanium surfaces (27).
Therefore, chitosan has a large number of amine functional
groups that enhanced immobilization of BMP-2 with the
Fig. 11 - Quantitative analysis of reverse transcription-polymerase
chain reaction (RT-PCR) amplicons on digitized gel images mea-
sured by Image J software (*p<0.1, **p<0.05).

© 2013 Wichtig Editore - ISSN 0391-3988
516
Immobilization of BMP-2 using a chitosan calcium chelating agent
Chitosan enhanced binding ability and cell adhesion of
BMP-2 and also reduced cytotoxicity. We also demon-
strated that chitosan had an excellent autonomous osseo-
integration effect. In addition, BMP-2 immobilization on a
chitosan chelated surface increased hydrophilicity of the
surface. BM-MSC differentiation to bone on a BMP-2 im-
mobilized surface chelated with chitosan was better than
that on a chitosan chelated surface.
CONCLUSIONS
More amine groups were found on the chitosan chelated
surface than on the alendronate chelated surface. The
quantity of BMP-2 on the BMP-2 immobilized surface che-
lated with chitosan was higher than that on the BMP-2 im-
mobilized surface chelated with alendronate. Hydrophilicity
of the BMP-2 immobilized surface chelated with chitosan
showed a significantly greater increase than that of any
other group. BM-MSCs on a BMP-2 immobilized surface
chelated with chitosan appeared to have a significant abil-
ity to differentiate into bone-forming cells. Based on these
results, we suggest that chitosan is an effective calcium
chelating agent for implants. Our future work will focus on
methods to immobilize various bioactive molecules on sur-
faces grafted with bioinert material. In addition, we will in-
vestigate various analytical tests.
Financial Support: This study was supported by the R & D Program
of the Ministry of Knowledge and Economy/Korea Evaluation Institute
of Industrial Technology (MKE/KEIT 10033290, Development of sur-
face activation technology for best function modification of implants
containing bioactive materials).
conflict of interest: The authors declare no conflict of interest.
Address for correspondence:
Young-Kwon Seo
Department of Medical Biotechnology
Dongguk University
3-26, Pil-dong, Chung-gu
Seoul 100-715, Korea
bioseo@dongguk.edu
cross-linker. We also found that the quantity of BMP-2 on
the chito + BMP group was higher than that on the alen +
BMP group. Thus, we demonstrated an association be-
tween the number of amine groups on the surface and that
of BMP-2 immobilized onto an amine-grafted surface.
We found that the hydrophilicity of the BMP-2 immobilized
surface chelated with chitosan could be improved. Bone-
inducing activity was enhanced when BMP-2 was immo-
bilized on the chitosan chelated surface. The correlation
between surface hydrophilicity and osteogenic activity of
cells has been demonstrated in other studies. Our results
suggest that hydrophilic titanium can lead to an alterations
in osteogenic activity (28). However, we found no significant
difference in cell proliferation among the samples. Other re-
searchers have already shown that cell proliferation on both
titanium grafted with carboxymethyl chitosan and BMP-2
functionalized substrates does not increase compared with
that on pristine titanium (29). We propose that although we
did not detect a significant increase in cell proliferation, en-
hanced cell adhesion due to the presence of chitosan and
BMP-2 would lead to more complete osseointegration.
Research has shown that chitosan has a wide range of ap-
plications, including antibacterial activity (30, 31). Analogous
studies of immobilizing BMP-2 to enhance the osteocon-
ductive effect of a titanium surface using chitosan have also
been reported. Shi et al immobilized BMP-2 on a titanium
surface, which was functionalized by covalent grafting with
carboxymethyl chitosan, and showed an antibacterial effect
(29). Shi et al grafted chitosan on titanium by immobilizing
L-DOPA on its surface. Their results showed that bacterial ad-
hesion on both the carboxymethyl chitosan-grafted (CMCS)
surface and BMP-2 immobilized CMSC surfaces decreased
significantly, compared with that on pristine substrates. In
addition, BMP-2 immobilized CMSC surfaces promote
significant attachment, alkaline phosphatase activity, and
calcium mineral deposition in both osteoblasts and human
BM-MSCs. However, we immobilized BMP-2 on chitosan,
which was chelated on an nHAp-coated titanium surface.
We adopted an nHAp-coated titanium surface, as it contains
a large number of calcium ions for chelation with chitosan.
REFERENCES
1.
Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface
treatments of titanium dental implants for rapid osseointe-
gration. Dent Mater. 2007;23(7):844-854.
2.
Fujishiro Y, Sato T, Okuwaki A. Coating of hydroxy-
apatite on metal plates using thermal dissociation of
calcium-EDTA chelate in phosphate solutions under hy-
drothermal conditions. J Mater Sci Mater Med. 1995;6(3):
172-176.

© 2013 Wichtig Editore - ISSN 0391-3988
517
Kim et al
18. Mao CB, Li HD, Cui Fz, Feng qL, Ma CL. The functionaliza-
tion of titanium with EDTA to induce biomimetic mineralization
of hydroxyapatite. J Mater Chem. 1999;9:2573-2582.
19. Suri S, Mönkkönen J, Taskinen M, et al. Nitrogen-containing
bisphosphonates induce apoptosis of Caco-2 cells in vitro
by inhibiting the mevalonate pathway: a model of bisphos-
phonate-induced gastrointestinal toxicity. Bone. 2001;29(4):
336-343.
20. Chen XG, Liu CS, Liu CG, Meng XH, Lee CM, Park HJ.
Preparation and biocompatibility of chitosan microcarriers
as biomaterial. Biochem Eng J. 2006;27(3):269-274.
21. Muzzarelli RAA, Ramos V, Stanic V, et al. Osteogenesis pro-
moted by calcium phosphate N,N-dicarboxymethyl chito-
san. Carbohydr Polym. 1998;36(4):267-276.
22. Dash C, Ahmad A, Nath D, Rao M. Novel bifunctional in-
hibitor of xylanase and aspartic protease: implications for
inhibition of fungal growth. Antimicrob Agents Chemother.
2001;45(7):2008-2017.
23. McDermott C, O’Donoghue MH, Heffron JJ. n-Hexane toxic-
ity in Jurkat T-cells is mediated by reactive oxygen species.
Arch Toxicol. 2008;82(3):165-171.
24. Jung UW, Kim SK, Kim CS, Cho KS, Kim CK, Choi SH. Effect
of chitosan with absorbable collagen sponge carrier on bone
regeneration in rat calvarial defect model. Curr Appl Phys.
2007;7:e68-e70.
25. Rogers MJ. From molds and macrophages to mevalonate:
a decade of progress in understanding the molecular mode
of action of bisphosphonates. Calcif Tissue Int. 2004;75(6):
451-461.
26. Yan z, Haijia S, Tianwei T. Adsorption behaviors of the
aminated chitosan adsorbent. Korean J Chem Eng. 2007;
24(6):1047-1052.
27. Adden N, Gamble LJ, Castner DG, Hoffmann A, Gross G,
Menzel H. Phosphonic acid monolayers for binding of bioac-
tive molecules to titanium surfaces. Langmuir. 2006;22(19):
8197-8204.
28. Park JA, Leesungbok R, Ahn SJ, Lee SW. Effect of etched
microgrooves on hydrophilicity of titanium and osteoblast re-
sponses: A pilot study. J Adv Prosthodont. 2010;2(1):18-24.
29. Shi z, Neoh KG, Kang ET, Poh CK, Wang W. Surface
functionalization of titanium with carboxymethyl chitosan
and immobilized bone morphogenetic protein-2 for en-
hanced osseointegration. Biomacromolecules. 2009;10(6):
1603-1611.
30. Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut
W. Chitosan as antimicrobial agent: applications and mode
of action. Biomacromolecules. 2003;4(6):1457-1465.
31. Bratskaya S, Marinin D, Simon F, et al. Adhesion and viability
of two enterococcal strains on covalently grafted chitosan
and chitosan/kappa-carrageenan multilayers. Biomacromol-
ecules. 2007;8(9):2960-2968.
3.
Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Os-
seointegrated titanium implants. Requirements for ensuring
a long-lasting, direct bone-to-implant anchorage in man.
Acta Orthop Scand. 1981;52(2):155-170.
4.
Morra M. Biochemical modification of titanium surfaces:
peptides and ECM proteins. Eur Cell Mater. 2006;12:1-15.
5.
Puleo DA, Kissling RA, Sheu MS. A technique to immobi-
lize bioactive proteins, including bone morphogenetic pro-
tein-4 (BMP-4), on titanium alloy. Biomaterials. 2002;23(9):
2079-2087.
6.
Luginbuehl V, Meinel L, Merkle HP, Gander B. Localized de-
livery of growth factors for bone repair. Eur J Pharm Bio-
pharm. 2004;58(2):197-208.
7.
Jennissen HP. Accelerated and improved osteointegration
of implants biocoated with bone morphogenetic protein 2
(BMP-2). Ann N Y Acad Sci. 2002;961:139-142.
8.
Akazawa T, Itabashi K, Murata M, et al. Osteoinduction
by Functionally Graded Apatites of Bovine Origin Loaded
with Bone Morphogenetic Protein-2. J Am Ceram Soc.
2005;88(12):3545-3548.
9.
Sachse A, Wagner A, Keller M, et al. Osteointegration of hy-
droxyapatite-titanium implants coated with nonglycosylated
recombinant human bone morphogenetic protein-2 (BMP-2)
in aged sheep. Bone. 2005;37(5):699-710.
10. Jarcho M, Bolen CH. Hydroxylapatite synthesis and char-
acterization in dense polycrystalline form. J Mater Sci.
1976;11:2027-2035.
11. de Groot K, Wolke JGC, Jansen JA. Calcium phosphate
coatings for medical implants. Proc Inst Mech Eng H. 1998;
212(2):137-147.
12. Daculsi G, Laboux O, Malard O, Weiss P. Current state of the
art of biphasic calcium phosphate bioceramics. J Mater Sci
Mater Med. 2003;14(3):195-200.
13. Chai CS, Ben-Nissan B. Bioactive nanocrystalline sol-gel
hydroxyapatite coatings. J Mater Sci Mater Med. 1999;10(8):
465-469.
14. Akao M, Aoki H, Kato K. Mechanical properties of sintered
hydroxyapatite for prosthetic applications. J Mater Sci.
1981;16(3):809-812.
15. Fujishiro Y, Fujimoto A, Sato T, Okuwaki A. Coating of Hy-
droxyapatite on Titanium Plates Using Thermal-Dissociation
of Calcium-EDTA Chelate Complex in Phosphate Solutions
Under Hydrothermal Conditions. J Colloid Interface Sci.
1995;173(1):119-127.
16. Schuessele A, Mayr H, Tessmar J, Goepferich A. Enhanced
bone morphogenetic protein-2 performance on hydroxyapa-
tite ceramic surfaces. J Biomed Mater Res A. 2009;90(4):
959-971.
17. Durrieu MC, Pallu S, Guillemot F, et al. Grafting RGD con-
taining peptides onto hydroxyapatite to promote osteoblastic
cells adhesion. J Mater Sci. 2004;15(7):779-786.
Wyszukiwarka
Podobne podstrony:
Ćwiczenie 3 immobilizacja na chitynie
bmp 511e20c05e252
bmp 4a2e19bce747c
Ćwiczenie 1. immobilizacja, Mikrobiologia przemysłowa
trusek hołownia, procesy membranowe, IMMOBILIZACJA BIOKATALIZATORÓW
Lab PŁ, nr 6 immobilizowane biokatalizatory
IMMOBILIZACJA
IMMOBILIZOWANE BIOKATALIZATORY
Ćwiczenie nr 3 immobilizacja
mapka sniegowa BMP(1)
Engine Immobilizer System
10 Cellular phone Immobilzer ITK 02P
immobiliser PROXIMA KIDNAPER
bmp 5469e3d645384
2754A IMMOBILISER PRE BII
Format BMP okiem hakera
20130327131616!Immobilizacja
więcej podobnych podstron