Received: 2 April 2009,
Revised: 23 June 2009,
Accepted: 29 June 2009,
Published online in Wiley Online Library: 3 August 2009
New (PP/EPR)/nano-CaCO
3
based formulations
in the perspective of polymer recycling. Effect
of nanoparticles properties and
compatibilizers
Nizar Mnif
a , b
*, Vale´rie Massardier
a
, Tasnim Kallel
b
and Boubaker Elleuch
b
Phase structure of composite polypropylene (PP)/ethylene–propylene–rubber (EPR)/coated nano-CaCO
3
composites,
used in the manufacture of bumpers, with and without compatibilizers has been investigated using scanning electron
microscopy (SEM), dynamic mechanical analysis (DMA) mechanical tests, and differential scanning calorimetry (DSC).
Blends of various compositions were prepared using a corotating twin-screw extruder. The experimental results
indicated that the dispersion of nanoparticles in (PP/EPR) depends on their surface (stearic acid and fatty acid
coatings). In both cases, the final morphology is the core–shell structure in which EPR acts as the shell part
encapsulating coated nano-CaCO
3
. In this case, EPR-g-MAH copolymer does not improve the interface between
(PP/EPR) and nanoparticles but PEP propylene ethylene copolymer should be preferentially localized at the interface
of PP and (EPR/nano-CaCO
3
) phases generating an improved adherence, which will ensure a better cohesion of the
whole material. According to the nature of the compatibilizers and surface treatment, it is believed that the synergistic
effect of both the EPR elastomer and CaCO
3
nanoparticles should account for the balanced performance of the ternary
composites. Copyright
ß 2009 John Wiley & Sons, Ltd.
Keywords: polypropylene; EPR; calcium carbonate; compatibilization; recycling
INTRODUCTION
With an annual production of more than 250,000 tons in Europe,
polypropylene is one of the most widely used polymers.
Polypropylene is used in automobiles, household appliances,
etc. In order to overcome its high flammability, tendency to
brittleness at temperatures below its glass transition tempera-
ture, and low stiffness, polypropylene can be modified by fillers
(CaCO
3
, alumina, silica, etc.) and elastomers such as ethylene
propylene rubber (EPR) and ethylene propylene diene monomer
(EPDM). However, EPR particles decrease tensile properties of PP
such as yield strength and Young’s modulus. So, to compensate
the effect of EPR content, the addition of rigid fillers such as
calcium carbonate (CaCO
3
) is recommended.
[1]
Nanofillers, such
as nanosilica for example, can be used as compatibilizer instead
of copolymers.
[2]
This prospective study aims at studying new
(PP/EPR) based formulations containing nano-CaCO
3
fillers in the
perspective of processing more complex blends when recycling.
Indeed, as polymer sorting often produces complex mixtures (a
polymer in major proportions and others in minor proportions)
for being economy efficient, it may be interesting to substitute
conventional fillers by their homologous nanosized ones in order
to facilitate the recycling of complex blends using nano-CaCO
3
based compatibilization.
[3]
Postconsumer recycled polypropylene containing elastomers
(EPR, EPDM, EOC, etc.) and fillers (CaCO
3
, silica, etc.) from
automobile bumpers represent considerable amount of waste. To
recover high volumes of polymer materials with economic
efficiency, one solution is to recover polymers with lower degrees
of purity. In this perspective, complex formulations as well as
associated parts can be recovered with simplified sorting.
Usually, to counterbalance or to take advantage of some
mixtures, it is necessary to find the chemical formulation that will
upgrade mechanical properties. To this end, compatibilizers, such
as copolymers (PEP: copolymer of ethylene and propylene, EP-g-
MAH: copolymer of ethylene and propylene grafted maleic
anhydride, etc.)
[3]
and more recently nanofillers,
[2]
are often used.
Considering this strategy of recycling, (PP/EPR) formulations
could be mixed with nano-CaCO
3
instead of micron size CaCO
3
that is often used as a filler for polymer composites.
The produced blends are called hybrid composites. The
mechanical properties of hybrid composites are dictated not only
by their composition but also by their phase morphology.
(wileyonlinelibrary.com) DOI: 10.1002/pat.1520
Research Article
* Correspondence to: N. Mnif, Laboratoire des Mate´riaux Macromole´culaires,
Inge´nierie des Mate´riaux Polyme`res, IMP, UMR CNRS 5627, Universite´ de Lyon,
INSA de Lyon, Baˆt Jules Verne, 20 Av. A. Einstein, 69621 Villeurbanne Cedex,
France.
E-mail: nizar.mnif@insa-lyon.fr
a N. Mnif, V. Massardier
Universite de Lyon, Lyon, F-69003, France; INSA de Lyon, IMP/LMM
Laboratoire des Mate´riaux Macromole´culaires, Villeurbanne, F-69621, France;
CNRS, UMR 5223. Inge´nierie des Mate´riaux Polyme`res, Villeurbanne, F-69621,
France
b N. Mnif, T. Kallel, B. Elleuch
Laboratoire Eau/Energie/Environnement, Ecole Nationale des Inge´nieurs de
Sfax (ENIS), Rte Soukra 3038 —Sfax—Tunisie
Polym. Adv. Technol. 2010, 21 896–903
Copyright
ß 2009 John Wiley & Sons, Ltd.
896

According to the literature, two kinds of phase morphologies are
normally found: either the rubber and rigid particles are
dispersed separately in the polypropylene matrix, or the rigid
particles are encapsulated by rubber particles.
[2]
Earlier works
showed that the most important factor that can determine phase
morphology is the surface treatment of the rigid particles. Most of
the previous studies have focused on the relationship between
phase morphology and mechanical properties of PP–rubber–filler
systems.
[3–7]
Adhesive forces acting among the particles depend
on the free energy surface of the filler.
[8]
The coating of CaCO
3
with stearic acid leads to a significant decrease in surface
tension,
[9–14]
which results in decreased interactions and hope-
fully to limited aggregation.
The phase structure of ternary phase composites is influenced by
the melt rheology of the system, compounding techniques, and the
surface characteristics and mutual wettability of the fillers and
polymer components. In PP/EPDM/calcium carbonate composites,
the surface treatment of filler was found to result in a separate
dispersion of phases (EPDM and CaCO
3
), whereas encapsulation
occurred when untreated calcium carbonate was used.
[15,16]
To
promote the adhesion between the polymer and filler particles, the
functionalization of polymer phases was carried out. In PP/EPR/filler
systems, it was shown that incorporating PP functionalized with
maleic anhydride resulted in separate dispersions of elastomer and
filler. Using maleated EPR and nonfunctionalized PP gave a filler
encapsulation structure.
[17,18]
In the present work, the properties of (PP/EPR)/nano-CaCO
3
ternary composites, with and without compatibilizers (EPR-g-
MAH and PEP), are investigated. The research is focused on the
synergistic toughening effect of compatibilizer and nano-CaCO
3
alongside the influence of particles’ surface treatment. The
rheological and mechanical analyses of the components and
the ternary blends of (PP/EPR)/nano-CaCO
3
as well as the effect of
the compatibilizers were studied.
EXPERIMENTAL
Materials
(PP/EPR) (78/22) (PP108MF97), supplied by Sabic, is a blend of an
isotactic polypropylene matrix (78 wt%) and an EPR dispersed
phase (random copolymer containing 50% of ethylene) (22 wt%);
specific gravity, 0.905 g/cm
3
; melt flow index (MFI), 10 g/10 min
under 2.16 kg at 230
8C.
EPR-g-MAH was supplied by Exxon Mobil (Exxelor VA 1801;
M
n
¼ 80.000 g/mol; ethylene content, 69.6 wt%; propylene con-
tent, 29.8 wt%; and MAH content, 0.6 wt%).
Vistamaxx 6200 (PEP), a copolymer of ethylene and propylene
(%propylene
> 80 wt%), is the result of a specially controlled
metallocene
polymerization
process
(density
¼ 0.861 g/m
3
;
MFI
¼ 20 g/10 min (2308C/2.16 Kg)).
Calcium carbonate (CaCO
3
) was supplied by Solvay
1
. Proper-
ties are described in Table 1.
Preparation of blends
Blends with and without compatibilizer and nano-CaCO
3
were
premixed as pellets to the required proportions prior to
processing in a corotating twin-screw extruder (Clextral BC 21:
D
¼ 25 mm, L/D ¼ 36). The screw and the temperature profiles
used in this study are given in Fig. 1. The rotational speed of twin-
screw extruder is between 180 and 200 rpm (Fig. 1). Table 2
illustrates the composition of the blends.
Table 1. The characteristics of nanoparticles
Particle diameter
Surface coating
Specific surface (BET)
Socal 322V
30–50 nm
Stearic acid (3.5 wt%)
coating content
¼ 33 g/kg
coating ratio
¼ 1.1 mg/m
2
25 m
2
/g
Winnofil-SPM
100 nm
Stearic acid (2.5 wt%)
coating content
¼ 27 g/kg
coating ratio
¼ 1.5 mg/m
2
20 m
2
/g
Table 2. Composition of the blends
Blends
(PP/EPR) (wt%)
PEP (wt%)
EPR-g-MAH (wt%)
Nano-CaCO
3
(wt%)
Socal322V
WinnofilSPM
(PP/EPR)
(78/22)
0
0
0
0
(PP/EPR)/W
(74.5)/20.5
0
0
0
5
(PP/EPR)/W/EPR-g-MAH
(71)/20
0
4
0
5
(PP/EPR)/W/PEP
(71)/20
4
0
0
5
(PP/EPR)/S
(74.5)/20.5
0
5
0
(PP/EPR)/S/EPR-g-MAH
(71)/20
0
4
5
0
(PP/EPR)/W/PEP
(71)/20
4
0
5
0
Figure 1. Twin-screw extruder. Screw and temperature profile.
Polym. Adv. Technol. 2010, 21 896–903
Copyright
ß 2009 John Wiley & Sons, Ltd.
View this article online at wileyonlinelibrary.com
(PP/EPR)/NANO-CACO
3
BASED FORMULATIONS IN THE PERSPECTIVE OF POLYMER RECYCLING
897

A vertical injection-molding machine (Battenfeld Unilog B2;
350 Plus) was used for preparing the specimens for mechanical
tests. Conditions are: speed of injection: 15 mm/sec, pressure
limits: 50 bars, temperature: 220
8C, and time: 15 sec.
Morphologies
Morphologies were studied with a Hitachi S800 scanning electron
microscope at an accelerating voltage of 30 kV realized in the
Technological center of the Microstructures (CT
m) of Lyon
(France). The specimens were injection molded in a Battenfeld
Unilog B2 and fractured in liquid nitrogen and covered with gold
before being examined. All SEM micrographs are secondary
electron images. These images were treated using some special
software (image treatment, ImageTool; http://ddsdx.uthscsa.edu/
dig/itdesc.html) in order to estimate the diameter of particles.
The analyses as well as the calculation of the diameters of the
particles were carried out on 30 nodules dispersed on a surface of
120
mm
2
.
Thermal analysis
The thermal analyses were performed under argon using a
Mettler-Toledo SA differential scanning calorimeter (DSC).
Standard aluminum pans were used. Samples (10–20 mg) were
weighted directly in the pan and an empty pan was used as
reference. Temperature calibration was performed using indium.
Heating was carried out from room temperature to 200
8C and
samples were then held at 200
8C for 5 min before cooling to
1008C. Cooling and heating rates were both set to 10 K/min.
Dynamic mechanical analysis
The complex modulus (G
), its storage (G
0
), loss parts (G
00
), and the
mechanical loss factor (tand
¼ G
00
/G
0
), were analyzed as a function
of temperature (T) with dynamic mechanical analysis (DMA)
using a Rheometrics Model RDA700. DMA spectra were taken in a
tension mode at 1 Hz frequency in a broad temperature range
T
¼ 80 to 1708C (heating rate 28C/min).
Mechanical tests
Tensile tests were carried out on a MTS 2/M tester at a speed of
500 mm/min (preliminary tests on lower speeds were carried out
to find the test conditions of rupture). The mechanical behavior
was investigated using injection-molded samples. Charpy impact
tests were carried out at 21
18C using a falling-weight impact
tester (type Otto Wolpert–Werke Ludwigshafen a. Rh.) in
accordance with the French standard method NF T 51–035.
The results supplied correspond to an average of 20 measure-
ments for each blend. Tests were performed according to ISO
527–2 1BA (75
5 2 mm) standard methods (Temperature:
21
18C). A pre-crack of 2 mm was introduced at the center of
one edge of the rectangular bars. A pre-crack consisted of a saw
slot and a sharp crack tip, which was created with a mechanical
saw making of translatory movements high/low.
RESULTS AND DISCUSSION
Morphological properties
It is known that the dispersion of fillers in the polymer matrix can
have a significant effect on the mechanical properties of the
composites. The dispersion of an inorganic filler in the thermo-
plastic is not an easy process. The problem is even more severe,
when using nanoparticles as fillers, because the nanoparticles have
a strong tendency to agglomerate. Consequently, the homo-
geneous dispersion of the nanoparticles in the thermoplastic
matrix is a difficult process and a good dispersion can be achieved
by the surface modification of the filler particles and appropriate
processing conditions.
[19]
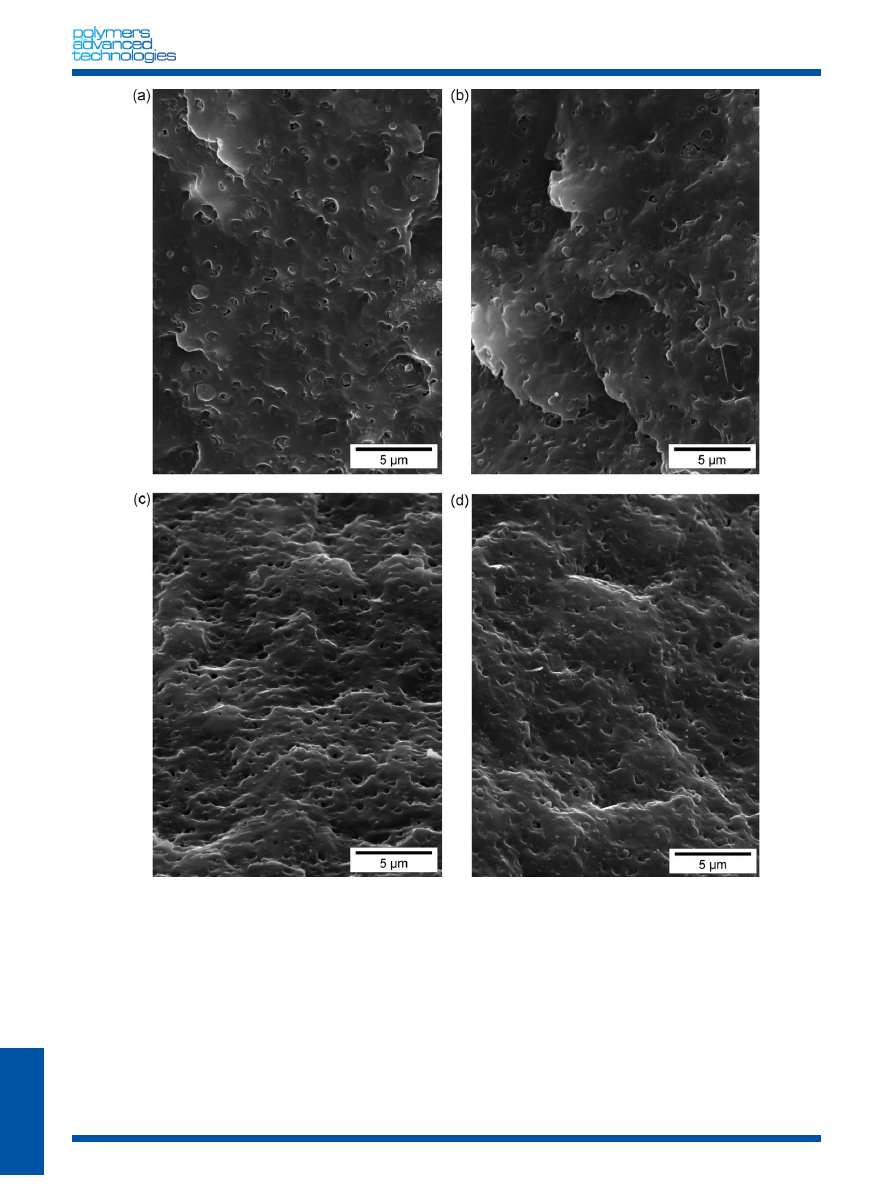
The observation of cryo-fractured
samples of the (PP/EPR) by SEM (Fig. 2(a)) displays a nodular
morphology, where the EPR morphology phase is dispersed in the
form of spherical particles (0.2–0.5
mm). In order to determine
whether stearic acid and fatty acid coatings of CaCO
3
particles really
help their dispersion in the PP/EPR matrix, SEM (Fig. 2(b) and 2(c))
was used to observe the fracture surfaces of samples fractured in
liquid nitrogen. According to these micrographs, the dispersion of
Winnofil CaCO
3
particles appears to be quite uniform; the size of
aggregates is lower than 2
mm. However, for specimens filled with
5 wt% of Socal322V [(PP/EPR)/S], the particles present large
agglomerates in the range of 3–12
mm (Fig. 2(c)). Indeed, the
presence of stearic acid decreased the adhesion between (PP/EPR)
and the particles of CaCO
3
. There are still presence of aggregates;
dispersion obviously is more difficult for the particles treated with
stearic acid. The particle–particle interaction is larger due to the
higher surface free energy. These aggregates are detrimental for
the toughening properties of these composites.
[20]
The large
aggregates create large voids upon loading and consequent
debonding, and could act as precursors for cracks.
[21]
The morphology of ternary blends with compatibilizers (EPR-g-
MAH and PEP) and nano-CaCO
3
is displayed in Fig. 3. Wang et al.
[22]
shows that according to routes were used to prepare the
PP/EPDM/nano-CaCO
3
tercomponent
composites
three
morphologies are observed: First, nano-CaCO
3
was randomly
distributed in the PP matrix and EPDM particles if the
components are premixed simultaneously. Second, nano-CaCO
3
particles and EPDM particles were separately distributed in the PP
matrix (PP and nano-CaCO
3
were mixed and EPRM is incorpor-
ated again). Finally, nano-CaCO
3
particles and their agglomerates
were embedded in EPDM particles to form a sandbag structure
like a sandbag for boxing. So two compatibilizers were used: the
PEP to improve PP/EPR interface and EPR-g-MAH for PP/nano-
CaCO
3
interface. In the case of the (PP/EPR)/W/EPR-g-MAH and
(PP/EPR)/S/EPR-g-MAH, Fig. 3(a) and 3(b) show that an increase of
the size of EPR particles (0.4–2
mm) and in the agglomerates of
CaCO
3
fillers (1–5
mm) and a bad adhesion between PP/EPR and
nanoparticles.
Figure 3(c) and 3(d) reveal the influence of PEP copolymer on
the dispersion of nano-CaCO
3
in the (PP/EPR) matrix. The
morphological properties reveal: a reduction in the size of the EPR
dispersed phase (0.1–0.5
mm) and an improvement of PP and
(EPR/nano-CaCO
3
) interface. Indeed, EPR and nano-CaCO
3
form a
core–shell structure in which elastomer may act as a shell part
encapsulating nanoparticules as suggested by Wang et al.
[22]
.
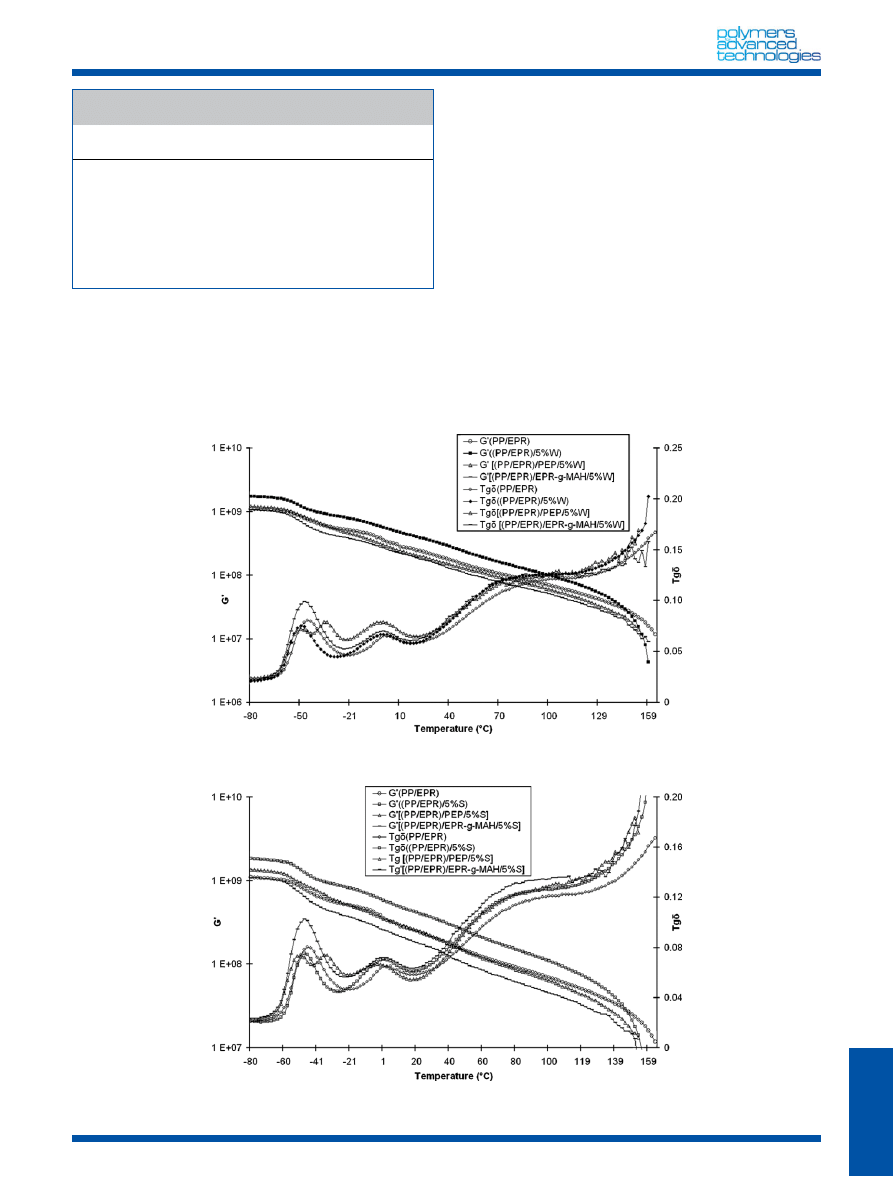
Dynamic mechanical properties
Dynamic mechanical properties of PP/EPR blends were studied
over a wide range of temperature (from
808C to 1608C) and the
results are reported in Table 3. As expected, three different
relaxations occur; the variation of the modulus and the tan d is
represented in Figs 4 and 5. The first corresponds to the
amorphous EPR component relaxation and is called b
EPR
(
468C).
The second (b
PP
(3
8C)) corresponds to the glass transition of
View this article online at wileyonlinelibrary.com
Copyright
ß 2009 John Wiley & Sons, Ltd.
Polym. Adv. Technol. 2010, 21 896–903
N. MNIF ET AL.
898

isotactic PP (iPP).
[23]
The last one, that is the a
PP
relaxation (78
8C)
is attributed to movements in the iPP crystalline regions.
[23]
The
rheological properties of (PP/EPR) show that a combination of
relaxation processes between those typical mechanisms from its
components, PP and EPR, is exhibited. Consequently, four
different relaxations are shown, two of them correspond to
the amorphous EPR component (at the lowest temperature and
labeled g
EPR
and b
EPR
) and the two others, namely b
PP
and a
PP
, are
stem from the semicrystalline PP homopolymer.
[24,25]
For (PP/EPR)/W and (PP/EPR)/S blends, Table 3 and Figs 4 and 5
show the displacement of the relaxation b
EPR
and a
PP
toward the
low temperatures (
DT ¼ 2–48C and 88C, respectively). The result is
attributed to a supplementary thermomechanical treatment for the
introduction of nano-CaCO
3
that generates a variation of the chain
length because without nanoparticles the dynamic mechanical
properties of (PP/EPR) blends are unchanged even after extrusion.
On the other hand, the presence of EPR-g-MAH copolymer does not
change the relaxation peaks b
EPR
and b
PP
but the displacement of
a
PP
toward higher temperatures shows that the presence of the
copolymer decreases the mobility of polymer chains.
In the case of (PP/EPR)/W/PEP and (PP/EPR)/S/PEP, Table 3 and
Figs 4 and 5 display a second peak of relaxation respectively at
Figure 2. Morphologies of the blends (a) (PP/EPR), (b) (PP/EPR)/W, and (c) (PP/EPR)/S.
Polym. Adv. Technol. 2010, 21 896–903
Copyright
ß 2009 John Wiley & Sons, Ltd.
View this article online at wileyonlinelibrary.com
(PP/EPR)/NANO-CACO
3
BASED FORMULATIONS IN THE PERSPECTIVE OF POLYMER RECYCLING
899
38 and 368C in the presence of PEP copolymer. Tsagaropoulos
and Eisenberg
[26,27]
studying a wide range of polymers, including
poly(dimethylsiloxane), filled with silica nanoparticles, observe in
dynamic mechanical measurements a second peak in tan d, 50–
100
8C above the glass transition, which is attributed to the glass
transition of an interfacial polymer layer with restricted mobility.
Their results are interpreted in terms of a model where there are
three types of polymer: a strongly bound, immobile layer
immediately surrounding the particle, which does not participate
in the glass transition; a second, loosely bound interfacial layer
which is responsible for the second glass transition; and quasi-
bulk polymer unaffected by the particle.
Thermal properties
The mechanical properties of the composites can be significantly
changed if the crystallization characteristics of PP have been
altered. Table 4 summarizes the differential scanning calorimetry
(DSC) results for unmodified (PP/EPR) matrix and its ternary
blends with either calcium carbonate or compatibilizers. The DSC
Figure 3. Morphologies of the blends (a) (PP/EPR)/W/EPR-g-MAH, (b) (PP/EPR)/S/EPR-g-MAH, (c) (PP/EPR)/W/PEP, and (d) (PP/EPR)/S/PEP.
View this article online at wileyonlinelibrary.com
Copyright
ß 2009 John Wiley & Sons, Ltd.
Polym. Adv. Technol. 2010, 21 896–903
N. MNIF ET AL.
900
thermograms of (PP/EPR) show two peaks: a single-peak
characteristic of the melting of a-PP was observed at 167
8C
(T
mPP
) and a second peak associated with the crystallinity of PP at
126
8C (T
cPP
). The presence of compatibilizers and nano-CaCO
3
does not change the percentage of crystallinity of the PP. The loss
of nucleating efficiency of filler in the presence of EPR on the PP
matrix is attributed to its encapsulated structure as the filler
particles in this system were surrounded by the EPR phase.
According to Zuiderduin et al.
[21]
the melting enthalpy is lowered
when the particle content is increased. The CaCO
3
particles
(treated and untreated) do not act as a nucleating agent in
polypropylene since the crystallinity is not increased.
Tensile properties
The extensive analysis of the mechanical properties of ternary-
phase composites was carried out.
[5,28–31]
Most of the studies
have focused on the influence of the phase morphology. Ternary
composites containing encapsulated fillers have been reported
to have somewhat higher tensile impact strength but lower
modulus than those with a separation structure, because the
effect of the dispersed elastomer is extended by the filler.
[17]
However, composites having a separation structure yielded a
marked increase in the composite modulus.
[17]
Although some
Figure 4. Dynamic mechanical spectra of (PP/EPR), (PP/EPR)/W, (PP/EPR)/W/EPR-g-MAH, and (PP/EPR)/W/PEP blends.
Figure 5. Dynamic mechanical spectra of (PP/EPR), (PP/EPR)/S, (PP/EPR)/S/EPR-g-MAH, and (PP/EPR)/S/PEP blends.
Table 3. Rheological characteristics of the blends
Blends
b
EPR
(
8C)
b
PP
(
8C) a
PP
(
8C)
(PP/EPR)
46
3
78
(PP/EPR)/W
50
0
70
(PP/EPR)/W/EPR-g-MAH
50
0
86
(PP/EPR)/W/PEP
52 and 38
5
78
(PP/EPR)/S
48
0
70
(PP/EPR)/S/EPR-g-MAH
48
0
85
(PP/EPR)/S/PEP
52 and 36
4
72
Polym. Adv. Technol. 2010, 21 896–903
Copyright
ß 2009 John Wiley & Sons, Ltd.
View this article online at wileyonlinelibrary.com
(PP/EPR)/NANO-CACO
3
BASED FORMULATIONS IN THE PERSPECTIVE OF POLYMER RECYCLING
901
experimental data on the mechanical properties of such ternary
composites have been published in the literature, contradictory
results on the influence of the filler on the microstructure and
crystallization of the ternary composites were reported.
[5,28–31]
Some tensile properties of PP/EPR blends with and without
nano-CaCO
3
and compatibilizers are summarized in Table 5. The
decrease in the yield stress is due to the early debonding of the
filler particles from the polymer matrix. Debonded particles do
not contribute to the yield stress.
[21]
The particle size does not
seem to influence the tensile yield stress for these composites.
Although debonding becomes increasingly more difficult with
smaller particles
[32]
this is not seen in this particle size regime. The
addition of copolymer elastomer (4% of EPR-g-MAH and PEP) into
PP/CaCO
3
blends led to a decrease of tensile stress about 15–
20%. The bad dispersion and adhesion of the filler (particularly
Socal particles) as well as the brittleness of the (PP/EPR) matrix in
the presence of EPR-g-MAH copolymer cause a reduction in the
elongation at break.
Energy at break represents surface with the lower part of the
traction diagram s
¼ f (e) limited by the elongation at break. The
presence of fillers increases the energy at break about 100% by
widening of the maximum of the peak of the traction diagram
but the addition of copolymers (PEP and EPR-g-MAH) decreases
its value with reduction in yield stress and elongation at break.
Impact properties
The impact strength for the different blends as a function of
composition is shown in Table 5. The mechanical properties of
hybrid composites are dictated not only by their composition, but
also by their phase morphology. According to the literature, two
kinds of phase morphologies are normally found. Either
the rubber particles and rigid particles are dispersed in the
polypropylene matrix separately, or the rigid particles are
encapsulated by rubber particles. Earlier work showed that
the most important factor that can determine phase morphology
is the surface treatment of the rigid particles. Most of the previous
studies have focused on the relationship between phase
morphology and mechanical properties in PP–rubber–filler
systems.
[1,5–8]
According to the morphological observations,
the bad adhesion and dispersion of fillers (particularly Socal
particles) in the (PP/EPR) matrix decrease the impact strength of
the blends. The addition of the copolymer EPR-g-MAH increases
impact strength; indeed, the presence of 4% of elastomer tends
to increase the impact properties of the blends. On the other
hand, a better adhesion between PP and EPR in the presence of
PEP copolymer considerably improves the impact properties
compared to the noncompatibilized (by 45% and 60%,
respectively, in the case of (PP/EPR)/W/PEP and (PP/EPR)/S/PEP).
CONCLUSION
The influence of surface treatment and compatibilizers on the
microstructure and mechanical properties of complex (PP/EPR)
composites containing calcium carbonate was investigated. A
study of the phase morphology by SEM, DMA, DSC, and
mechanical properties revealed that the dispersion of calcium
Table 4. Thermal properties of (PP/EPR) blends
Blends
T
c
(
8C)
T
c onset
(
8C)
T
mPP
(
8C)
Crystallinity (x
c
)
(PP/EPR)
126
134
169
48
(PP/EPR)/W
127
134
169
50
(PP/EPR)/W/EPR-g-MAH
127
133
170
51
(PP/EPR)/W/PEP
125
134
172
50
(PP/EPR)/S
126
135
170
50
(PP/EPR)/S/EPR-g-MAH
128
134
169
49
(PP/EPR)/S/PEP
125
136
172
49
Table 5. Mechanical properties of (PP/EPR) blends (21
18C)
Blends
Traction test
Charpy impact
Yield stress
(MPa)
Elongation at
break (%)
Energy at
break (J)
Impact strength
(kJ/m
2
)
(PP/EPR)
20.3
0.40
180
25
8.5
0.9
75
8
(PP/EPR)/W
19.4
0.35
175
20
16
0.8
60
10
(PP/EPR)/W/EPR-g-MAH
16.5
0.45
115
20
14.5
1.1
70
9
(PP/EPR)/W/PEP
17.5
0.40
182
25
13.9
1.3
88
7
(PP/EPR)/S
19.4
0.35
135
35
15.9
1.2
51
8
(PP/EPR)/S/EPR-g-MAH
17.8
0.45
130
40
15.5
1.1
73
10
(PP/EPR)/S/PEP
17.8
0.35
195
30
14.8
1.3
82
9
View this article online at wileyonlinelibrary.com
Copyright
ß 2009 John Wiley & Sons, Ltd.
Polym. Adv. Technol. 2010, 21 896–903
N. MNIF ET AL.
902

carbonate on the (PP/EPR) matrix depends on surface treatment.
Indeed, calcium carbonate with fatty acids is dispersed better
than nano-CaCO
3
with stearic acid. Second, in ternary poly-
propylene/elastomer/calcium carbonate, the final structure is
determined by the dynamic equilibrium of encapsulation. Third,
the presence of the PEP copolymer considerably improves the
adherence at the interface between the PP and EPR phases and
consequently PP and (EPR/nano-CaCO
3
) interface. Finally, the
EPR-g-MAH copolymer does not present interaction with the (PP/
EPR) and nanoparticles but affects the mechanical properties of
the composites.
Acknowledgements
The authors gratefully acknowledge the Technological Center of
Microstructures (CTm) of University Claude Bernard-69622-Lyon
(France) for SEM observations.
REFERENCES
[1] J. Jancar, A. DiAnselmo, A. T. DiBenedetto, J. Kucera, Polymer 1993, 34,
1684.
[2] L. Elias, F. Fenouillot, J. C. Majeste, P. h. Cassagnau, Polymer 2007, 48,
6029.
[3] N. Mnif, V. Massardier, T. Kallel, B. Elleuch, Polym. Comp. 2008, DOI
10.1002
[4] S. M. Zebarjad, S. A. Sajjadi, M. Tahani, J. Mat. Proc. Tech. 2006, 175,
446.
[5] J. Jancar, A. T. J. Dibenedetto, J. Mater. Sci. 1994, 29, 4651.
[6] R. Premphet, P. Horanont, J. Appl. Polym. Sci. 2000, 76, 1929.
[7] D. Benderly, M. Siegmann, M. Narksis, Polym. Comp. 1996, 17, 86.
[8] S. E. Barbosa, J. Kenny, N. J. Capiti, Polym. Comp. 2000, 21, 377.
[9] J. Jancar, J. Mater. Sci. 1996, 31, 3983.
[10] B. Puka´nszky, E. Fekete, F. Tu¨do’’s, Makromol. Chem. Macromol. Symp.
1989, 28, 165.
[11] B. Puka´nszky, E. Fekete, Adv. Polym. Sci. 1999, 139, 109.
[12] E. Fekete, J. Mo´czo´, B. Puka´nszky, J. Colloid. Interf. Sci. 2004, 269, 143.
[13] J. Mo´czo´, E. Fekete, B. Puka´nszky, Prog. Colloid. Polym. Sci. 2004, 125,
134.
[14] H. Balard, E. Papirer, Prog. Org. Coat. 1993, 22, 1.
[15] B. Pukanszky, J. Kolarik, F. Lednicky, 1986, 553.
[16] J. Kolarik, F. Lednicky, Polym. Compos. 1986, 537.
[17] J. Kolarik, F. Lednicky, J. Jancar, B. Pukanszky, Polym. Commun. 1990,
31, 201.
[18] J. Kolarik, J. Jancar, Polymer 1992, 33, 4961.
[19] C. M. Chan, J. Wu, J. X. Li, Y. K. Cheung, Polymer 2002, 43, 2981.
[20] G. H. Michler, J. M. Tovmasjan, Plaste Kautschuk 1988, 35, 73.
[21] W. C. J. Zuiderduin, C. Westzaan, J. Hue´tink, R. J. Gaymans, Polymer
2003, 44, 261.
[22] X. Wang, J. Sun, R. Huang, J. Appl. Polym. Sci. 2006, 99, 2268.
[23] L. D’Orazio, R. Greco, C. Mancarella, E. Martuscelli, G. Ragosta, G.
Silvestre, Polym. Eng. Sci. 1982, 22, 536.
[24] N. Mnif, V. Massardier, M. Jaziri, J. Appl. Polym. Sci. 2007, 104,
3220.
[25] M. Jaziri, N. Mnif, V. Massardier, H. Perier-Camby, Polym. Eng. Sci. 2007,
47, 1009.
[26] G. Tsagaropoulos, A. Eisenberg, Macromolecules 1995, 28, 6067.
[27] G. Tsagaropoulos, A. Eisenberg, Macromolecules 1995, 28, 396.
[28] J. Jancar, A. T. Dibenedetto, J. Mater. Sci. 1995, 30, 1601.
[29] W. Y. Chiang, W. D. Yang, B. Pukanszky, Polym. Eng. Sci. 1992, 32,
641.
[30] J. Wang, J. F. Tung, M. Y. Ahmad Fuad, P. R. Hornsby, J. Appl. Polym. Sci.
1996, 60, 1425.
[31] K. Premphet, P. Horanont, J. Appl. Polym. Sci. 1999, 74, 3445.
[32] B. Pukanszky, Polypropylene: Structure, Blends and Composites, (Eds. J.
Karger-Kocsis), Chapter 1, Chapman & Hall, London, 1995,
Polym. Adv. Technol. 2010, 21 896–903
Copyright
ß 2009 John Wiley & Sons, Ltd.
View this article online at wileyonlinelibrary.com
(PP/EPR)/NANO-CACO
3
BASED FORMULATIONS IN THE PERSPECTIVE OF POLYMER RECYCLING
903
Wyszukiwarka
Podobne podstrony:
Regulaminy T 11.2.nowy, PP i K
FIZ22, studia, studia, sprawozdania, Ćw 22, Nowy folder
10 Rejestr gruntów dz 10,22 nowy
czesc I, studia, nano, 3rok, 6sem, projektowanie wyrobów z materiałów polimerowych
projekt - elastomery o optymalnym usieciowaniu, studia, nano, 3rok, 6sem, projektowanie wyrobów z ma
radiacja spr3-polimeryzacja radiacyjna, studia, nano, 3rok, 5sem, chemia i technologia radiacyjna po
Dozymetr alaninowy, studia, nano, 3rok, 5sem, chemia i technologia radiacyjna polimerów, lab
Chemia i technologia radiacyjna polimerow Cw2 - Dozymetria CalorymetriaAlanina, studia, nano, 3rok,
radiacja spr1-dozymetr Frickego, studia, nano, 3rok, 5sem, chemia i technologia radiacyjna polimerów
Ćw. 22, chemia fizyczna, Nowy folder
sciaga egzamin III[1][1][1].1 by luke, aaa, studia 22.10.2014, całe sttudia, III semestr, teoria obw
22-27, weterynaria, Nowy folder, k2, studia materialy, Interna Duża
Nowy Dokument programu Microsoft Word (22)
22 Jądro atomowe i Czarnobyl NOWY
spis, aaa, studia 22.10.2014, całe sttudia, III semestr, teoria obwodów wyk, Wszystko, Nowy folder,
Nowy Dokument programu Microsoft Office Word (22)
Nowy Dokument programu Microsoft Word 22
program nauczania nowy 22 07 09
więcej podobnych podstron