
394
Journal of Basic Microbiology 2007, 47, 394 – 399
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Research Paper
Influence of soil compaction on microfungal community
structure in two soil types in Bartin Province, Turkey
Ömer Kara and
İlyas Bolat
Department of Soil Science and Forest Ecology, Faculty of Forestry, University of Zonguldak Karaelmas,
Turkey
Soil compaction negatively influences physical properties of soil (bulk density and pore space),
and may consequently limit soil microfungi, which are significant for nutrient bioavailability.
We measured microfungal community responses to compaction in a sandy loam and a clay
loam soil at picnic sites. Soil bulk density increased significantly in the compacted samples.
However, microfungal numbers and community composition were unrelated to changes in soil
bulk density. With increases in bulk density from 1.22 to 1.37 g cm
–3
in the clay soil and from
1.38 to 1.54 g cm
–3
in the sandy loam soil, the total number of fungi declined or showed
insignificant increases. In the compacted samples as well as the control sites, the most
frequently occurring genera in the clay soil were Penicillium, Aspergillus, and Gliocladium.
However, the most prominent feature occurring within the sandy loam soil was the exhibition
of the greatest increase in the frequency of the Fusarium genus. When comparing compacted
and control soils, fungal community composition corresponded more closely within each soil
texture. The two microfungal soil communities, therefore, tolerated compaction. In contrast, a
difference occurred in the fungal communities between the two soil textures. This is more
likely due to the variability in the controlling factors of microfungal abundance and
composition, such as soil characteristics, tree species, and
competitive ability of fungal genera.
Keywords: Microfungal community / Soil compaction / Soil texture / Soil microorganisms / Soil organic matter
Received: March 02, 1007; returned for modification: June 14, 2007; accepted June 19, 2007
DOI 10.1002/jobm.200710341
Introduction
*
Excessive compaction disrupts important physical
properties by modifying porosity and bulk density.
Affected soils with higher strength, higher bulk den-
sity, and decreased pore space have lower infiltration
rate, reduced water retention capacity, and increased
runoff (Horton et al. 1994). As shown by Moffat (1991),
soil compaction also decreases nutrient availability due
to biomass removals or erosion.
Soil microfungi are important for the degradation of
organic matter hence providing nutrients for plants to
grow. Therefore, the reduction of microfungal activity
by compaction is of great concern. Soil compaction also
Correspondence: Ö. Kara, Department of Soil Science and Forest
Ecology, Faculty of Forestry, University of Zonguldak Karaelmas, 74100
Bart
ın, Turkey
E-mail: omerkara@karaelmas.edu.tr
Fax: +90 378 2277421
Tel.: +90 378 2277422
negatively affects soil physical properties. This, in turn,
may limit the activity of soil micro-organisms and bio-
chemical processes important for nutrient bioavailabil-
ity in soil.
Soil compaction is generally believed to decrease mi-
crobial activity, but the results of a few studies show
variances. For example, several investigations have
found decreases in microbial activity or biomass due to
compaction (Dick et al. 1988, Li et al. 2003), while others
report no relationship between compaction and a de-
crease in microbial activity (Jordan et al. 1999, Ponder
and Tadros 2002). However, the number, weight and
activity of micro-organisms may be good indicators
of soil quality (Nielsen and Winding 2002). Micro-
organisms maintain soil quality because they play an
active role in soil fertility as a result of their involve-
ment in the cycle of nutrients like carbon and nitrogen,
which are required for plant growth. Micro-organisms
provide a valuable sensitive biological indicator for soil
health and fertility (Turco et al. 1994).

Journal of Basic Microbiology 2007, 47, 394 – 399
Influence of soil compaction on fungal community structure
395
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Specific studies have indicated that microfungi are a
dominant component of total soil microbial biomass,
accounting for up to 90% of the biomass in the soil
(Anderson and Domsch 1975). Microfungi also function
as the main organic matter decomposers in soil and
play an essential role in humus formation (Christensen
1989). Any physical manipulation to the soil ecosystem
may affect the distribution, activities, and ecological
environment of soil microfungi. However, few studies
have examined the impact of soil compaction on micro-
fungi in Turkish soil.
The purpose of the present study is to assess the
abundance and the diversity of microfungi in the soil
at a picnic area, which is subjected to compaction as a
result of pedestrian traffic.
Materials and methods
Site description
The research area was located in the Bartın province
(41
°38′
N, 32
°20′
E) situated on the Western Black Sea in
Turkey. This region has a humid mesothermal climate
characterized by warm summers. Based on climatologi-
cal data over the past 30 years, the annual mean tem-
perature in this region is 12.6
°C. The mean tempera-
tures of the hottest months, July and August, are 22.4
and 21.9
°C, respectively. The mean temperature of the
coldest months, January and February, are 4.0 to 4.1
°C,
respectively. Annual mean precipitation in the region is
1087.0 mm, and annual relative humidity is 80%. The
principal geological formation in the Bartın province is
calcareous rock. Sandy loam and clay textured soils are
covered with Euroamerican Poplars (Populus x euroameri-
cana (Dode) Guinier) and Black Locust (Robinia pseudoaca-
cia L.) trees, respectively, at the picnic sites.
Sampling and isolation methods
Soil cores were collected from the top soil (0 – 10 cm
depth) of compacted and control plots, oven dried at
105
°C for 24 h, and weighed to determine bulk density.
Bulk density was calculated from mass and volume.
Pore space was calculated from the bulk and particle
density. The additional larger samples were collected
from each soil profile to be used in the determination
of particle size distribution, pH, CaCO
3
content, particle
density, soil moisture, and organic carbon. The soil
samples were air-dried and screened with a 2 mm sieve.
The following selected soil physical and chemical prop-
erties were determined by means of appropriate meth-
ods: soil particle size distribution by the hydrometer
method, particle density by pycnometer method, soil
moisture content by gravimetric method, pH in 1 : 2.5
(w/v) of soil:water suspension by pH-meter, soil organic
matter by Walkley-Black wet oxidation method, CaCO
3
content by Scheibler calcimeter method (Rowell 1994).
For soil microfungi analysis, soil samples were taken
with a sterile trowel, placed in clean polyethylene bags,
and stored in a refrigerator at +4
°C until analyzed by
plating. The isolation of soil microfungi was performed
using the suspension plating technique (Bills et al.
2004). Fungal colonies were determined
by plating 1 ml
of 1 : 10 000 soil suspension dilutions (three replicates)
onto peptone dextrose agar (PDA) supplemented with
Rose-Bengal and streptomycin. After 7 days of incuba-
tion, the numbers of viable propagules of microfungi
were counted. The concentrations of viable fungi in the
soil were expressed as colony-forming units (CFU) per
gram of dry weight (d.w.) soil. Representative micro-
fungi isolates were sub-cultivated and maintained on
PDA slants. The classification of soil microfungi at the
genus level followed the method of Barnett and Hunter
(1999). For identification at the species level, the follow-
ing literature sources were consulted: Pitt (1979) for
penicillia; Raper and Fennell (1965), Klich (2002) for
aspergilli; Ellis (1971) for Alternaria and Cladosporium;
and Nelson et al. (1983) for Fusarium. For other species,
the monograph of Domsch et al. (1993) was used. Refer-
ence strains identified to species level have been depos-
ited in the Culture Collections of Kukens (WDCM101),
Centre for Research and Application of Culture Collec-
tions of Microorganisms.
Data analysis
To evaluate similarity among the microfungi commu-
nity of the different textured soils both from the com-
pacted and control site, the following similarity indices
were used: (i) Sorenson’s similarity index (S
s
) (Krebs
1989):
=
+ +
2
2
s
a
S
a
b
c
where “a” is the number of species common to both
samples, “b” is the number of species found only in
sample B, and c is the number of species found only in
sample A. Coefficients vary between 0, which indicates
that the two communities have no species in common,
and 1.0, which indicates that have all species in com-
mon.
(ii) The percentage similarity index (PSC) (Washing-
ton 1984):
1
T
i
i
n
n
=
⎛
⎞
−
−
⎜
⎟
⎝
⎠
∑
1
2
1
2
PSC = 100
0.5
100
i
n
n
396
Ö. Kara and
İ. Bolat
Journal of Basic Microbiology 2007, 47, 394 – 399
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Table 1. Characteristics of fine and coarse textured soils from picnic areas.
Properties
Clay soil
Sandy loam soil
Compacted
Control
Compacted
Control
Particle density, g cm
–3
Porosity %
Organic C, %
pH
Lime content, %
Sand, %
Silt, %
Clay, %
Soil moisture %
2.40
a,
*
42.80
a
4.24
a
7.77
a
26.07
a
24.01
a
28.00
a
47.99
a
14.94
a
2.30
a
46.77
b
2.80
b
7.92
a
12.69
bc
25.24
a
22.96
a
51.80
a
17.59
a
2.55
b
39.34
c
1.10
c
8.24
b
11.34
bc
80.16
b
12.23
b
7.61
b
3.95
b
2.53
b
45.25
b
2.15
d
8.05
c
8.41
d
65.28
c
21.86
c
12.86
c
11.15
c
* Within each row, values with different letters are significantly different (p = 0.05; n = 6).
where n
1i
and n
2i
are the number of individuals of the
ith taxon in the first and second samples, respectively,
and n
1
and n
2
are the total numbers of individuals in the
first and second samples, respectively. The scale for the
percentage similarity indices ranges from 0 to 100, in
which 100 represents identical taxa at each site, and 0
reflects two completely dissimilar communities. These
two indices characterize the similarity of communities
in different respects; species presence and absence ver-
sus species relative to abundance.
Significant differences for clay and sandy loam soils
from both the compacted and control sites were deter-
mined by the independent-samples t test. Statistical
calculations were performed using SPSS 8.0 for Win-
dows.
Results and discussion
Table 1 shows that the soil properties are different in
the different textured soils. The compaction of our
cores shows major changes in bulk density for both
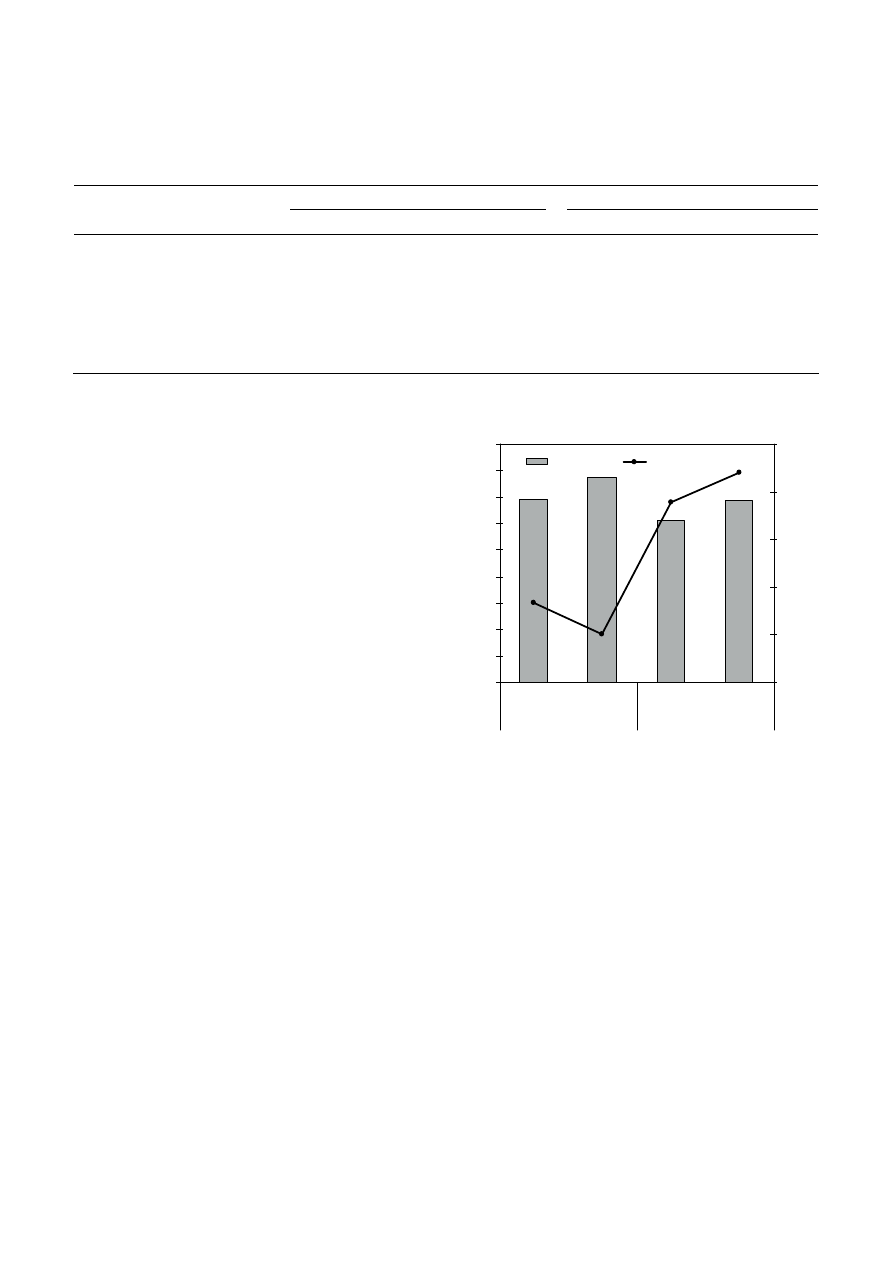
soils (Fig. 1). Bulk density increases significantly from
1.22 to 1.37 g cm
–3
in the clay soil and from 1.38 to
1.54 g cm
–3
in the sandy loam soil. This, in turn, causes
a significant (p < 0.01) decline in the general porosity of
the soil. Therefore, the reduction of the total porosity
between the compacted and control soils is 9.27% for
the clay soil and 13.06% for the sandy loam soil.
Håkansson and Lipiec (2000) reported that soil sensitiv-
ity to compaction depends on soil properties, particu-
larly on soil texture (especially clay content) and struc-
ture. Our results show that picnic sites of clay soils
with rich organic matter compact less than sandy loam
soils with low organic matter. The reduced compactibil-
ity due to high organic carbon in the clay soil suggests
that the organic carbon content of the soil may be the
1,38
1,54
1,22
1,37
8,3
5,0
18,9
22,0
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
1.8
Control Compacted
Control Compacted
Sandy loam
Clay
Bul
k
de
ns
it
y
g
c
m
-3
0
5
10
15
20
25
CF
U
x
1
0
4
g
-1
dw
so
il
Bulk density
CFU
Figure 1. A comparison of bulk density and CFU in compacted and
noncompacted clay and sandy loam soils.
most important factor in determining the degree of soil
compaction.
No significant differences (p > 0.05) were observed in
CFUs per gram of dry soil between the compacted and
control samples within each soil textures (Fig. 1). The
mean number of microfungi from clay soil samples
from the compacted site was 21.94
× 10
4
CFU/g, which
was not statistically greater than the observed
18.96
× 10
4
CFU/g counted from control soils. The mean
numbers of microfungi from sandy loam soil samples –
4.95
× 10
4
and 8.58
× 10
4
CFU/g for compacted and con-
trol sites, respectively – were not statistically differ-
ent. The results of our study show that moderate com-
paction is not detrimental to microfungal abundance,
regardless of soil texture. The compaction may favour
smaller, habitable-sized pores without a reduction in

Journal of Basic Microbiology 2007, 47, 394 – 399
Influence of soil compaction on fungal community structure
397
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Table 2. Microfungi species abundance in clay and sandy loam soils.
Clay soil
Sandy loam soil
Fungi species
Compacted Control
Compacted Control
Acremonium salmoneum Gams & Lodha
Aspergillus flavus Link
A. fumigatus Fresen.
A. niveus Blochwitz
A. sydowi Thom&Church
A. versicolor (Vuill.) Tirab.
Cladosporium cladosporioides (Fresen.)G.A.
C. oxysporium Berk. & Curt
C. sphaerospermum Penz
Fusarium aquaeductuum (Rabenh.&Radlk) Sacc
.
F. avenaceum (Fr.) Sacc.
F. oxysporium Schltdl.
F. sporotrichioides Sherb.
Fusarium Link ex Fr.
Gliocladium roseum Bainier
G. vermoesenii (Biourge) Thom
Gliocladium Corda
Mucor circinelloides Tiegh.
M. racemosus Bull.
Mucor Mich. Ex Fr.
Neosartorya fischeri (Wehmer) Malloch & Cain
Penicillium chrysogenum Thom
P. glandicola (Oudem.) Seifert & Samson
P. melinii Thom
P. rugulosum Thom
P. verrucosum Dierckx
Penicillium Link ex. Gray sp.
Penicillium sp.1
Rhizopus Ehrenberger
Trichoderma koningii Oudem.
T. viride Pers
Trichoderma Pers ex Fr.
Verticillium biguttatum Gams
V. catenulatum Gams
Verticillium Nees ex Link
0.33*
1.00
1.00
0.66
2.00
1.00
2.33
0.33
1.00
1.66
1.00
0.33
2.33
3.33
0.66
1.00
0.66
1.33
0.66
2.00
1.33
1.00
1.66
0.33
2.00
0.66
3.33
1.66
3.66
0.66
0.66
0.66
0.33
0.33
0.33
0.33
0.66
0.33
0.33
0.66
0.33
0.33
0.66
0.66
0.33
0.33
0.66
0.66
0.66
0.33
0.33
0.33
0.33
0.66
0.33
0.33
0.33
0.33
0.33
0.33
0.33
* Values are
× 10
4
CFU/g dry weight soil.
microfungal numbers. This finding agrees with the
results of Shestak and Busse (2005), who found that
habitat condition was improved by compaction and
apparently offset any detrimental effects of restricted
air, water, or nutrient flow (Table 2).
However, fungal counts are significantly greater
(p < 0.05) in the clay soil as compared to the fungal
counts in the sandy loam soil (Fig. 1). We believe that
this result may be related to soil texture which is
known to play a role in determining microbial commu-
nity composition by modifying soil moisture and or-
ganic carbon content (Table 1, Schutter and Dick 2000).
The differences observed in CFU between the clay
and sandy loam soil supports the idea that increased
clay content in soil leads to more stabilization of soil
organic carbon content and higher microbial counts
(Fig. 1, Ladd et al. 1985).
In both the compacted and control sites, the most
frequently occurring genera in the clay soil were Penicil-
lium sp., Aspergillus sp., and Gliocladium sp. Also associ-
ated with these genera were Trichoderma sp. and Verticil-
lium spp. However, the greatest increase in the fre-
quency of Fusarium species occurred in the sandy loam
soil. Among the fungal genera, Fusarium
includes phy-
topathogenic species, while
Penicillium includes
antago-
nistic species to plant pathogens (Knudsen et al.
1995,
Luque et al.
2005)
. Fusarium sp.
increases
in the sandy
loam soil when the
relative number of
Penicillium sp.
diminishes, suggesting
the
strong competitive ability of
these fungal genera.
In this study, clay and sandy loam soils are respec-
tively covered with Black Locust and Euroamerican
Poplar trees. Tree species in picnic areas influence the
microfungal composition, which are most likely de-

398
Ö. Kara and
İ. Bolat
Journal of Basic Microbiology 2007, 47, 394 – 399
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
pending on the availability and quality of organic sub-
strates. Previous studies have shown that tree cover is
one of the major factors determining the characteris-
tics of soil (Berg and Wessén 1984). Therefore, consid-
eration has been given to the possibility that the tree
species had obvious differential effects on the microbial
community in the surface soil. Christensen (1969 and
1981) showed that the composition of microfungal
species in soil was strongly correlated with the plant
species composition in the overlying vegetation.
Microfungal community structure varies considera-
bly between clay and sandy loam soils but not between
compacted and control soils in each soil texture. The
greatest similarity was found between the fungal flora
of the compacted and control soils within each soil
texture. Therefore, the highest coefficients in Table 3
are for compacted clay soil compared to control clay
soil (0.533 and 34.22 Sorenson’s and PSC, respectively)
and compacted sandy loam to control sandy loam
(0.580 and 50.80 Sorenson’s and PSC, respectively). This
result shows that compaction does not impact the mi-
crofungal community composition. In fact, similarity
coefficients increase with compaction, suggesting a
rapid and successful adaptation to the altered environ-
ment. Therefore, compaction of medium intensity,
which does not reduce fungal diversity in an area, may
create new habitat conditions that allow microfungi to
live. Similarly, nominal changes in microbial activity
and diversity due to compaction have been found by
other studies (Li 2000, Ponder and Tadros 2002).
A difference is observed between the microfungal
communities of the different soil textures when they
are compared using the Sorenson’s index and the per-
centage index (Table 3). The similarity values between
clay and sandy loam soil from both the compacted and
control sites are lesser than the ones within each soil
texture. In general, low similarity values indicate the
existence of different community compositions be
Table 3. Similarity coefficients for microfungi communities in
compacted and noncompacted clay and sandy loam soils.
Similarity coefficients
Soil texture comparison
Sorenson’s PSC
Compacted clay to control clay
Compacted clay to control sandy
loam
Compacted clay to compacted sandy
loam
Control clay to control sandy loam
Control clay to compacted sandy
loam
Compacted sandy loam to control
sandy loam
0.533
0.142
0.303
0.214
0.303
0.580
34.22
8.82
20.20
16.40
23.71
50.80
tween soil textures. This result suggests that edaphic
effects on microfungal community composition are
much greater than soil compaction effects. Most likely,
this is due to the variability in the controlling factors
for microfungal composition, such as soil organic mat-
ter, texture, moisture, and pH, which is more impor-
tant to microfungal growth and sustainability than soil
physical disturbance. Many studies found that distinct
microbial communities were associated with different
soil types (Bossio et al. 1998, Grayston et al. 2001). Fun-
gal communities in the clay soil of both the compacted
and control sites are more similar to each other than
the communities in their corresponding sandy loam
soil, which illustrates the difference in the soil charac-
teristics between the soil textures regardless of com-
paction.
Conclusions
The abundance and community composition of soil
microfungi in clay and sandy loam textured soils were
not detrimentally affected by the pedestrian traffic.
We suggest that the fundamental explanation for
these observations is that the associated impact of soil
properties, tree species, and
competitive ability of fun-
gal genera lead to the differences in qualitative and
quantitative distribution of microfungal community on
a greater basis as compared to the impact of soil com-
paction. Therefore, according to the results of this
study, soil physical disturbances associated with com-
paction appear to be of little concern for the microfun-
gal community. Further studies will be required to
more clearly separate true differences in microbial
community under compacted conditions at picnic
grounds.
Acknowledgements
We would like to thank the Zonguldak Karaelmas Uni-
versity Scientific Research Projects Committee for fi-
nancial support (Project No:
2004-59-03-07).
References
Anderson, J.P.E. and Domsch, K.H., 1975. Measurement of bac-
terial and fungal contributions to respiration of selected
agricultural and forest soils. Can. J. Microbiol., 21, 314 – 322.
Barnett, H.L. and Hunter, B.B., 1999. Illustrated Genera of
Imperfect Fungi. APS Press, The Amer. Phytopathol. Soc. St.
Paul, Minnesota, USA, 218 pp.

Journal of Basic Microbiology 2007, 47, 394 – 399
Influence of soil compaction on fungal community structure
399
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Berg, B. and Wessén, B., 1984. Changes in organic-chemical
components and ingrowth of fungal mycelium in decompo-
sing birch leaf litter as compared to pine needles. Pedobiol.,
26, 285 – 298.
Bills, G.F., Christensen, M., Powell, M. and Thorn, G., 2004.
Saprobic soil fungi. In: Biodiversity of Fungi (G.M. Mueller,
G.F. Bills and M.S. Foster, eds.) pp. 273 – 294. Elsevier Aca-
demic Press New York.
Bossio, D.A., Scow, K.M., Gunapala, N. and Graham, K.J., 1998.
Determinants of soil microbial communities: effects of ag-
ricultural management, season, and soil type on phos-
pholipid fatty acid profiles. Microbial Ecol., 36, 1 – 12.
Christensen, M., 1969. Soil microfungi of dry to mesic conifer-
hardwood forests in Northern Wisconsin. Ecology, 50,
9 – 27.
Christensen, M., 1981. Species diversity and dominance in
fungal communities. In: The fungal community: Its organi-
zation and role in the ecosystem (D.T. Wicklow and G.C.
Caroll, editors), pp. 201 – 232. Marcel Dekker New York.
Christensen, M., 1989. A view of fungal ecology. Mycologia,
81, 1 – 19.
Dick, R.P., Myrold, D.D. and Perle, E.A., 1988. Microbial bio-
mass and enzyme activities in compacted and rehabilitated
skid trail soils. Soil Sci. Soc. Am. J., 52, 512 – 516.
Domsch, K.H., Gams, W. and Anderson, T.H., 1993. Compen-
dium of Soil Fungi. IHW-Verlag, Eching, Germany, 860 pp.
Ellis, M.B., 1971. Dematiaceus Hypomycetes. Commonwealth
Mycological Institute, Kew, Surrey,UK, 608 pp.
Grayston, S.J., Griffith, G.S., Mawdsley, J.L., Campbell, C.D.
and Bardgett, R.D., 2001. Accounting for variability in soil
microbial communities of temperate upland grassland eco-
systems. Soil Biol. Biochem., 33, 533 – 551.
Hådansson, I. and Lipiec, J., 2000. A review of the usefulness
of relative bulk density values in studies of soil structure
and compaction. Soil Till. Res., 53, 71 – 85.
Horton, R., Ankeny, M.D., Allmaras, R.R., 1994. Effects of
compaction on soil hydraulic properties. In: Soil Compac-
tion in Crop Production (B.D. Soane and C. von Ouverkerk,
eds.), pp. 141 – 165. Elsevier Amsterdam.
Jordan, D., Li, F., Ponder, F., Berry, Jr.E.C., Hubbard, V.C. and
Kim, K.Y., 1999. The effects of forest practices on
earthworm populations and soil microbial biomass in a
hardwood forest in Missouri. Appl. Soil Ecol., 13, 31 – 38.
Klich, M.A., 2002. Identification of common Aspergillus species.
Centraalbureau voor Schimmelcultures, 122 pp.
Knudsen, I.M.B., Elmhont, S., Hockenhull, J. and Jensen, D.F.,
1995. Distribution of saprophytic fungi antagonistic to Fu-
sarium culmorum in two differently cultivated field soils,
with special emphasis on the genus Fusarium. Biol. Agr.
Hort., 12, 61 – 79.
Krebs, C.J., 1989. Ecological Methodology. Harpner & Row
Publishers, New York, 654 pp.
Ladd, J.N., Amato, M. and Oades, J.M., 1985. Decomposition of
plant material in Australian soils. III.Residual organic and
microbial biomass C and N from isotope-labeled legume
material and soil organic matter, decomposing under field
conditions. Aust. J. Soil Res., 23, 603 – 611.
Li, Q., 2000. The effects of silvicultural treatments on soil
microbial biomass and functional diversity. Dissertation,
North Carolina State University.
Li, Q., Allen, H.L. and Wilson, C.A., 2003. Nitrogen mineraliza-
tion dynamics following the establishment of a loblolly pi-
ne plantation. Can. J. Forest Res., 33, 364 – 374.
Luque, A., Pioli, R.N., Bonel, B. and Alvarez, D., 2005. Cellulo-
lytic fungi populations in stubble and soil as affected by
agricultural management practices. Biol. Agr. Hort., 23,
121 – 142.
Moffat, A.J., 1991. Forestry and soil protection in the UK. Soil
Use Man., 7, 145 – 151.
Nelson, P.E., Tousson, T.A. and Marasas, W.F.O., 1983. Fusari-
um Species. An Illustrated Manual for Identification 193 pp.
The Penn. State Univ. Press, Pennsylvania, USA.
Nielsen, M.N. and Winding, A., 2002. Microorganisms as indi-
cators of soil health. Technical report no. 338, National En-
vironmental Research Institute, Denmark.
Pitt, J.I., 1979. The Genus Penicillium and Its Teleomorphic States
Eupenicillium and Talaromyces, 634 pp. Academic Press INC,
London, UK.
Ponder, F. and Tadros, M., 2002. Phospholipid fatty acids in
forest soil four years after organic matter removal and soil
compaction. Appl. Soil Ecol., 19, 173 – 182.
Raper, K.B. and Fennell, D.I., 1965. The Genus Aspergillus,
686 pp. The Williams and Wilkins Comp. Baltimore, USA.
Rowell, D.L., 1994. Soil science: Methods and Applications,
350 pp. Longman Scientific and Technical, Singapore.
Schutter, M.E. and Dick, R.P., 2000. Comparison of fatty acid
methyl ester (FAME) methods for characterizing microbial
communities. Soil Sci. Soc. Am. J., 64, 1659 – 1668.
Shestak, C.J. and Busse, M.D., 2005. Compaction alters physi-
cal but not biological indices of soil health. Soil Sci. Soc.
Am. J., 69, 236 – 246.
Turgo, R.F., Kennedy, A.C. and Jawson, M.D., 1994. Microbial
indicators of soil quality. In: Defining soil quality for a sus-
tainable environment (J.W. Doran et al., eds.), pp. 73 – 90
SSSA Special Publication 35. Madison.
Washington, H.G., 1984. Diversity, biotic, and similarity indi-
ces. Water Res., 18, 653 – 694.
((Funded by:
● the Zonguldak Karaelmas University Scientific Re-
search Projects Committee; grant number: 2004-59-03-
07))
Wyszukiwarka
Podobne podstrony:
jobm 200710325
jobm 200710320
jobm 200710313
jobm 200710333
jobm 200710111
jobm 200710318
jobm 200710317
jobm 200710310
jobm 200710132
jobm 200710337
jobm 3620260101
200710s11 OgarnijTemat comid 26410 (2)
20071010
20071002CV PL Prof Grudzewski, a
20071002CV PL Prof Grudzewski, a
200710311013330 Coatedproductsu Nieznany (2)
jobm 3620250801
20071031
jobm 3620260805
więcej podobnych podstron