
Journal of Basic Microbiology 2007, 47, 417 – 425
417
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Research Paper
Alpha-amylase production by Bacillus subtilis CM3
in solid state fermentation using cassava fibrous residue
M.R. Swain and R.C. Ray
Central Tuber Crops Research Institute (Regional Centre), Dumuduma Housing Board,
Bhubaneswar – 751019, India
In extraction of starch from cassava (Manihot esculenta Crantz), one of the major solid waste
released is fibrous residues which constitute 15 – 20% by weight of the cassava chips/tuber
processed. Production of
α-amylase under solid state fermentation by Bacillus subtilis CM3 has
been investigated using cassava fibrous residue. Response surface methodology (RSM) was used
to evaluate the effect of the main variables, i.e. incubation period, initial medium pH, moisture
holding capacity and temperature on enzyme production. A full factorial Central Composite
Design (CCD) was applied to study these main factors that affected
α-amylase production. The
experimental results showed that the optimum incubation period, initial medium pH,
moisture holding capacity and temperature were 6 days, 8.0, 70% and 50 °C, respectively.
Keywords:
α-Amylase / Bacillus subtilis CM3 / Cassava fibrous residue / Response surface methodology /
Solid state fermentation
Received: April 23, 2007; returned for modification: May 21, 2007; accepted: June 01, 2007
DOI 10.1002/jobm.200710132
Introduction
*
Cassava (Manihot esculanta Cranz.) is a starchy tropical
tuber crop having 20 – 30% extractable starch. In India,
more than 1500 cottage and small industries crush over
5000 tonnes of cassava per day during harvest season
(October – February) (Edison et al. 2006). Industrial pro-
cessing of cassava is done mainly to produce flour and
starch, and in the process generates huge solid wastes
in form of peels and fibrous residue with high moisture
content (85%). These solid wastes are generally dis-
carded in the landfill with out treatment. Cassava fi-
brous residue (CFR) contains about 10 – 15% crude fibre,
55 – 65% starch and very low ash content (1 – 1.2%) (on
dry weight basis) (Jyothi et al. 2005). Because of its low
ash content, CFR could offer numerous advantages in
comparison to other crop residues such as rice straw
and wheat straw, which have 17.5% and 11.0% ash
contents, respectively, for uses in bioconversion pro-
cesses using microbial cultures (Pandey et al. 2000a, b).
The CFR has been successfully put to use under SSF for
Correspondence: R.C. Ray, Central Tuber Crops Research Institute
(Regional Centre), Dumuduma Housing Board, Bhubaneswar – 751019,
India
E-mail: rc_ray@rediffmail.com
Fax: 91-674-2470528
various end products such as animal feed after enrich-
ing the protein content using fungi (Ray et al. 2006),
enzymes (Pandey et al. 2000b); organic acids (Kolichsky
et al. 1995); aroma compounds (Christen et al. 1997,
Bramorski et al. 1989), gibberllic acid (Tomasini et al.
1997), etc.
Approximately 90% of all industrial enzymes are
produced in submerged fermentation (SmF), frequently
using specifically optimized and genetically manipu-
lated microorganisms. However, SSF constitutes an
interesting alternative since the metabolites so pro-
duced are concentrated and purification process costs
less over SmF (Nigam and Singh 1995, Pandey et al.
2000a). SSF is defined as the cultivation of microorgan-
isms on moist solid support, either on inert carriers or
insoluble substrates that can, in addition be used as
carbon and energy source. SSF takes place in the ab-
sence and near absence of free water, thus being close
to the natural environment to which microorganisms
are adapted (Holker et al. 2004). The aim of SSF is to
bring the cultivated microorganisms into tight contact
with the insoluble substrate and thus to achieve the
highest substrate concentration during fermentation.
Response surface methodology (RSM) is an experi-
mental strategy for seeking the optimum conditions for
a multivariable system (He et al. 2004) and is used for

418
M. R. Swain and R. C. Ray
Journal of Basic Microbiology 2007, 47, 417 – 425
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
optimization of culture conditions (Rao et al. 1993). RSM
consists of a group of mathematical and statistical pro-
cedures that can be used to study relationships between
one or more responses and a number of independent
variables. In addition to analyzing the effect of inde-
pendent variables, this experimental methodology gen-
erates a mathematical model that accurately describes
the over all process (Senanayake and Shahidi 2002,
He et al. 2004). Statistical optimization not only allows
quick screening of large experimental domain, but also
reflects the role of each of the components. RSM has
already been successfully applied for optimization of
the media and culture conditions in many cultivation
processes for the production of primary and secondary
metabolites (Shirai et al. 2001, Boyaci 2005) i.e., amino
acid (Xiong et al. 2005), ethanol (Carvalho et al. 2003),
flavouring compound (acetoin) (Xian et al. 2007) and
enzymes (Rao and Satyanarayana 2003).
In our earlier study it was found that Bacillus subtilis
strains were one of the predominant groups of bac-
teria isolated from the culturable cowdung microflora
(Swain and Ray 2007). These strains exhibit several
beneficial agricultural activities like biocontrol against
Fusarium oxysporum and Botryodiplodia theobromae (Swain
and Ray 2007), production of indole-3-acetic acid in
enhancing sprouting of yam minisetts (Swain et al.
2007) and thermostable enzyme (
α-amylase) in sub-
merged fermentation (Swain et al. 2006). The present
study was carried out to investigate the
α-amylase pro-
duction by B. subtilis strain CM3 isolated from cowdung
microflora in SSF using CFR as the substrate and opti-
mization of the fermentation parameters (incubation
period, medium initial pH, moisture holding capacity
(MHC) and temperature) by applying RSM.
Materials and methods
Bacillus subtilis strain
Bacillus subtilis strain CM3 earlier isolated from cultur-
able cowdung microflora (Swain and Ray 2007) was
found to produce thermostable (
≈60 °C) α-amylase in
submerged fermentation (Swain et al. 2006). In SSF
study, this strain (CM3) was used. The culture was
maintained on NA (nutrient agar) slants at 4 °C.
Cassava fibrous residue (CFR): CFR [(g/100 g dry resi-
due); moisture: 11.2; starch: 63.0; crude fibre: 10.8;
crude protein: 0.9 and total ash: 1.2] was used as solid
substrate (support and nutrient source) for SSF. CFR
was collected during starch extraction (October –
November, 2006) from cassava using the mobile starch
extraction plant, developed by our institute (Edison
et al. 2006). Because of its high water content (70–80%)
and presence of high quantity of starch (63% on dry
weight basis), the residues were de-watered, sun-dried
for 6 – 8 days and then oven – dried at 80 °C for 24 h to
prevent microbial deterioration. The dried CFR was
stored in air-tight container until required.
Optimization of incubation period, initial medium pH,
MHC and temperature by applying RSM
The characterization of different factors for
α-amylase
production was optimized by applying RSM. The statis-
tical model was obtained using Central Composite De-
sign (CCD) with four independent variables [incubation
period (A), initial medium pH (B), moisture holding
capacity (C) and temperature (D)]. Each factor in this
design was studied at five different levels (Table 1).
A set of 30 experiments was performed. All variables
were taken at a central coded value considered as zero.
The minimum and maximum ranges of variables were
used and the full experimental plan with respect to
their values in coded form is listed in Table 2. Upon
completion of the experiments, the average of
α-amy-
lase production was taken as the dependent variable or
response.
Statistical analysis and modeling
The data obtained from RSM on
α-amylase production
were subjected to the analysis of variance (ANOVA). The
results of RSM were used to fit a second order polyno-
mial equation (1) as it represents the behaviour of such
a system more appropriately.
Y= β
0
+
β
1
A + β
2
B + β
3
C + β
4
D + β
1
β
1
A
2
+
β
2
β
2
B
2
+
β
3
β
3
C
2
+
β
4
β
4
D
2
+
β
1
β
2
AB + β
1
β
3
AC + β
2
β
3
BC
+
β
1
β
4
AD + β
2
β
4
BD + β
3
β
4
CD (1)
where Y is response variable,
β
0
is intercept,
β
1
,
β
2
,
β
3
and
β
4
are linear coefficients,
β
1,1
,
β
2,2
,
β
3,3
and
β
4,4
are
squared coefficient,
β
1,2
,
β
1,3
,
β
2,3
,
β
1,4
,
β
2,4
and
β
3,4
are
interaction coefficient and A, B, C, D, A
2
, B
2
, C
2
, D
2
, AB,
AC, BC, AD, BD and CD are level of independent vari-
ables. Statistical significance of the model equation was
Table 1. Range of the values for the response surface metho-
dology.
Levels
Independent
variables
–
α
–1
0 +1 +
α
Incubation period
(Days)
– 2
2
6
10
14
Initial medium pH
3
5
7
9
11
Moisture holding
capacity (%)
20
40
60
80
100
Temperature (°C)
0
20
40
60
80

Journal of Basic Microbiology 2007, 47, 417 – 425
Alpha-amylase production by B. subtilis 419
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Table 2. Experimental design and result of CCD of response surface methodology.
Std.
A: Incubation period
(h)
B: pH
C: Moisture holding apacity
(%)
D: Temperature
(°C)
Enzyme production
(U/gds)
Predicted
Experimental
1
–1
–1
–1
–1
3232
3039
2
1
–1
–1
–1
3375
2992
3
–1
1
–1
–1
3610
3613
4
1
1
–1
–1
3785
3613
5
–1
–1
1
–1
3500
3340
6
1
–1
1
–1
3631
3293
7
–1
1
1
–1
3890
3962
8
1
1
1
–1
4033
3915
9
–1
–1
–1
1
3716
3502
10
1
–1
–1
1
3847
3455
11
–1
1
–1
1
4106
4123
12
1
1
–1
1
4248
4076
13
–1
–1
1
1
3972
3803
14
1
–1
1
1
4091
3756
15
–1
1
1
1
4374
4425
16
1
1
1
1
4505
4378
17 –
α
0
0
0
2812
2543
18
α
0
0
0
3630
3529
19
0
–
α
0
0
4980
5809
20
0
α
0
0
5702
5722
21
0
0
–
α
0
5402
5254
22
0
0
α
0
5398
5598
23
0
0
0
–
α 2150
2057
24
0
0
0
α 3328
3052
25
0
0
0
0
6386
6462
26
0
0
0
0
6386
6430
27
0
0
0
0
6386
6380
28
0
0
0
0
6386
6480
29
0
0
0
0
6386
6256
30
0
0
0
0
6386
6311
determined by Fisher’s test value, and the production
of variance explained by the model was given by the
multiple coefficient of determination, R squared (R
2
)
value. Design Expert (ver, 7.1; Statease Inc. Minneapo-
lis, MN, USA) was used in this investigation.
Effect of incubation period on enzyme production
The inoculum was prepared in soluble starch-peptone
broth (soluble starch: 2%; peptone: 1%; MgSO
4
: 0.05%;
NaCl: 0.05%, pH: 7.0) (Swain et al. 2006) by transferring
a loop full of organism (B. subtilis CM3) from a stock
culture and incubating at 50 °C and 120 rpm for 24 h in
an orbital incubator shaker (Remi Pvt. Ltd, Bombay,
India). The inoculum contained 1
× 10
7
CFU/ml.
CFR (20
g) was taken in Roux bottles (132
mm
× 275 mm), moistened with 27 ml of distilled water
containing 1% peptone to provide 70% moisture hold-
ing capacity (MHC) and were mixed thoroughly. The
bottles were autoclaved at 15 lb pressure for 30 min.
After autoclaving the bottles were taken out and cooled
at room temperature, 30
± 2 °C and inoculated with
15% (w/v) inoculum (1
× 10
7
CFU/ml). Then the inocu-
lated substrates were incubated under static condition
at 50 °C for 10 days in an incubator (Beautex Instru-
ments, New Delhi, India). Triplicate flasks were main-
tained for each treatment. The contents in the bottle
were periodically mixed by gentle tapping. At interval
of two days, the bottles were taken out and the enzyme
was extracted with 25 ml of distilled water [1 : 2 (CFR:
Water) ratio] and squeezed through wet cheesecloth.
The pooled enzyme extract was centrifuged at 8000 g
for 20 min in a refrigerated centrifuge (Remi India Pvt
Ltd, Bombay, India) and the clear supernatant (volume
made up to 25 ml) was used for enzyme assay.
Effect of MHC and initial medium pH
on enzyme production
The influence of MHC on the enzyme titre was evalu-
ated by varying the moisture content of the substrate
from 40 to 80% MHC, and initial medium pHs were
adjusted to 5 – 9 by using 0.1 N HCl or NaOH. The sam-
ples (n = 3) were incubated for six days at 50 °C.

420
M. R. Swain and R. C. Ray
Journal of Basic Microbiology 2007, 47, 417 – 425
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Effect of temperature on enzyme production
The effect of temperature was studied by evaluating the
organism at different temperatures (20 – 60 °C) main-
tained in an incubator.
Amylase assay
The amylase assay was based on the reduction in blue
colour intensity resulting from enzymatic hydrolysis of
starch and formation of starch-iodine complex (Swain
et al. 2006). The reaction mixture consisted of 0.2 ml
enzyme (cell free supernatant), 0.25 ml of 0.1% starch
solution and 0.5 ml of phosphate buffer (0.1 M, pH 6.0)
incubated at 50 °C for 10 min. The reaction was stopped
by adding 0.25 ml of 0.1 N HCl and the colour was de-
veloped by adding 0.25 ml of I/KI solution (2% KI in
0.2% I). The optical density (OD) of the blue colour solu-
tion was determined using a UV-Vis Spectrophotometer
(Model no CE 7250, Cecil Instrument, UK) at 690 nm.
One unit of enzyme activity is defined as the quantity
of enzyme that causes 0.01% reduction of blue colour
intensity of starch iodine solution at 50 °C in one min-
ute per ml (Swain et al. 2006). In SSF, units of enzyme
activity are calculated as units per gram of dry sub-
strate (gds).
Determination of moisture of the substrate
The moisture content of the substrate was analyzed by
a Mettler LP16 Infra – Red analyzer.
Rate of hydrolysis of starch
A 2% (w/v) solution of soluble starch and cassava starch
were incubated with 2 – 4 ml of B. subtilis crude enzyme
at 50 °C in an incubator. The degradation of starch was
evaluated at one-hour interval up to 5 h.
Results
Optimization of incubation period, initial medium pH,
MHC and temperature by applying RSM
The results of CCD experiments for studying the effect
of four independent fermentation variables (incubation
period, initial medium pH, MHC and temperature) are
presented along with the mean predicted and observed
responses in Table 2. The regression equations obtained
after the ANOVA gave the level of
α-amylase production
as a function of the initial values of incubation period,
pH, MHC and temperature. The final response equation
that represented a suitable model for
α-amylase pro-
duction is given below:
Y = 79.91
+
0.64
× A + 1.64 × B + 1.04 × C + 2.11
× D – 6.74 × A
2
– 2.05
× B
2
– 2.05
× C
2
– 7.89
× D
2
+ 0.033
× AB – 0.020 × AC + 0.026
× BC – 0.018
× AD – 0.056 × BD – 0.064 × CD
where Y is enzyme production, A is incubation period
(days), B is initial medium pH, C is MHC (%) and D is
temperature (°C).
The coefficient of determination (R
2
) was calculated
as 0.9587 for
α-amylase production (Table 3), indicating
that the statistical model can explain 95.87% of vari-
ability in the response. The R
2
value is always between
0 and 1. The closer the R
2
is to 1.0, the stronger the
model and the better it predicts the response (Rao and
Satyanarayana 2003). An adequate precision of 17.850
for
α-amylase production was recorded. The predicted
R
2
(0.7646) is in reasonable agreement with the adjusted
R
2
(0.9202). This indicated a good agreement between
the experimental and predicted value for
α-amylase
production.
The
model
F-value of 24.89 and values of “prob > F”
less than 0.05 indicated that the model terms are sig-
nificant. For
α-amylase production B, D, A
2
, B
2
, C
2
and D
2
are significant model. The “lack of fit F-value” of 38.78
implied that the “lack of fit” is significant.
Response surface was generated by plotting the re-
sponse (
α-amylase production) on the z-axis against any
two independent variables while keeping the other
independent variables at zero level. Therefore, six re-
sponse surfaces were obtained by considering all the
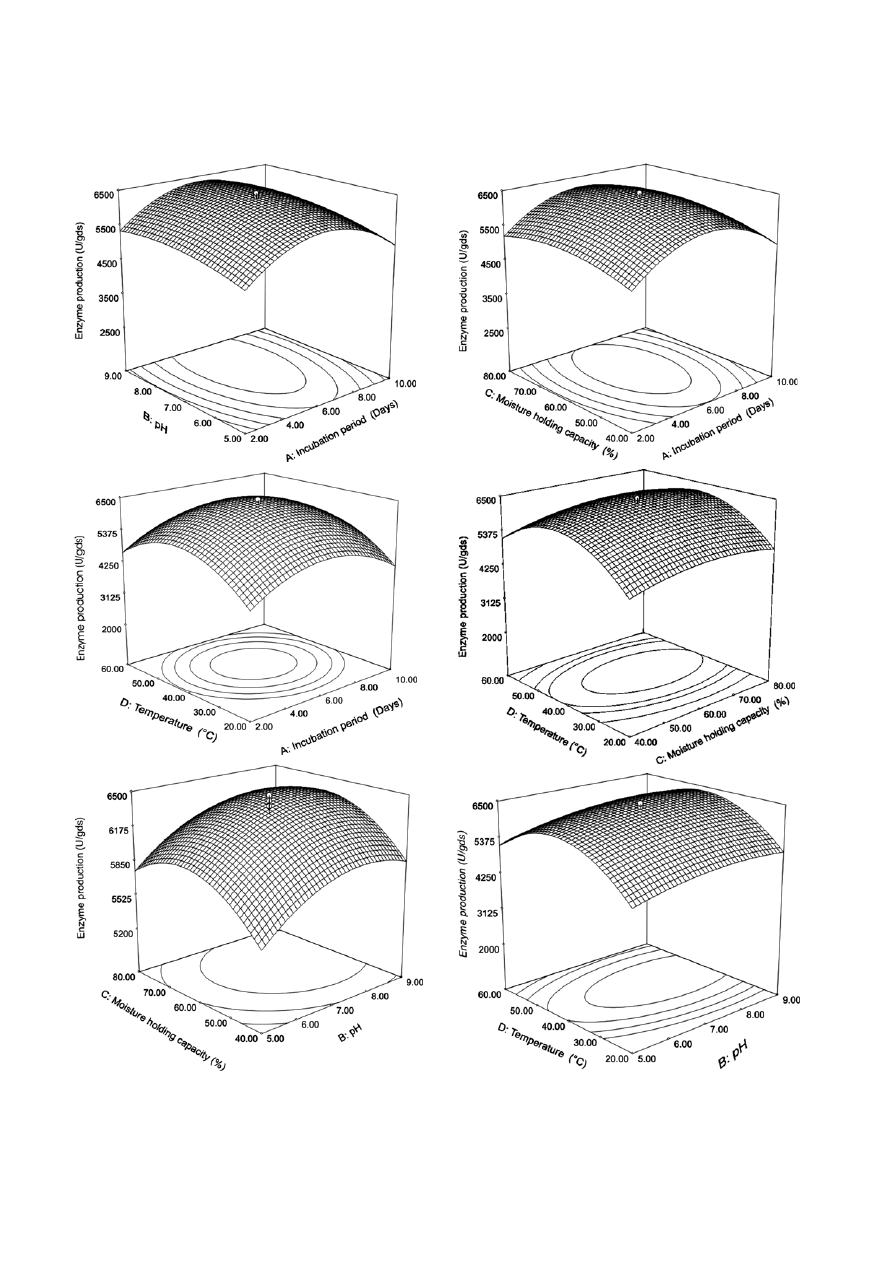
possible combinations. Fig. 1A depicts three dimen-
sional diagram and a contour plot of calculated re-
sponse surface from the interaction between incuba-
tion period and pH while keeping the other two vari-
ables (MHC and temperature) at ‘0’ level. A linear in-
crease in
α-amylase production was observed when
Table 3. ANOVA for
α-amylase production in solid state fermentation.
Source
Sum of Squares
Degree of freedom
Mean Square
F-Value
p-value
Model 1387.73 14
200.15 24.89 0.0001
Pure error
1.54
5
0.31
Total 2922.65 29
R
2
= 0.9587, Adjusted
R
2
= 0.9202, Predicted
R
2
= 0.7646, Adequate precision = 17.850, Lack of Fit
F-value = 38.78.
Journal of Basic Microbiology 2007, 47, 417 – 425
Alpha-amylase production by B. subtilis 421
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 1. Statistical optimization of enzyme production using RSM. A: incubation period; B: pH; C: moisture holding capacity and
D: temperature holding capacity.
A
B
C
D
E
F
422
M. R. Swain and R. C. Ray
Journal of Basic Microbiology 2007, 47, 417 – 425
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
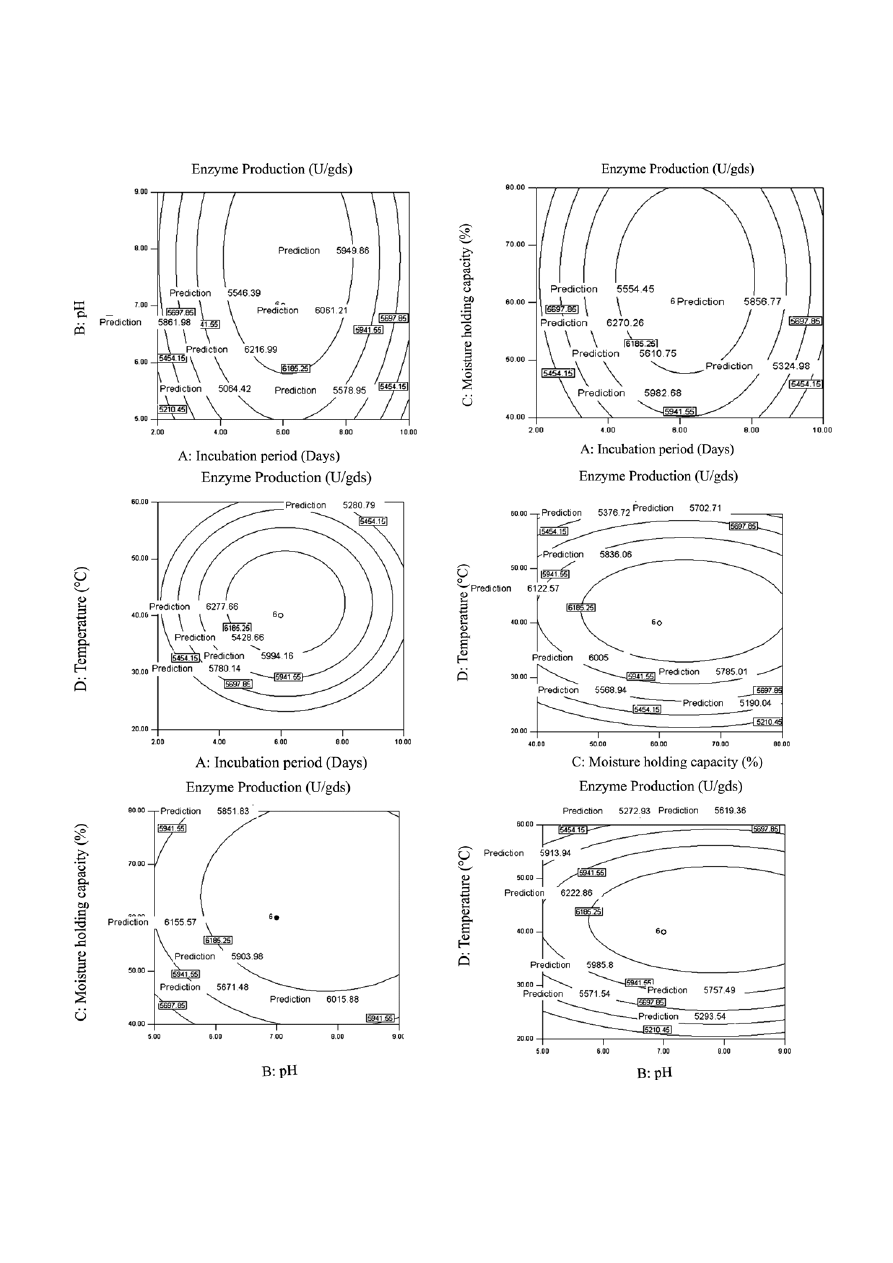
Figure 2.
Contour plot of the effect of A: pH and incubation period; B: incubation period and moisture holding capacity; C: incubation
period and temperature; D: pH and moisture holding capacity; E: pH and temperature and F: temperature and moisture holding.
A
B
C
D
E
F
Journal of Basic Microbiology 2007, 47, 417 – 425
Alpha-amylase production by B. subtilis 423
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
incubation period was increased up to 6 days, and there
after, it declined. In case of medium pH,
α-amylase
production was increased up to pH 8.0 and then de-
clined. When the level of MHC (%) was increased from
40 to 70%, a linear increase in
α-amylase production
was recorded up to day 6 and declined there after
(Fig. 1B). The response between incubation period and
temperature indicated that temperature at 50 °C was
optimum with 6 days incubation period for
α-amylase
production (Fig. 1C). The response surface was mainly
used to find out the optima of the variables for which
the response was maximized. An interaction between
the remaining two parameters (MHC and temperature)
(Fig. 1D) suggested a little difference with the earlier
responses. Fig. 1E and F represented the three dimen-
sional diagram and contour plots of calculated re-
sponse surface from the interaction between MHC and
pH, and temperature and pH, respectively. The six con-
tour plots proved the significance of earlier response
i.e. incubation period with pH, incubation period with
MHC, Incubation period with temperature, MHC with
temperature, pH with MHC, pH with temperature, and
MHC with temperature (Fig. 2A, B, C, D, E and F). Thus
Incubation period (6 days), initial medium pH (8.0),
MHC (70%) and temperature (50 °C) were adequate for
attaining maximum enzyme titre (6462 U/gds) as shown
in Table 2.
Validation of model
Validation was carried out in shake flasks under condi-
tions predicted by the model. The experimental values
were found to be very close to the predicted values and
hence, the model was successfully validated. Validation
of the statistical model and regression equation were
performed by taking A (6 days), B (8.0), C (70%) and D
(50 °C) in the experiment. The predicted response for
α-amylase production was 6362 U/gds, while the actual
(experimental) response was 6462 U/gds, thus proving
the validity.
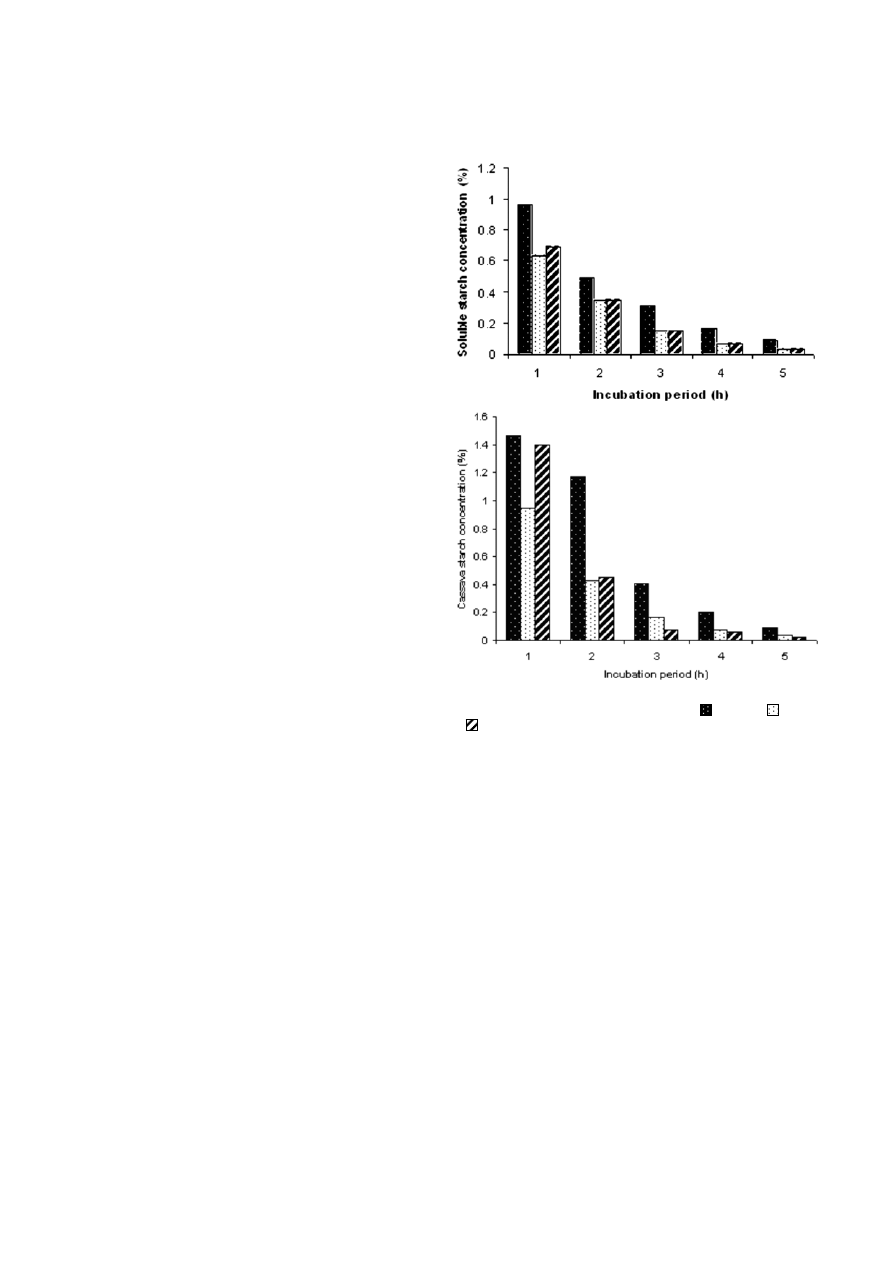
Rate of hydrolysis of starch
The rate of hydrolysis of 2% (w/v) soluble starch and
cassava starch by
B. subtilis CM3 α-amylase is shown in
Fig. 3. There was a gradual hydrolysis of starches with
increase in incubation period from 1 to 5 h and the rate
of hydrolysis also increased with the increase in en-
zyme concentration. With application of 4 ml crude
enzyme (
≈24,800 U/gds) there was 99% hydrolysis of
soluble as well as cassava starch.
Figure 3. Hydrolysis of commercial starch (A) and cassava starch
(B) by different
α-amylase concentrations (– – 2 ml, – – 3 ml,
– – 4 ml) from B. subtilis.
Discussion
B. subtilis is one of the best characterized organisms in
gram positive bacteria. It is safe, stable and widely used
in industrial fermentation process (Schallmey
et al.
2004). CFR is widely used as solid substrate for produc-
tion of several industrially important enzymes, i.e.,
amylase, cellulase, protease, lipases, etc. (Pandey
et al.
2000a) and other value added products (Ray
et al. 2006,
Ray and Moorthy 2007). In our previous study,
B. sub-
tilis strain CM3 was chosen as the best α-amylase pro-
ducer in SmF among five selected strains (CM1 – CM5)
isolated from cowdung microflora (Swain
et al. 2006).
The optimum temperature, pH and incubation
temperature for
α-amylase production by B. subtilis
CM3 were 50 – 70%, 5 – 9 and 36 h, respectively, in SmF.
The purified enzyme was in two forms with mole-
cular mass of 18.0 and 43.0 kDa in native SDS-PAGE.
However, optimization of culture parameters in
A
B

424
M. R. Swain and R. C. Ray
Journal of Basic Microbiology 2007, 47, 417 – 425
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
SSF often vary from SmF due to several physiologi-
cal factors (mainly water potential) (Durand
et al.
1997, Pandey
et al. 2000b), as evident from this
study.
RSM used in this investigation suggested the impor-
tance of various fermentation parameters at different
levels. The methodology employed will be successful to
any process, where an analysis of the effects and inter-
action of many experimental factors are required. CCD
maximizes the amount of information that can be ob-
tained, while limiting the numbers of individual ex-
periments (Kunamneni and Singh 2005). Thus, smaller
and less time consuming experimental designs could
generally suffice for the optimization of many fermen-
tation processes. The results of this study endorse this
viewpoint. In this study, a high similarity was observed
between the predicted and experimental results, which
reflected the accuracy and applicability of RSM to op-
timize the process for enzyme production in SSF. In
this study, an incubation period of 6 days, initial me-
dium pH of 8.0, moisture-holding capacity of 70%
and temperature at 50°C were the major factors that
influenced the enzyme titre. The decrease in enzyme
production above 50% MHC was probably due to inacti-
vation of the enzyme at higher (>50 °C) temperature
(Baysal
et al. 2003). Moisture holding capacity is another
important factor in SSF that influence the growth
of the microorganisms and there by enzyme production
(Durand
et al. 1997, Yang and Wang 1999). In general,
MHC between 50 to 70% is found suitable for
α-amylase
production by various microorganisms (Pandey
et al.
2000b, Ray
et al. 2006). In this context, our results
corroborated with these reports. Beyond 70% MHC,
the enzyme activity by
B. subtilis CM3 in this study
declined. The decline of enzyme activity might be
attributed to poor porosity, lower oxygen transfer, poor
aeration and adsorption of enzyme to the substrate
particle (Pandey
et al. 2000a, b). When the applicability
of the
B. subtilis amylase in liquefying starch was
studied at its optimum temperature of 50 °C, the crude
enzyme (4 ml) could hydrolyze starch (
≈99%) after
5 h of incubation. Further study is in progress in our
laboratory to utilize the
B. subtilis crude enzyme
for liquefaction of cassava starch for production of
ethanol.
References
Bramorski, A., Soccol, C.R., Christen, P. and Revah. S., 1989.
Fruity aroma production by Ceratacystis fimbriata in solid
culture from agro-industrial wastes. Rev. Microbiol.,
29,
208 – 212.
Baysal, Z., Uyar, F. and Aytekin, C., 2003. Solid-state fermenta-
tion for production of
α-amylase by a thermotolerant Bacil-
lus subtilis from hot spring water. Process Biochem.,
38,
1665 – 1668.
Boyaci, I.H., 2005. A new approach of determination of enzy-
me kinetic constants using response surface methodology.
Biochem. Eng. J.,
25, 55–62.
Carvalho, J.C.M., Vitolo, M., Sato, S. and Aquarone, E., 2003.
Ethanol production by Saccharomyces cerevisiae grown in su-
garcane blackstrap molasses through a feed batch process:
optimization by response surface methodology. Appl. Bio-
chem. Biotechnol.,
110, 151–164.
Christen, P., Meza, J.C. and Revah, S., 1997. Fruity aroma
production in solid state fermentation by Ceratacystis fimbri-
ata: influence of the substrate type and the presence of pre-
cursors. Mycol. Res.,
101, 911–919.
Durand, A., Almanzaa, S., Renaud, R. and Maratray, J., 1997.
Solid state fermentation: attractive alternative to sub-
merged liquid fermentations. Agro Food Ind. Hi-Tech.,
8,
39 – 42.
Edison, S., Anantharaman, M. and Srinivas, T., 2006. Status of
cassava in India – an overall view. Tech. Bull. Ser. 46, Cent-
ral Tuber Crops Research Institute, Thiruvanathapuram,
India, p. 79.
He, G.Q., Kong, Q. and Dingm, L.X., 2004. Response surface
methodology for optimizing the fermentation medium of
Clostridium butyricum. Lett. Appl. Microbiol.,
39, 363–368.
Holker, U., Hofer, M. and Lenz, J. 2004. Biotechnological ad-
vantages of laboratory-scale solid state fermentation with
fungi. Appl. Microbiol. Biotechnol.,
64, 175–186.
Jyothi, A.N., Sasikiran, K., Nambisan, B. and Balagopalan, C.,
2005. Optimization of glutamic acid production from cas-
sava starch factory residues using Brevibacterium divaricatum.
Process Biochem.,
40, 3576–3579.
Kolicheski, M.B., Soccal, C.R., Marin, B., Medeiros, E. and
Raimbault, M., 1995. Citric acid production on three cellu-
losic support in solid state fermentation. In: Advance in
Solid State Fermentation (S. Roussas, B.K. Lonsane, M. Rai-
mabult, G. Viniegra-Gonzalez, eds.), pp. 447 – 460. Kluwer
Academic Publisher Dordrecht.
Kunamneni, A. and Singh, S., 2005. Response surface optimi-
zation of enzymatic hydrolysis of maize starch for higher
glucose production. Biochem. Eng. J.,
27, 179–190.
Nigam, P. and Singh, D., 1995. Enzyme and microbial systems
involved in starch processing. Enz. Microb. Technol.,
17,
770 – 778.
Pandey, A., Soccol, C.R. and Mitchell, W., 2000a. New deve-
lopment in solid state fermentation: I. Bioprocess and pro-
ducts. Process Biochem.,
35, 1153–1169.
Pandey, A., Soccol, C.R., Nigam, P., Soccol, V.T., Vandenberg-
he, L.P.S. and Mohan, R., 2000b. Biotechnological potential
of agro-individual residues. II. Cassava bagasse. Biores.
Technol.,
74, 81–87.
Rao, J.L.M. and Satyanarayana, T., 2003. Statistical optimiza-
tion of a high maltose-forming, hyperthermostable and
Ca
2+
-independent
α-amylase production by an extreme ther-
mophile Geobacillus thermoleovorans using response surface
methodology. J. Appl. Microbiol.,
95, 712–718.
Rao, P.V. Jayaraman, K. and Lakshmanan, C.M., 1993. Produc-
tion of lipase by Candida rugosa in solid-state fermentation,

Journal of Basic Microbiology 2007, 47, 417 – 425
Alpha-amylase production by B. subtilis 425
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
medium optimization and effect of aeration. Process Bio-
chem.,
28, 391–395.
Ray, R.C. and Moorthy, S.N., 2007. Exopolysaccharide (pull-
ulan) production from cassava starch residue by Aureobasi-
dium pullulans strain MTTC 1991. J. Sci. Ind. Res.,
66, 252–
255.
Ray, R.C., Sahoo A.K., Asana, K. and Tomita, F., 2006. Micro-
bial processing of agricultural residues for production of
food, feed and food-additives. In: Microbial Biotechnology
in Agriculture and Aquaculture Vol. II (R.C. Ray, ed.),
pp. 511 – 552. Science Publishers Inc. Enfield, New Hamp-
shire.
Schallmey, M., Singh, A. and Ward, O.P., 2004. Developments
in the use of Bacillus species for industrial production.
Can.
J. Microbiol.,
50, 1–17.
Senanayake, S.P.J.N and Shahidi, F., 2002. Lipase catalysed
incorporation of docosahexaenoic acid (DHA) in to borage
oil: optimization using response surface methodology. Food
Chem.,
77, 115–123.
Shirai, K., Guerrero, I., Huerta, S., Saucedo, G., Castillo, A.,
Gorzalez, R.O. and Hall, G.M., 2001. Effect of initial glucose
concentration and inoculum level of lactic acid bacteria in
shrimp waste ensilation. Enz. Microb. Technol.,
28, 446–
452.
Swain, M.R. and Ray, R.C., 2007. Biocontrol and other benefi-
cial activities of Bacillus subtilis isolated from cowdung mi-
croflora. Microbiol. Res., doi:10.1016/j.micres.2006.10.009.
Swain, M.R., Naskar, S.K. and Ray, R.C., 2007. Indole-3-acetic
acid production and effect on sprouting of yam (Dioscorea
rotundata L.) minisetts by Bacillus subtilis isolated from cul-
turable cowdung microflora. Polish J. Microbiol.,
56, 103–
110.
Swain, M.R., Kar, S., Padmaja, G. and Ray, R.C., 2006. Partial
characterization and optimization of production of ex-
tracellular
α-amylase from Bacillus subtilis isolated from cul-
turable cowdung microflora. Polish J. Microbiol.,
55, 289–
296.
Tomasini, A., Fajardo, C. and Barrios-Gonzalez, J., 1997. Gib-
berllic acid production using different solid state fermenta-
tions. Process Biochem.,
37, 637–641.
Xian, J.Z., Lin, P.H., Qin, J.Y. and Xu, P., 2007. Statistical opti-
mization of medium composition for enhanced acetoin
production from molasses and soybean meal hydrolysate
Appl. Microbiol Biotechnol.,
74, 61–68.
Xiong, C., Shouwen, C., Ming, S. and Ziniu, Y., 2005. Medium
optimization by response surface methodology for poly-y-
glutamic acid production using dairy manure as the basis
of a solid substrate. Appl. Microbiol. Biotechnol.,
69, 390–
396.
Yang, S.S and Wang, J.Y., 1999. Protease and amylase produc-
tion of Streptomyces rimosus in submerged and solid state
cultivations. Bot. Bull. Acad. Sin.,
40, 259–265.
Wyszukiwarka
Podobne podstrony:
jobm 200710325
jobm 200710320
jobm 200710313
jobm 200710333
jobm 200710111
jobm 200710341
jobm 200710318
jobm 200710317
jobm 200710310
jobm 200710337
jobm 3620260101
200710s11 OgarnijTemat comid 26410 (2)
20071010
20071002CV PL Prof Grudzewski, a
20071002CV PL Prof Grudzewski, a
200710311013330 Coatedproductsu Nieznany (2)
jobm 3620250801
20071031
jobm 3620260805
więcej podobnych podstron