
358
Journal of Basic Microbiology 2007, 47, 358 – 362
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Short Communication
Phosphate supply and arsenate toxicity
in ectomycorrhizal fungi
Serena H. Chen and Mark Tibbett
Centre for Land Rehabilitation, School of Earth and Geographical Science (M087),
Faculty of Natural and Agricultural Sciences, The University of Western Australia, Crawley, Australia
Three species of ectomycorrhizal fungi (Hebeloma crustuliniforme, Suillus variegatus and Cenococcum
geophilum) were grown in axenic culture amended with range of AsO
4
3–
concentration under
three different PO
4
3–
regimes. The fungi exhibited different growth responses to AsO
4
3–
that
varied with PO
4
3–
concentration. Suillus variegatus showed the greatest sensitivity to AsO
4
3–
, with
growth almost completely inhibited in the presence of AsO
4
3–
under the lower two PO
4
3–
treatments. Under the highest PO
4
3–
treatment however, growth was enhanced and S. variegatus
was able to persist at AsO
4
3–
concentrations of up to 4 mM. Hebeloma crustuliniforme also showed
high sensitivity to AsO
4
3–
especially at low PO
4
3–
concentration. The two higher PO
4
3–
treatments
had an ameliorating effect on AsO
4
3–
toxicity in H. crustuliniforme. This demonstrates the ability
of PO
4
3–
to alleviate AsO
4
3–
toxicity. The response from S. variegatus and H. crustuliniforme, both
basidiomycetes, was in contrast to the ascomycete C. geophilum. This fungus demonstrated
tolerance to AsO
4
3–
when grown in culture solution and PO
4
3–
did not have an ameliorating effect
on AsO
4
3–
toxicity in C. geophilum.
Keywords: Ectomycorrhiza / Arsenic / Phosphate / Arsenate / Ascomycete / Toxicity
Received: January 15, 2007; accepted February 23, 2007
DOI 10.1002/jobm.200710320
Introduction
*
Arsenate (AsO
4
3–
) is the dominant form of arsenic in
aerobic soils and is an approximate analogue of phos-
phate (PO
4
3–
). AsO
4
3–
enters cells via the same membrane
transport system as PO
4
3–
, allowing for high concentra-
tions of arsenic to be taken up into cells of receptor
organisms (Meharg and Hartley-Whitaker 2002) and
competes with PO
4
3–
for binding to ADP. The formation
of the unstable ADP-As complex deprives the cell of
its energy source, ATP (Meharg and Hartley-Whitaker
2002, Quaghebeur and Rengel 2003). This disruption of
energy flow can ultimately lead to cell death.
A number of studies have examined the effects of
varying concentrations of AsO
4
3–
and PO
4
3–
on higher
plants. The plant species investigated were generally
those found growing naturally in As-contaminated sites
Correspondence: Mailing address: Dr.
M. Tibbett, Centre for Land
Rehabilitation, School of Earth and Geographical Science, Faculty of
Natural and Agricultural Sciences, The University of Western Australia,
35 Stirling Highway, Crawley, WA 6009, Australia
E-mail: Mark.Tibbett@uwa.edu.au
including Holcus lanatus (Meharg and Macnair 1992,
1990, Quaghebeur and Rengel 2003), Leymus cinereus
(Knudson et al. 2003) and Silene vulgaris (Sneller et al.
1999). The studies generally showed that increasing the
supply of AsO
4
3–
to these species resulted in decreases in
biomass. These reductions in growth rate were allevi-
ated with higher concentrations of PO
4
3–
.
Mycorrhizal symbiosis plays a significant role in the
establishment and survival of plants in contaminated
sites (Lepp and Dickinson 1998). Some ectomycorrhizal
(ECM) fungi have been shown to not only increase the
nutrient status of their host plant but also improve the
ability of the plant to tolerate toxic elements (Aggangan
et al. 1998). Very few studies have examined the role of
mycorrhizal symbiosis on the tolerance of plants to
arsenic. Ericoid mycorrhizal fungal colonisation of
Calluna vulgaris has been reported to have a significant
effect on AsO
4
3–
uptake by the plant (Sharples et al.
2000a). Arbuscular mycorrhizal (AM) fungi have also
been shown to enhance AsO
4
3–
resistance on plant Holcus
lanatus (Gonzalez-Chavez et al. 2002). Detailed research
on the effect of AsO
4
3–
on the growth of mycorrhizal

Journal of Basic Microbiology 2007, 47, 358 – 362
Effect of phosphate on arsenate toxicity
359
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
fungi in axenic culture has been limited to studies by
Sharples et al. (1999, 2000 b, 2001) primarily on the
ericoid fungus Hymenoscyphus ericae. Due to this paucity
of research, the current study was conducted to deter-
mine the sensitivity of three ECM fungal species to
AsO
4
3–
. The species selected were: Hebeloma crustulini-
forme, Suillus variegatus and Cenococcum geophilum. We
tested two related hypotheses: (1) AsO
4
3–
would be toxic
to all three ECM fungal species tested, especially at high
concentrations, and (2) PO
4
3–
in the growth medium
would diminish the toxic effects of AsO
4
3–
in the ECM
fungi. This was achieved by measuring the fungal
growth of each ECM fungal species in response to a
range of AsO
4
3–
concentrations at three different con-
centrations of PO
4
3–
.
Materials and methods
Two species of basidiomycete, Hebeloma crustuliniforme
(Bull.) Quél. and Suillus variegatus (Swartz: Fr) O.K. and
one species of ascomycete, Cenococcum geophilum Fr.
were used in this study. Four 3 mm circular plugs were
removed from the edges of actively growing fungal
colonies and transferred to Petri dishes containing 25
ml modified Melin-Norkrans (MMN) liquid medium.
The MMN medium had the following composition with
different combinations of AsO
4
3–
and PO
4
3–
: 6.51 mM
NH
4
NO
3
, 0.57
mM MgSO
4
⋅ 7 H
2
O, 0.23
mM CaCl
2
,
0.01 mM ZnSO
4
, 0.30 mM Thiamine, 0.035 mM Ferric
EDTA and 5.55 mM d-glucose. AsO
4
3–
was supplied as
Na
2
HAsO
4
at concentrations of 0, 0.5, 1, 2, 4 and 8 mM.
PO
4
3–
was supplied as KH
2
PO
4
at concentrations of 20
(low), 200 (medium) and 2000 µM (high). Concentrations
of Na
+
and K
+
were balanced for all treatments through
additions of NaCl and KCl. The solution pH was ad-
justed to 5.5. All treatments were replicated four times
for each fungal species.
The fungal cultures were incubated at 25
°C for 20 days.
The mycelial mats were removed from the solution me-
dia and rinsed with DI water. These were placed in alu-
minium trays and oven-dried overnight at 60
°C. The dry
weight of each sample was determined gravimetrically.
Fungal dry weights were normalised in order to fac-
tor out the effect of differential PO
4
3–
supply on growth.
Accordingly, fungal dry weight values were divided by
the mean dry weight achieved in the absence of AsO
4
3–
at the corresponding PO
4
3–
treatment. Statistical analysis
was carried out on the original dry weight data and on
the normalised data using SPSS 11.0.1 (Chicago USA).
All the datasets were normally distributed (Kolmo-
gorov-Smirnov), however they did not meet the as-
sumptions set forth by Levene’s test of homogeneity of
variances. Accordingly, non-parametric statistics were
generated using the Kruskall-Wallis H-test. When the
Kruskall-Wallis statistical results indicated that differ-
ences existed between the three PO
4
3–
treatments
(p < 0.05), the Mann-Whitney U-test was performed to
determine where pair wise differences occurred.
Results
In the absence of AsO
4
3–
S. variegatus produced the great-
est biomass of the three ECM species. The fungal dry
weight of S. variegatus was 70 mg at high PO
4
3–
, 48 mg at
medium PO
4
3–
and 12 mg at low PO
4
3–
. Under the same
conditions H. crustuliniforme produced 20, 17 and 9 mg,
and C. geophilum produced 7, 4 and 2 mg respectively
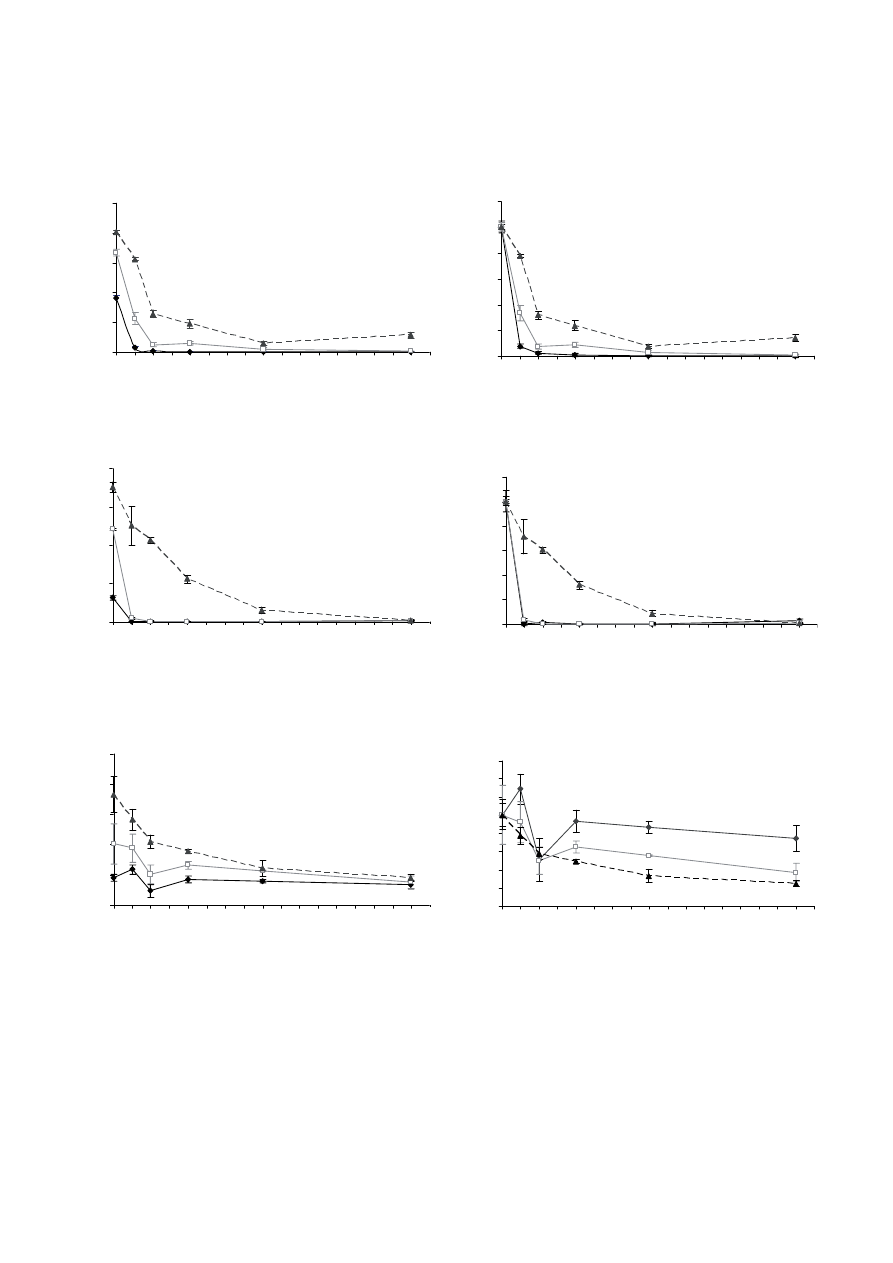
[Fig. 1a (i)]. In response to increasing AsO
4
3–
concentra-
tion, the fungal dry weights of H. crustuliniforme and
S. variegatus decreased exponentially. C. geophilum show-
ed a general decrease in fungal dry weight with increas-
ing AsO
4
3–
concentration, however the response was less
marked than the other two species. The extent of the
effect of AsO
4
3–
and PO
4
3–
concentrations on fungal dry
weight varied with each species.
Hebeloma
crustuliniforme made significant responses to
the different PO
4
3–
treatments when the AsO
4
3–
concen-
trations were below 4 mM [Fig. 1a (ii)]. At 0.5, 1 and
2 mM AsO
4
3–
, the relative biomasses were significantly
greater with higher PO
4
3–
concentrations (p < 0.01). At
4 mM AsO
4
3–
, a difference was observed between the low
and high PO
4
3–
treatments (p < 0.05). At 8 mM AsO
4
3–
,
fungal biomass was significantly greater at high PO
4
3–
(p < 0.05).
Suillus
variegatus exhibited the greatest sensitivity to
AsO
4
3–
. Suillus variegatus generated little or no biomass
under the two lower PO
4
3–
treatments in the presence of
AsO
4
3–
[Fig. 1b (i)]. The fungal dry weights decreased by
more than 95% under the low and medium PO
4
3–
treat-
ments at the lowest AsO
4
3–
addition (0.5 mM). Under the
high PO
4
3–
treatment however, the addition of 0.5 mM
AsO
4
3–
decreased fungal dry weight by only 30%. The
high PO
4
3–
treatment also resulted in S. variegatus being
able to grow in the presence of AsO
4
3–
up to the second
highest concentration 4 mM, at which the relative dry
weight was 9% of growth in AsO
4
3–
free media. Fungal
biomass at high PO
4
3–
was significantly greater (p < 0.05)
than at the two lower PO
4
3–
treatments in the presence
of all concentrations of AsO
4
3–
, with the exception of the
highest (8 mM) treatment.
C. geophilum grew under all AsO
4
3–
treatments in-
cluding 8 mM. The fungal dry weights were generally
360
S. H. Chen and M. Tibbett
Journal of Basic Microbiology 2007, 47, 358 – 362
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
a. Hebeloma crustuliniforme
b. Suillus variegates
c. Cenococcum geophilum
0
5
10
15
20
25
0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 8 8.5
AsO
4
3-
in culture medium (mM)
funga
l
d
ry
w
t
(m
g)
a
b
c
a
b
c
a
b
c
a
b
c
a
b
b
a
b
b
(i)
0
20
40
60
80
100
120
0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 8
AsO
4
3-
in culture medium (mM)
re
la
tiv
e
d
ry
wt
(%)
a
b
b
a
ab
b
a
b
c
a
b
c
a
b
c
(ii)
0
20
40
60
80
0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 8 8.5
AsO
4
3-
in culture medium (mM)
funga
l
d
ry
w
t
(m
g)
a
b
c
a
b
c
a
b
c
a
b
b
a
b
b
(i)
0
20
40
60
80
100
120
0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 8
AsO
4
3-
in culture medium (mM)
re
la
ti
v
e
d
ry
w
t
(%)
a
b
c
a
b
b
a
b
b
a
b
b
(ii)
0
2
4
6
8
10
0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 8 8.5
AsO
4
3-
in culture medium (mM)
funga
l
d
ry
w
t
(m
g)
a
ab
b
a
b
b
a
b
c
(i)
0
20
40
60
80
100
120
140
160
0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 8
AsO
4
3-
in culture medium (mM)
re
la
ti
ve
dr
y
w
t
(%
)
a
a
b
a
b
c
a
ab
b
(ii)
Figure 1. (i) Fungal dry weight and (ii) relative dry weight [fungal biomass / fungal biomass at zero AsO
4
3–
(at corresponding PO
4
3–
treatment)]
of a. Hebeloma crustuliniforme, b. Suillus variegatus and c. Cenococcum geophilum. Fungi were exposed to a range of AsO
4
3–
concentrations at low (20
µM) PO
4
3–
(–
䉬
–), medium (200 µM) PO
4
3–
(–
ⵧ
–) and high (2000 µM) PO
4
3–
(
–
䉱
–
). At each AsO
4
3–
concentration,
data with different letters are significantly different (p < 0.05). The absence of letters denotes no significant difference. Bars represent
2 standard errors of the mean, n = 4.

Journal of Basic Microbiology 2007, 47, 358 – 362
Effect of phosphate on arsenate toxicity
361
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
greater at higher concentrations of PO
4
3–
[Fig. 1c (i)].
However, the relative dry weights had the opposite
response, with higher values at low PO
4
3–
than at the
medium and high PO
4
3–
treatments [Fig. 1c (ii)]. This is in
contrast to the other two ECM fungal species, which
had relative dry weights following the same trends as
the fungal dry weights. At 0, 4 and 8 mM AsO
4
3–
, the
PO
4
3–
treatments had no significant effect on the fungal
dry weights of C. geophilum. At 0.5 mM AsO
4
3–
, the fungal
dry weight at high PO
4
3–
was greater than at low PO
4
3–
(p < 0.05). At 1 and 2 mM AsO
4
3–
, the high PO
4
3–
treat-
ment produced significantly greater fungal dry weights
than the lower two PO
4
3–
treatments. The relative dry
weights for C. geophilum showed no significant differ-
ences between the PO
4
3–
treatments at the lower AsO
4
3–
concentrations of 0.5 and 1 mM AsO
4
3–
. Between 2 to
8 mM AsO
4
3–
, the high PO
4
3–
treatment resulted in rela-
tive dry weights lower than with the low PO
4
3–
treat-
ment.
Discussion
The current study found large differences in the fungal
dry weights between the ECM fungal species. Suillus
variegatus produced dry weight values an order of mag-
nitude greater than C. geophilum. Suillus variegatus has
been previously found to produce a high biomass, rela-
tive to other ECM fungal species (Meharg et al. 1997). It
has also been previously noted that the growth of
C. geophilum is quite slow (LoBuglio 1999). The biomass
of H. crustuliniforme was comparable to that found by
Sharples et al. (1999). Different experimental conditions
as well as different isolates were used, thus the slight
variance that existed was expected.
The extent of AsO
4
3–
toxicity in the ECM fungal spe-
cies studied was dependent on the PO
4
3–
concentration
in solution. Cenococcum geophilum was the most tolerant
isolate and S. variegatus was the most sensitive. The
results demonstrated an ameliorating effect of PO
4
3–
on
As-toxicity in H. crustuliniforme, with increasing PO
4
3–
concentration. Suillus variegatus required the highest
concentration of PO
4
3–
to produce an ameliorating effect
on AsO
4
3–
toxicity.
The ameliorating effect of PO
4
3–
on As-toxicity has
been demonstrated in other studies on fungi and
higher plants (Sharples et al. 1999, Sneller et al. 1999).
Sharples et al. (1999) tested the effect of PO
4
3–
on AsO
4
3–
sensitivity in H. crustuliniforme and also an ericoid fun-
gus Hymenoscyphus ericae. The isolates were grown in
cultures containing a range of PO
4
3–
concentrations with
and without AsO
4
3–
(at 1.33 mM AsO
4
3–
for H. ericae and
0.33 mM AsO
4
3–
for H. crustuliniforme) (Sharples et al.
1999). The reduction in growth caused by AsO
4
3–
toxicity
seemed to be completely alleviated at the 1000 µM PO
4
3–
treatment. The amelioration effect demonstrated in the
present study was not as strong as that found in the
study by Sharples et al. (1999) despite using PO
4
3–
con-
centrations up to 2000 µM. However, a different isolate
of H. crustuliniforme was used in the present study and
there were other variations in experimental conditions
such as higher concentrations of AsO
4
3–
, which may
account for differences in results.
It is likely that the ameliorating effect demonstrated
here, and elsewhere, was due to competition between
AsO
4
3–
and PO
4
3–
for transport carrier sites across the
plasma membrane. A higher PO
4
3–
concentration would
result in less AsO
4
3–
being taken up into the fungi. Al-
though AsO
4
3–
and PO
4
3–
utilise the same membrane
transport system, there is a higher affinity for PO
4
3–
(Meharg and Macnair 1990, Sneller et al. 1999). This can
help explain the persistence of H. crustuliniforme at ex-
posure to AsO
4
3–
as high as 8 mM when supplied with a
PO
4
3–
concentration of 2 mM (high treatment).
Cenococcum
geophilum
demonstrated resistance to AsO
4
3–
,
growing at all concentrations tested. In contrast to the
other two ECM fungal species studied, C. geophilum ex-
hibited greater sensitivity to AsO
4
3–
with increased PO
4
3–
supply (Fig. 1c [ii]). At the low PO
4
3–
treatment, the fun-
gus appeared to be relatively unaffected by increasing
AsO
4
3–
exposure. The results showed that exposure of
C. geophilum to higher AsO
4
3–
concentrations (
≥2 mM)
produced greater relative dry weights under low PO
4
3–
supply than under high PO
4
3–
supply. These results how-
ever, should be interpreted with caution as the relative
dry weights were calculated using mean dry weights (at
0 mM AsO
4
3–
) for each PO
4
3–
treatment that were not
significantly different [Fig. 1c (i)]. Thus it is possible
that the relative values calculated for C. geophilum do
not accurately represent normalised growth values.
Nonetheless, this study has demonstrated that increas-
ing PO
4
3–
supply did not have an ameliorating effect on
the growth of C. geophilum exposed to AsO
4
3–
.
Although this response to PO
4
3–
in C. geophilum is con-
trary to most studies, a lack of an ameliorating effect of
PO
4
3–
has been previously reported in the plant Pteris
vittata (Wang et al. 2002). Wang et al. (2002) found that
the biomass production of P. vittata, a hyperaccumula-
tor of AsO
4
3–
, was not significantly affected by concen-
trations of PO
4
3–
or AsO
4
3–
. However, it was found that
increasing the supply of PO
4
3–
significantly decreased
the concentration of AsO
4
3–
within the plant. Further
studies on C. geophilum, encompassing the measurement
of AsO
4
3–
and PO
4
3–
uptake will help reveal the exact

362
S. H. Chen and M. Tibbett
Journal of Basic Microbiology 2007, 47, 358 – 362
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
effect of AsO
4
3–
and PO
4
3–
on the fungus and may help
determine the possible mechanisms used by this spe-
cies to achieve tolerance to AsO
4
3–
.
Organisms have been shown to achieve AsO
4
3–
toler-
ance through various mechanisms. Meharg and Mac-
nair (1990) found that the tolerance of the grass Holcus
lanatus to AsO
4
3–
was achieved through the suppression
of the high-affinity uptake system for PO
4
3–
/AsO
4
3–
, which
lowers the influx rate of the toxic element. Sneller et al.
(1999) reported the production of phytochelatins (PCs)
to be one of the primary mechanisms for As-detoxi-
fication in Silene vulgaris. The PCs can bind to the re-
duced AsO
3
3–
, forming PC-complexes that can accumu-
late within the root vacuole at high concentrations
(Meharg and Hartley-Whitaker 2002). AsO
4
3–
tolerance is
achieved by the fungus H. ericae through the reduction
of AsO
4
3–
to AsO
3
3–
within fungal cells, followed by en-
hanced efflux of the AsO
3
3–
into the external environ-
ment (Sharples 2000 b). This enhanced AsO
3
3–
efflux has
also been reported to be the mechanism used by As-
resistant bacteria and yeasts (Meharg and Hartley-
Whitaker 2002).
The effect of AsO
4
3–
and PO
4
3–
on the growth of ECM
fungi depends on the species. The response from
H. crustuliniforme and S. variegatus to increasing concen-
trations of AsO
4
3–
and PO
4
3–
were relatively similar, com-
pared to the very different response from C. geophilum.
Hebeloma crustuliniforme and S. variegatus (basidiomy-
cetes) belong to a different phylum from C. geophilum
(ascomycete). Antibus et al. (1992) studied the effects of
phosphatase activities and phosphorus uptake by six
species of ECM fungi, C. geophilum was the only ascomy-
cete. As with the present study, Antibus et al. (1992)
found that the response by C. geophilum was quite dif-
ferent from that of the basidiomycetes. The response of
C. geophilum to AsO
4
3–
appears more similar to that of the
ericoid fungus H. ericae, also an ascomycete, than of
other basidomycete ECM species. We hypothesise that
some ascomycetes may have natural AsO
4
3–
tolerance
mechanisms (see Sharples et al. 2000 b) that are re-
flected in our observed responses of C. geophilum in the
current study.
References
Aggangan, N.S., Dell, B. and Malajczuk, N., 1998. Effects of
chromium and nickel on growth of the ectomycorrhizal
fungus Pisolithus and formation of ectomycorrizas on Euca-
lyptus urophylla S.T. Blake. Geoderma, 84, 15–27.
Antibus, R.K., Sinsabaugh, R.L. and Linkins, A.E., 1992.
Phosphatase activities and phosphorus uptake from inositol
phosphate by ectomycorrhizal fungi. Can. J. Bot.,
70, 794–
801.
Gonzales-Chavez, C., Harris, P.J., Dodd, J. and Meharg, A.A.,
2002. Arbuscular mycorrhizal fungi confer enhanced arse-
nate resistance on Holcus lanatus. New Phytol.,
155, 163–
171.
Knudson, J.A., Meikle, T. and DeLuca, T.H., 2003. Ecosystem
restoration: Role of mycorrhizal fungi and phosphorus in
the arsenic tolerance of basin wildrye. J. Environ. Qual.,
32,
2001 – 2006.
Lepp, N.W. and Dickinson, M.D., 1998. Biological interactions:
The role of woody plants in phytorestoration. In: Metal-
Contaminated Soils: In Situ Inactivation and Phytorestora-
tion, pp. 67 – 73. Springer-Verlag and R.G. Landes Company.
LoBuglio, K.F., 1999. Cenoccum. In: Ectomycorrhizal Fungi.
Key Genera in Profile (J.W.G. Cairney and S.M. Chambers,
Editors), pp. 287 – 309. Springer-Verlag Berlin.
Meharg, A.A., Dennis, G.R. and Cairney, J.W.G., 1997. Bio-
transformation of 2.4.6-trinitrotoluene (TNT) by ectomy-
corrhizal basidiomycetes. Chemosphere,
35, 513–521.
Meharg, A.A. and Macnair, M.R., 1990. An altered phosphate
uptake system in arsenate-tolerant Holcus lanatus. New Phy-
tol.,
116, 29–35.
Meharg, A.A. and Macnair, M.R., 1992. Suppression of the
high affinity phosphate uptake system: A mechanism of ar-
senate tolerance in Holcus lanatus. L. J. Exp. Bot.,
43, 519–
524.
Mehart, A.A. and Hartley-Whitaker, J., 2002. Arsenic uptake
and metabolism in arsenic resistant and nonresitant plant
species. New Phytol.,
154, 29–43.
Quaghebeur, M. and Rengel, Z., 2003. The distribution of
arsenate and arsenite in shoots and roots of Holcus lanatus is
influenced by arsenic tolerance and arsenate and phospha-
te supply. Plant Physiol.,
132, 1600–1609.
Sharples, J.M., Meharg, A.A., Chambers, S.M. and Cairney,
J.W.G., 1999. Arsenate sensitivity in ericoid and ectomy-
corrhizal fungi. Environ. Toxicol. Chem.,
18, 1848–1855.
Sharples, J.M., Meharg, A.A., Chambers, S.M. and Cairney,
J.W.G., 2000a. Evolution – Symbiotic solution to arsenic
contamination. Nature,
404, 951–952.
Sharples, J.M., Meharg, A.A., Chambers, S.M. and Cairney,
J.W.G., 2000b. Mechanism of arsenate resistance in the eri-
coid mycorrhizal fungus Hymenoscyphus ericae. Plant Physi-
ol.,
124, 1327–1334.
Sharples, J.M., Meharg, A.A., Chambers, S.M. and Cairney,
J.W.G., 2001. Arsenate resistance in the ericoid mycorrhizal
fungus Hymenoscyphus ericae. New Phytol.,
151, 265–270.
Sneller, F.E.C., Van Heerwaarden, L.M., Kraaijeveld-Smit, F.J.L.,
Ten Bookum, W.M., Koevoets, P.L.M., Schat, H. and
Verkleij, J.A.C., 1999. Toxicity of arsenate in Silene vulgaris,
accumulation and degradation of arsenate-induced phyto-
chelatins. New Phytol.,
144, 223–232.
Wang, J., Zhao, F.J., Meharg, A.A., Raab, A., Feldmann and
McGrath, P., 2002. Mechanisms of arsenic hyperaccumula-
tion in
Pteris vittata. Uptake kinetics, interactions with
phosphate, and arsenic speciation. Plant Physiol.,
130,
1552 – 1561.
Wyszukiwarka
Podobne podstrony:
jobm 200710325
jobm 200710313
jobm 200710333
jobm 200710111
jobm 200710341
jobm 200710318
jobm 200710317
jobm 200710310
jobm 200710132
jobm 200710337
jobm 3620260101
200710s11 OgarnijTemat comid 26410 (2)
20071010
20071002CV PL Prof Grudzewski, a
20071002CV PL Prof Grudzewski, a
200710311013330 Coatedproductsu Nieznany (2)
jobm 3620250801
20071031
jobm 3620260805
więcej podobnych podstron