
9
Microbial Nanoparticle Production
Murali Sastry, Absar Ahmad, M. Islam Khan, and Rajiv Kumar
9.1
Overview
Inorganic materials in the form of hard tissues are an integral part of most multicellular
biological systems. Hard tissues are generally biocomposites containing structural bioma-
cromolecules and some 60 different kinds of minerals that perform a variety of vital struc-
tural, mechanical, and physiological functions [1]. Unicellular organisms such as bacteria
and algae also are capable of synthesizing inorganic materials, both intra- and extracellu-
larly [2]. Examples of such organisms include magnetotactic bacteria which synthesize
magnetite particles [3–5] (see also Chapter 10), diatoms and radiolarians that synthesize
siliceous materials [6, 7], and S-layer bacteria that synthesize gypsum and calcium carbo-
nate as surface layers [8]. These bioinorganic materials can be extremely complex both in
structure and function, and also exhibit exquisite hierarchical ordering from the nan-
ometer to macroscopic length scales which has not even remotely been achieved in labora-
tory-based syntheses. While the study of inorganic structures in biological systems would
impact both the physical sciences (geology, mineralogy, physics, chemistry) and biological
sciences (zoology, microbiology, evolution, physiology, cellular biology), one area where
there is perhaps the greatest potential for application is that of materials science, particu-
larly nanomaterials. Indeed, one branch of materials science where considerable develop-
ment has already taken place is in the design and crystal growth of minerals such as cal-
cium carbonate, hydroxyapatites, and gypsum by the use of biomimetic methods [9].
An important aspect of nanotechnology concerns the development of experimental pro-
cedures for the reproducible synthesis of nanomaterials of controlled size, polydispersity,
chemical composition, and shape. Though solution-based chemical methods enjoy a long
history dating back to the pioneering work of Faraday on the synthesis of aqueous gold
colloids [10], increasing pressure to develop green chemistry, eco-friendly methods for
nanomaterial synthesis has resulted in researchers turning to biological organisms for
inspiration. It is interesting to note that while biotechnological applications such as reme-
diation of toxic metals have long employed microorganisms such as bacteria [11, 12]
and yeast [13], the detoxification process occurring by reduction of the metal ions or by
formation of insoluble complexes with the metal ion (e. g., metal sulfides) in the form
126
Nanobiotechnology. Edited by Christof Niemeyer, Chad Mirkin
Copyright
c 2004 WILEY-VCH Verlag GmbH & Co. K aA, Weinheim
ISBN 3-527-30658-7
G

of nanoparticles, the possibility of using such microorganisms in the deliberate synthesis
of nanomaterials is a recent phenomenon. An amalgamation of curiosity, environmental
compulsions, and conviction that nature has evolved the best processes for synthesis of
inorganic materials on nano- and macro-length scales has contributed to the development
of a relatively new and largely unexplored area of research based on the use of microbes in
the biosynthesis of nanomaterials.
Microbes are classically defined as microscopic organisms, the term most often having
been used to signify bacteria. In this chapter, we will use the general definition to include
in addition to bacteria, actinomycetes (both prokaryotes) and algae, yeasts, and fungi
(eukaryotes). Some of the earliest reports on the accumulation of inorganic particles in
microbes can be traced to the work of Zumberg, Sigleo and Nagy (gold in Precambrian
algal blooms) [14], Hosea and coworkers (gold in algal cells) [15], Beveridge and co-work-
ers (gold in bacteria) [16], Aiking and co-workers (CdS in bacteria) [11], Reese and co-work-
ers (CdS in yeast) [17, 18], Temple and LeRoux (ZnS in sulfate-reducing bacteria) [19], and
Blakemore, Maratea and Wolfe (magnetite in bacteria) [20]. More recent and detailed in-
vestigations into the use of microbes in the deliberate synthesis of nanoparticles of differ-
ent chemical compositions include bacteria for silver [21–24], gold [24, 25–27] CdS [28–30]
ZnS (sphalerite) [31], magnetite [3–5, 32], iron sulfide [33, 34], yeast for PbS [35] and CdS
[36], and algae for gold [37]. In all these studies, the nanoparticles are formed intracellu-
larly, but may be released into solution by suitable treatment of the biomass. Recently, we
have shown that fungi when challenged with aqueous metal ions lead to the formation of
nanoparticles both intra- and extracellularly [38–42]. Different genera of fungi have been
identified for the extracellular synthesis of gold [38], silver [39], and CdS quantum dots
[40], as well as the intracellular growth of nanocrystals of gold [41] and silver [42]. Extre-
mophilic actinomycetes such as Thermomonospora sp. have also been used to synthesize
fairly monodisperse gold nanoparticles extracellularly [43]. Yacaman and co-workers
have demonstrated the growth of gold nanoparticles in sprouts, roots and stems of live
alfalfa plants [44, 45].
Among the different microbes studied for the biosynthesis of nanoparticles, bacteria
have received the most attention [11, 16, 19–34]. In a series of papers, Tanja Klaus and
co-workers showed that the metal-resistant bacterium, Pseudomonas stutzeri AG259 (ori-
ginally isolated from a silver mine), when challenged with high concentrations of silver
ions during culturing resulted in the intracellular formation of silver nanoparticles of vari-
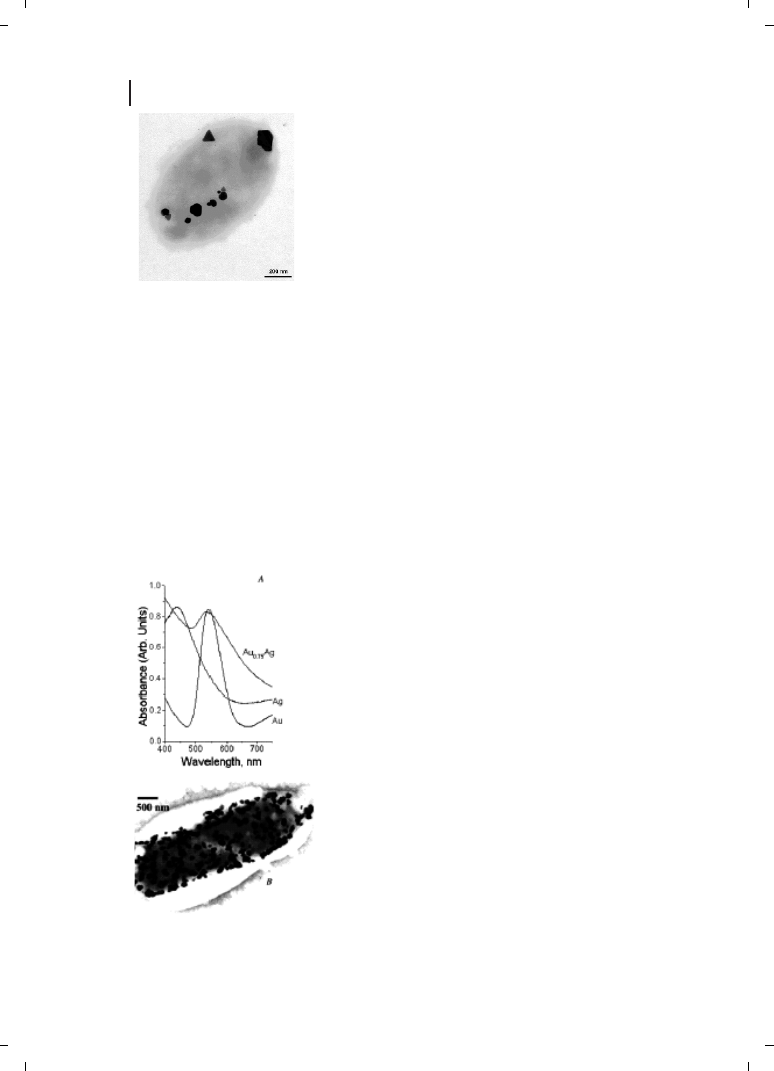
able shape [21–23]. This is illustrated in the transmission electron microscopy (TEM)
image of a Pseudomonas stutzeri AG259 cell with a number of silver particles located in-
tracellularly (Figure 9.1). The particles were crystalline, were often observed to form at
the poles of the bacteria, and were not particularly monodisperse, ranging in size from
a few nm to 200 nm [21]. Most of the nanoparticles were found to be composed of elemen-
tal silver, while occasionally the formation of Ag
2
S was observed [21]. The exact mechan-
ism leading to the formation of intracellular silver nanoparticles in P. stutzeri AG259 is yet
to be elucidated. Biofilms of metal nanoparticles embedded in a biological matrix may
have important applications in the synthesis of eco-friendly and economically viable cer-
met materials for optically functional thin film coatings [23]. Jorger, Klaus and Granqvist
showed that heat treatment of the Ag nano-bacteria biomass yielded hard coatings of a
cermet that was resistant to mechanical scratching with a knife and whose optical proper-
127
9.1 Overview
ties could be tailored by varying the silver loading factor [23]. The cermet material was
composed primarily of graphitic carbon and up to 5 % by weight (of the dry biomass)
of silver.
In an interesting recent study, Nair and Pradeep have demonstrated that bacteria not
normally exposed to large concentrations of metal ions also may be used to grow nanopar-
ticles [24]. These authors showed that Lactobacillus strains present in buttermilk, when ex-
posed to silver and gold ions, resulted in the large-scale production of nanoparticles within
the bacterial cells [24]. The exposure of lactic acid bacteria present in the whey of butter-
milk to mixtures of gold and silver ions could also be used to grow nanoparticles of alloys
of gold and silver [24]. The UV-visible spectra of the bacterial colloids after exposure to
pure silver and gold ions as well as a mixture of the two ions, are shown in Figure 9.2.
The surface plasmon vibrations from the silver and gold bacterial colloids occur at 439
128
9 Microbial Nanoparticle Production
Figure 9.1
Silver-based crystals with different morphology, size and
chemical composition produced by
P. stutzeri AG259. Triangular, hexa-
gonal and spheroid Ag-containing particles are accumulated at different
cellular binding sites in the periplasmic space of the bacterial cell.
(Reprinted with permission from Ref. [23];
c 2000 Wiley-VCH).
Figure 9.2
(A) Comparison of the UV/visible absorption
spectra of bacterial colloids of pure Au and Ag with an alloy
colloid of starting composition Au
0.75
Ag. The peak maxima are
547, 439, and 537 nm for Au, Ag and Au
0.75
Ag, respectively.
Note that there is no peak due to Ag colloid in the alloy. The
spectra have been moved vertically as there is a shift in
baseline from sample to sample. (B) TEM of a bacterium with
alloy crystallites. [111] Zone axis was seen in the electron
diffraction; smaller crystallites were also seen outside the
bacterium. (Reprinted with permission from Ref. [24];
c 2002
American Chemical Society).
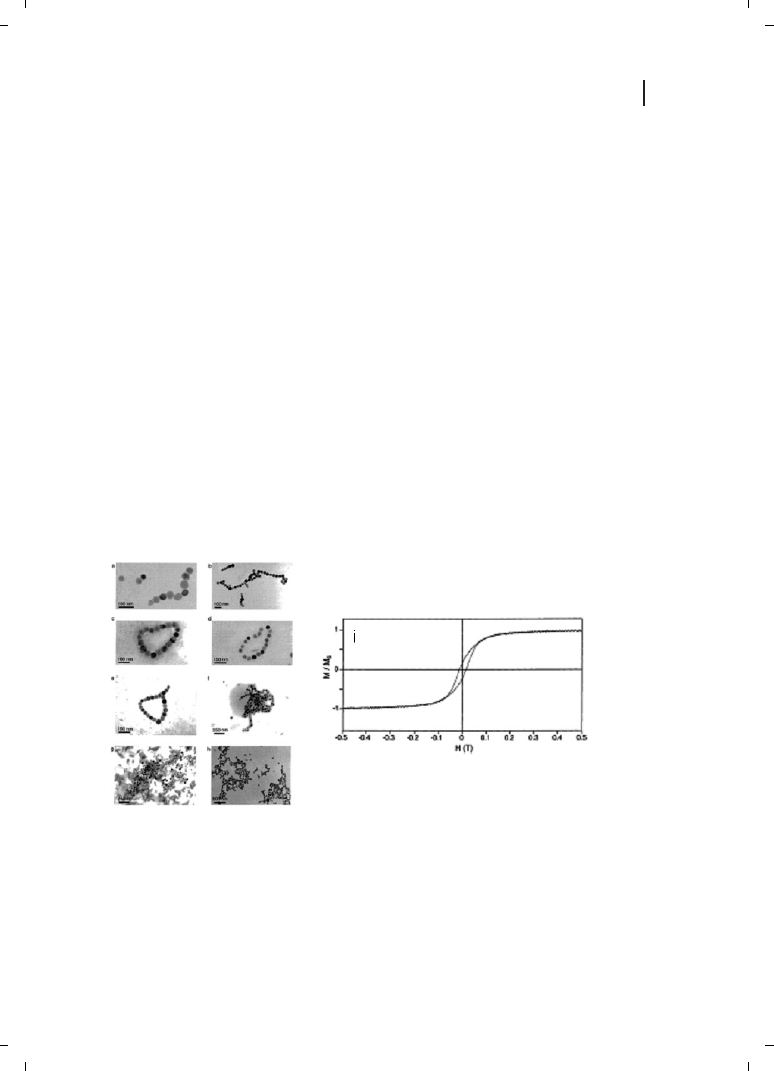
and 547 nm respectively, while for the mixed alloy case it is centered at 537 nm. In the
case of bacteria exposed to a mixture of the metal ions, the fact that the plasmon vibration
wavelength is within the range defined by pure silver and gold nanoparticles, together
with the absence of a distinct vibration corresponding to pure silver, was argued by
Nair and Pradeep to indicate the formation of an alloy of the composition Au
0.75
Ag,
and not a core-shell structure [24]. By using a series of time-dependent UV-visible spectro-
scopy and TEM measurements, Nair and Pradeep concluded that the nucleation of the
silver and gold nanoparticles occurs outside the bacterium (presumably on the cell surface
through sugars and enzymes in the cell wall), following which the metal nuclei are trans-
ported into the cell where they aggregate and grow to larger-sized particles. The presence
of noble metal nanocenters is known to enhance Raman spectroscopic signatures [46],
and this feature was used by the authors to probe the internal chemical environment
in the bacteria [24].
There is much interest in the development of protocols for the synthesis of semiconduc-
tor nanoparticles such as CdS for application as quantum-dot fluorescent biomarkers in
cell labeling [47]. Simple variation of the particle size enables tailoring of the band gap
and, consequently, the color of the quantum dots during UV-light irradiation [47]. Bacteria
have been used with considerable success in the synthesis of CdS nanoparticles [28–30].
Holmes and co-workers have shown that exposure of the bacterium Klebsiella aerogenes to
Cd
2+
ions resulted in the intracellular formation of CdS nanoparticles in the size range
20–200 nm [29]. They also showed that the composition of the nanoparticles formed
was a strong function of buffered growth medium for the bacterium. In an interesting
extension of the bacteria-based methodology for the growth of magnetic nanoparticles,
129
9.1 Overview
Figure 9.3
Representative selection of cluster
morphologies for magnetite (Fe
3
O
4
) colloids ex-
tracted from cells imaged by transmission electron
micrographs. Note the magnetic flux closure rings
in images (c), (d), and (e). The tendency to form
string-like aggregates can be clearly seen in for ex-
ample, images (b) and (f). (i) Normalized magne-
tization M/M
S
(M
S
is the measured saturation
magnetization at high field of dried magnetite par-
ticles extracted from cells) versus the applied
magnetic field H. (Reprinted with permission from
Ref. [4];
c 2002 American Chemical Society).
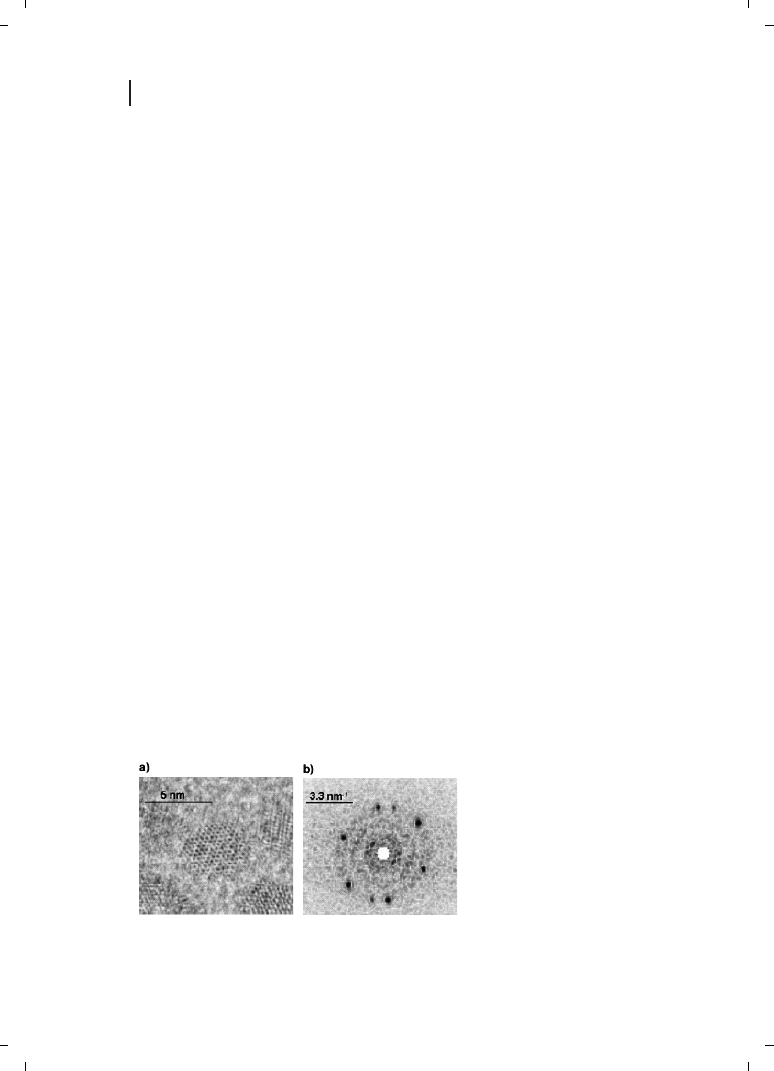
Roh and co-workers showed that metals such as Co, Cr, and Ni may be substituted into
magnetite crystals biosynthesized in the thermophilic iron-reducing bacterium Thermoa-
naerobacter ethanolicus (TOR-39) [5]. This procedure led to the formation of octahedral-
shaped magnetite nanoparticles in large quantities that co-existed with a poorly crystalline
magnetite phase near the surface of the cells. A more fundamental investigation into the
assembly of single-domain magnetite particles harvested from the bacterium Magnetospir-
illum magnetotacticum into folded-chain and flux-closure ring morphologies was carried
out by Philipse and Maas [4]. The TEM images in Figure 9.3a–h show the magnetite par-
ticles extracted from the bacterial biomass by sonication. The particles are
Z4.7 nm in dia-
meter and predominantly organized in the form of rings (Figure 9.3c–e) and, more infre-
quently, as linear superstructures. The magnetite crystals are single domains with large
magnetic moments that, when constrained to lie on a two-dimensional surface, are
responsible for the head-to-tail assembly. The circular structures were explained by the
authors to be flux-closure rings of in-plane dipoles. In conventional ferrofluids, the mag-
netic moments of the particles are much smaller than that observed for biogenic magne-
tite and therefore, such linear and ring-like structures have not been observed. Figure 9.3i
shows data obtained from magnetization measurements of dried magnetite particles har-
vested from the bacterial cells. Based on these measurements and the magnitude of the
remnant magnetization/coercive field, it was established that the biogenic magnetite
nanoparticles are not superparamagnetic [4].
It has long been recognized that the exposure of yeasts such as Candida glabrata
and Schizosaccharomyces pombe to Cd
2+
ions leads to the intracellular formation of CdS
quantum dots [17, 18]. In this particular case, the biochemical process resulting in the
nanoparticle formation is well understood [17, 18]. Yeast cells exposed to Cd
2+
ions
produce metal-chelating peptides (glutathiones),and this is accompanied by an increase
in the intracellular sulfide concentration and the formation of nanocrystalline CdS. The
biogenic CdS quantum dots are capped and stabilized by the peptides, glutathione and
its derivative phytochelatins with the general structure (g-Glu-Cys)
n
Gly [17, 18]. Based
on an extensive screening program, Kowshik and co-workers have identified the yeast,
Torulopsis sp. as being capable of intracellular synthesis of nanoscale PbS crystallites
when exposed to aqueous Pb
2+
ions [35]. The PbS nanoparticles were extracted
from the biomass by freeze–thawing, and analyzed using a variety of techniques. A
blue shift in the absorption edge suggested that the particles were indeed in the quantum
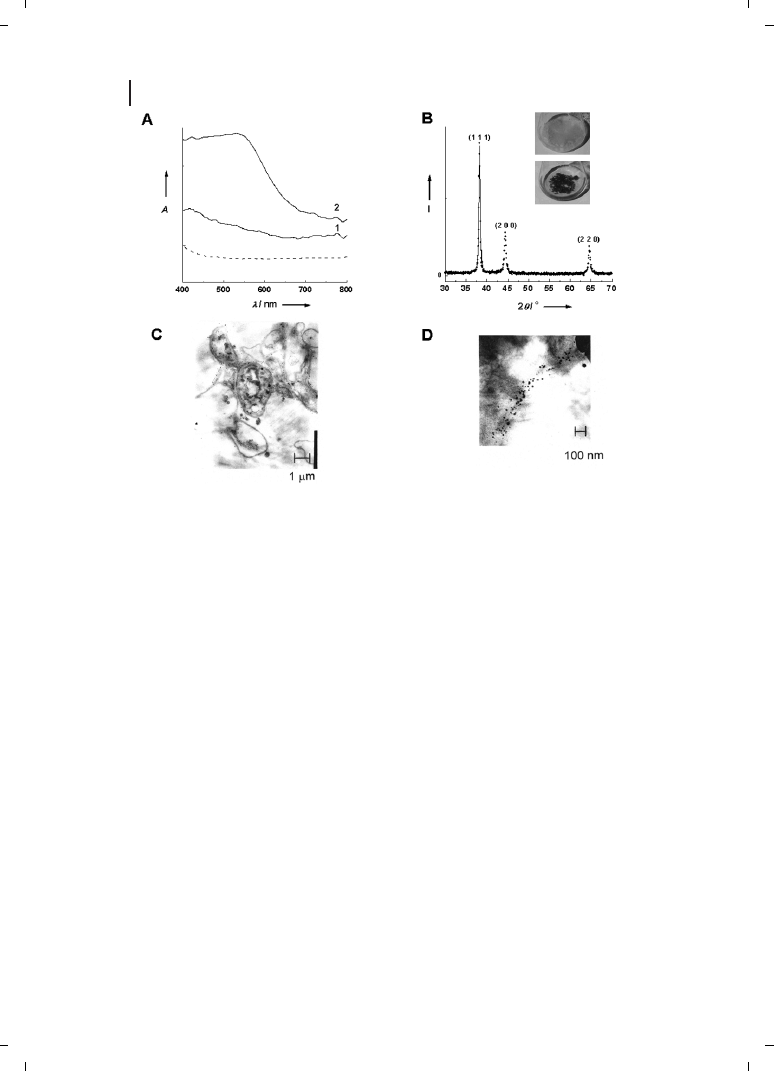
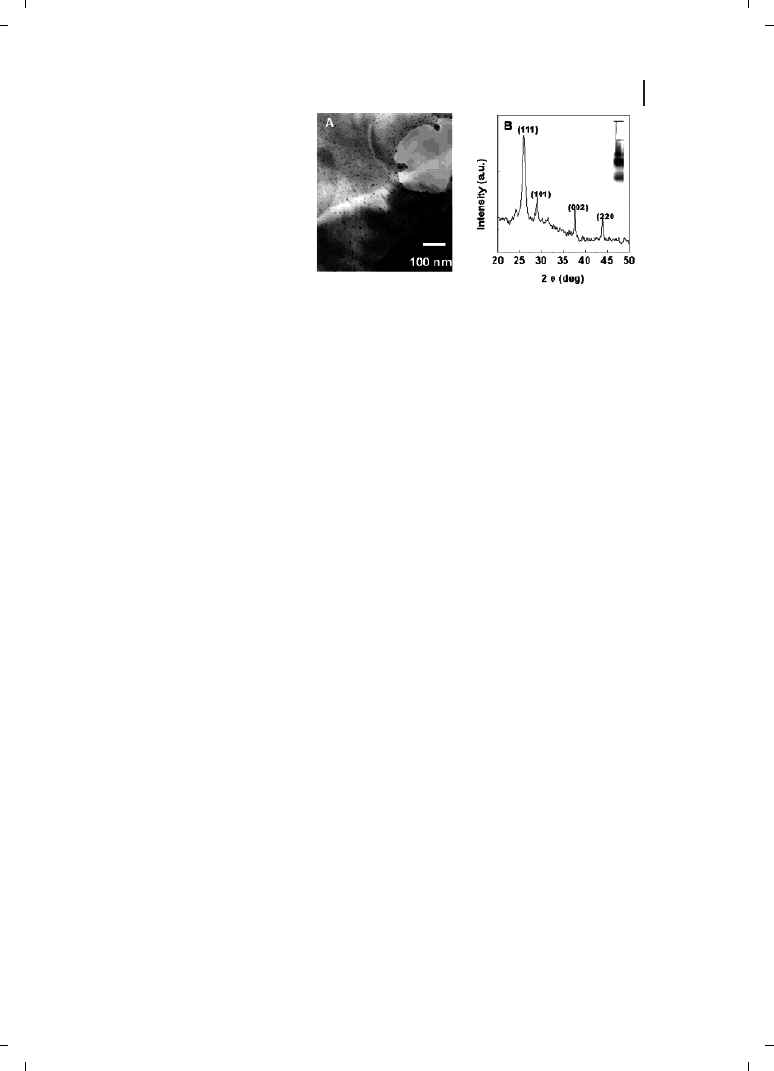
size regime. A HRTEM image of the PbS nanoparticles harvested from the Torulopsis sp.
biomass is shown in Figure 9.4, and shows clearly that the particles are quite spherical
130
9 Microbial Nanoparticle Production
Figure 9.4
(a) An HRTEM image
showing the near-spherical PbS nano-
crystallites; (b) their diffraction pattern.
(Reprinted with permission from Ref.
[35];
c 2002 Wiley-VCH).

in shape and range in size from 4 to 8 nm. The particles are crystalline and exhibit a well-
developed electron diffraction pattern (Figure 9.4b) with evidence for mixed cubic and hex-
agonal phases in the particles. Ultimately, biogenic nanoparticles would have to compete
with chemically synthesized nanoparticles in terms of performance in devices. As a
step in this direction, Kowshik et al. have shown that CdS quantum dots syn-
thesized intracellularly in Schizosaccharomyces pombe yeast cells exhibit ideal diode cha-
racteristics [36]. Biogenic CdS nanoparticles in the size range 1–1.5 nm were used in
the fabrication of a heterojunction with poly(p-phenylenevinylene). Such a diode exhibited
75 mA cm
–2
current in the forward bias mode at 10 V, while breakdown occurred at 15 V
in the reverse direction.
The use of fungi in the synthesis of nanoparticles is a relatively recent addition to the
list of microbes discussed above. A detailed screening process involving approximately 200
genera of fungi resulted in two genera which, when challenged with aqueous metal ions
such as AuCl
4
–
and Ag
2+
, yielded large quantities of metal nanoparticles either extracellu-
larly (Fusarium oxysporum) [38, 39] or intracellularly (Verticillium sp.) [41, 42]. The inset of
Figure 9.5B shows flasks containing the Verticillium sp. biomass before (flask on top) and
after exposure to 10
–4
M HAuCl
4
solution for 72 hours [41]. The appearance of a distinc-
tive purple color in the fungal biomass indicates formation of gold nanoparticles and can
clearly be seen in the UV-visible absorption spectrum recorded from the gold-loaded bio-
mass as a resonance at
Z540 nm (Figure 9.5A, curve 2). This resonance is clearly missing
in the biomass before exposure to gold ions (Figure 9.5A, curve 1) and in the filtrate
(dotted line, Figure 9.5A) after reaction of Verticillium with the gold ions. The gold ions
are thus reduced intracellularly, further evidence of which is provided by TEM analysis
of thin sections of the cells after formation of gold nanoparticles (Figure 9.5C and D).
In the low-magnification TEM image (Figure 9.5C), a number of nearly spherical gold na-
noparticles are seen very close to the surface of the cells. At higher magnification (Figure
9.5D), the nanoparticles ranging in size from 5 nm to 200 nm with an average size of
20
e 8 nm are clearly seen populating both the cell wall and cytoplasmic membrane of the
fungus. Furthermore, the gold nanoparticles are crystalline, as can be seen from the
powder X-ray diffraction pattern recorded from the biofilm (Figure 9.5B). The Bragg re-
flections are characteristic of face-centered cubic (fcc) gold structure. The reduction of
the gold ions is expected to be due to reaction with enzymes present in the cell walls
of the mycelia [41]. Exposure of Verticillium sp. to silver ions resulted in a similar intracel-
lular growth of silver nanoparticles [42].
From the application point of view, extracellular synthesis of nanoparticles would be
more important. We have observed that exposure of the fungus Fusarium oxysporum to
aqueous gold and silver ions leads to the formation of fairly monodisperse nanoparticles
in solution [38, 39]. Even more exciting was the finding that exposure of Fusarium oxy-
sporum to aqueous CdSO
4
solution yielded CdS quantum dots extracellularly [40]. Figure
9.6A shows the CdS nanoparticles formed after reaction of 10
–4
M CdSO
4
solution with
the Fusarium oxysporum biomass for 12 days. The particles are reasonably monodisperse,
and range in size from 5 to 20 nm. X-ray diffraction analysis of a film of the particles
formed on a Si (111) wafer clearly showed that the particles were nanocrystalline with
Bragg reflections characteristic of hexagonal CdS (Figure 9.6B). Reaction of the fungal bio-
mass with aqueous CdNO
3
solution for an extended period of time did not yield CdS
131
9.1 Overview
nanoparticles, indicating the possibility of release of a sulfate reductase enzyme into solu-
tion. The inset of Figure 9.6B shows the polyacrylamide gel electrophoresis (PAGE) results
of the aqueous extract exposed to the fungal biomass for 12 days. The electrophoresis
measurements indicate the presence of at least four protein bands in the extract. Reaction
of the protein extract after dialysis (using a dialysis bag with 3kDa molecular weight cut-
off) with CdSO
4
solution did not yield CdS nanoparticles. However, addition of ATP and
NADH to the dialysate restored the CdS formation capability of the protein extract. It is
believed that the same proteins are also responsible for the reduction of gold and silver
ions. The gold, silver, and CdS nanoparticles were stable in solution for many months
due to stabilization by surface-bound proteins [38, 40]. The development of a rational na-
noparticle biosynthesis procedure using specific enzymes secreted by fungi in both the
intra- and extracellular synthesis of nanoparticles has many attractive associated features.
Plant pathogenic fungi produce copious quantities of enzymes, are usually nonpathogenic
to humans, and are easily cultured in the laboratory.
132
9 Microbial Nanoparticle Production
Figure 9.5
(A) UV/Visible spectra recorded from
biofilms of the
Verticillium sp. fungal cells before
(curve 1) and after (curve 2) exposure to 10
–4
M
aqueous HAuCl
4
solution for 72 hours. The spec-
trum recorded from the HAuCl
4
solution after im-
mersion of the fungal cells for 72 hours is shown
for comparison (dashed line). (B) X-ray diffraction
pattern recorded from an Au nano-
Verticillium bio-
film formed on a Si (111) wafer. The principal Bragg
reflections are identified. The inset shows pictures
of the
Verticillium fungal cells after removal from the
culture medium (flask on top) and after exposure to
10
–4
M aqueous solution of HAuCl
4
for 72 hours
(flask at bottom). (C, D) TEM images at different
magnifications of thin sections of stained
Verticil-
lium cells after reaction with AuCl
4
–
ions for
72 hours. (Reprinted with permission from
Ref. [41];
c 2001 Wiley-VCH).
9.2
Outlook
A case for the serious investigation of microorganisms such as bacteria, algae, yeasts,
actinomycetes, and fungi as possible inorganic nanofactories has been made. A number of
issues from the nanotechnology and microbiology points of view require to be addressed
before such a biosynthesis procedure can compete with existing physical and chemical
synthesis protocols. The elucidation of biochemical pathways leading to metal ion reduc-
tion or formation of insoluble complexes in the different classes of microbes is essential in
order to develop a rational microbial nanoparticle synthesis procedure. Likewise, an un-
derstanding of the surface chemistry of the biogenic nanoparticles (i. e., the nature of cap-
ping surfactants/peptides/proteins) would be equally important. This would then lead to
the possibility of genetically engineering microbes to overexpress specific reducing mole-
cules and capping agents, thereby controlling not only the size of the nanoparticles but
also their shape. The rational use of constrained environments within cells such as the
periplasmic space and cytoplasmic vesicular compartments (e. g., magnetosomes) to mod-
ulate nanoparticle size and shape is an exciting possibility. The range of chemical compo-
sitions of nanoparticles currently accessible by microbial methods is currently extremely
limited and confined to metals, some metal sulfides and iron oxide. Extension of the pro-
tocols to enable reliable synthesis of nanocrystals of other oxides (TiO
2
, ZrO
2
, etc.) and
nitrides, carbides, etc. could make microbial synthesis a commercially viable proposition.
Equally intriguing are questions related to the metal ion reduction/reaction process in
cellular metabolism, and whether the nanoparticles formed as byproducts of the reduction
process have any role to play in cellular activity (e. g., magnetite in magnetotactic bacteria).
Plant organisms (e. g., fungi) are not normally exposed to high concentrations of metal
ions such as Cd
2+
, AuCl
4
–
and Ag
2+
. The fact that, when challenged, they secrete enzymes
that are capable of metal ion reduction – and indeed conversion of sulfates to sulfides –
suggests that evolutionary processes may be at play.
133
9.2 Outlook
Figure 9.6
(A) Bright-field TEM image of CdS na-
noparticles formed by reaction of CdSO
4
with the
fungal biomass for 12 days. (B) X-ray diffraction
pattern recorded from the CdS nanoparticle film
deposited on a Si (111) wafer. The inset shows the
native gel electrophoresis of aqueous protein
extract obtained from
Fusarium oxysporum mycelia;
10 % (w/v) polyacrylamide slab gel, pH 4.3
(Reprinted with permission from Ref. [40];
c 2002 American Chemical Society).

Acknowledgments
The authors would like to thank graduate students Priyabrata Mukherjee, Satyajyoti Sena-
pati, and Deendayal Mandal for their enthusiastic contributions to much of the experi-
mental work.
134
9 Microbial Nanoparticle Production
References
[1]
H. A. Lowenstam, Science 1981, 211, 1126.
[2]
K. Simkiss, K. M. Wilbur, Biomineraliza-
tion, Academic Press, New York, 1989.
[3]
R. B. Frankel, R. P. Blakemore (eds). Iron
Biominerals, Plenum Press, New York,
1991.
[4]
A. P. Philipse, D. Maas, Langmuir 2002, 18,
9977.
[5]
Y. Roh, R. J. Lauf, A. D. McMillan, C.
Zhang, C. J. Rawn, J. Bai, T. J. Phelps, Solid
State Commun. 2001, 118, 529.
[6]
N. Kröger, R. Deutzmann, M. Sumper,
Science 1999, 286, 1129.
[7]
J. Parkinson, R. Gordon, Trends Biotech.
1999, 17, 190.
[8]
S. Schultz-Lam, G. Harauz, T. J. Beveridge,
J. Bacteriol. 1992, 174, 7971.
[9]
S. Mann, Biomineralization. Principles
and Concepts in Bioinorganic Materials
Chemistry, Oxford University Press, Oxford,
2001.
[10]
M. Faraday, Philos. Trans. R. Soc. London
1857, 147, 145.
[11]
H. Aiking, K. Kok, H. van Heerikhuizen,
J. van’t Riet, Appl. Environ. Microbiol. 1982,
44, 938.
[12]
J. R. Stephen, S. J. Maenaughton, Curr.
Opin. Biotechnol. 1999, 10, 230.
[13]
R. K. Mehra, D. R. Winge, J. Cell. Biochem.
1991, 45, 30.
[14]
J. E. Zumberg, A. C. Sieglo, B. Nagy, Miner.
Sci. Eng. 1978, 10, 223.
[15]
M. Hosea, B. Greene, R. McPherson,
M. Heinzl, M. D. Alexander, D. W. Darnall,
Inorg. Chim. Acta. 1986, 123, 161.
[16]
T. J. Beveridge, R. J. Doyle, Metal Ions
and Bacteria, J. Wiley Sons, New York,
1989.
[17]
R. N. Reese, D. R. Winge, J. Biol. Chem.
1988, 263, 12832.
[18]
C. T. Dameron, R. N. Reese, R. K. Mehra,
A. R. Kortan, P. J. Carroll, M. L. Steiger-
wald, L. E. Brus, D. R. Winge, Nature 1989,
338, 596.
[19]
K. L. Temple, N. LeRoux, Econ. Geol. 1964,
59, 647.
[20]
R. P. Blakemore, D. Maratea, R. S. Wolfe,
J. Bacteriol. 1979, 140, 720.
[21]
T. Klaus, R. Joerger, E. Olsson, C.-G.
Granqvist, Proc. Natl. Acad. Sci. USA 1999,
96, 13611.
[22]
T. Klaus-Joerger, R. Joerger, E. Olsson,
C.-G. Granqvist, Trends Biotech. 2001, 19, 15.
[23]
R. Joerger, T. Klaus, C.-G. Granqvist, Adv.
Mater. 2000, 12, 407.
[24]
B. Nair, T. Pradeep, Cryst. Growth Des.
2002, 2, 293.
[25]
T. J. Beveridge, R. G. E. Murray, J. Bacteriol.
1980, 141, 876.
[26]
G. Southam, T. J. Beveridge, Geochim.
Cosmochim. Acta 1996, 60, 4369.
[27]
D. Fortin, T. J. Beveridge, in: Biominerali-
zation. From Biology to Biotechnology and
Medical Applications, E. Baeuerien (ed.),
Wiley-VCH, Weinheim, 2000, p. 7.
[28]
D. P. Cunningham, L. L. Lundie, Appl.
Environ. Microbiol. 1993, 59, 7.
[29]
J. D. Holmes, P. R. Smith, R. Evans-Gow-
ing, D. J. Richardson, D. A. Russell, J. R.
Sodeau, Arch. Microbiol. 1995, 163, 143.
[30]
P. R. Smith, J. D. Holmes, D. J. Richardson,
D. A. Russell, J. R. Sodeau, J. Chem. Soc.,
Faraday Trans. 1998, 94, 1235.
[31]
M. Labrenz, G. K. Druschel, T. Thomsen-
Ebert, B. Gilbert, S. A. Welch, K. M. Kem-
ner, G. A. Logan, R. E. Summons, G. De
Stasio, P. L. Bond, B. Lai, S. D. Kelly, J. F.
Banfield, Science 2000, 290, 1744.
[32]
D. R. Lovley, J. F. Stolz, G. L. Nord, E. J. P.
Phillips, Nature 1987, 330, 252.

135
References
[33]
J. H. P. Watson, D. C. Ellwood, A. K. Sper,
J. Charnock, J. Magn. Magn. Mater. 1999,
203, 69.
[34]
J. H. P. Watson, B. A. Cressey, A. P. Roberts,
D. C. Ellwood, J. M. Charnock, J. Magn.
Magn. Mater. 2000, 214, 13.
[35]
M. Kowshik, W. Vogel, J. Urban, S. K.
Kulkarni, K. M. Paknikar, Adv. Mater. 2002,
14, 815.
[36]
M. Kowshik, N. Deshmukh, W. Vogel,
J. Urban, S. K. Kulkarni, K. M. Paknikar,
Biotech. Bioeng. 2002, 78, 583.
[37]
M. G. Robinson, L. N. Brown, D. Beverley,
Biofouling 1997, 11, 59.
[38]
P. Mukherjee, S. Senapati, D. Mandal,
A. Ahmad, M. I. Khan, R. Kumar,
M. Sastry, ChemBioChem. 2002, 3, 461.
[39]
A. Ahmad, P. Mukherjee, S. Senapati,
D. Mandal, M. I. Khan, R. Kumar,
M. Sastry, Coll. Surf. B. 2003, 28, 313.
[40]
A. Ahmad, P. Mukherjee, D. Mandal,
S. Senapati, M. I. Khan, R. Kumar,
M. Sastry, J. Am. Chem. Soc. 2002, 124,
12108.
[41]
P. Mukherjee, A. Ahmad, D. Mandal,
S. Senapati, S. R. Sainkar, M. I. Khan,
R. Ramani, R. Parischa, P. V. Ajaykumar,
M. Alam, M. Sastry, R. Kumar, Angew.
Chem. Int. Ed. 2001, 40, 3585.
[42]
P. Mukherjee, A. Ahmad, D. Mandal,
S. Senapati, S. R. Sainkar, M. I. Khan,
R. Parischa, P. V. Ajayakumar, M. Alam, R.
Kumar, M. Sastry, Nano Lett. 2001, 1, 515.
[43]
A. Ahmad, S. Senapati, M. I. Khan,
R. Kumar and M. Sastry, Langmuir 2003,
19, 3550.
[44]
J. L. Gardea-Torresdey, J. G. Parsons,
E. Gomez, J. Peralta-Videa, H. E. Troiani,
P. Santiago, M. J. Yacaman, Nano Lett.
2001, 2, 374.
[45]
J. Gardea-Torresdey, E. Gomez, J. Peralta-
Videa, J. Parsons, H. Troiani, M. Jose-
Yacaman. Langmuir 2003, 19, 1357.
[46]
Y. C. Cao, R. Jin, C. A. Mirkin, Science 2002,
297, 1536.
[47]
W. C. W. Chan, D. J. Maxwell, X. Gao, R. E.
Bailey, M. Han, S. Nie, Curr. Opin. Biotech.
2002, 13, 40.
Wyszukiwarka
Podobne podstrony:
2013 09 23 Fuzja producentów bronii
2013 09 28 Producent makaronów nie chce gejów
2011 Galactoglucomannan oligosaccharide supplementation affects nutrient digestibility, fermentation
download Zarządzanie Produkcja Archiwum w 09 pomiar pracy [ www potrzebujegotowki pl ]
09 AIDSid 7746 ppt
09 Architektura systemow rozproszonychid 8084 ppt
TOiZ 09
Product presentation XC100FC
~$Production Of Speech Part 2
Wyklad 2 TM 07 03 09
09 Podstawy chirurgii onkologicznejid 7979 ppt
Wyklad 4 HP 2008 09
09 TERMOIZOLACJA SPOSOBY DOCIEPLEŃ
09 Nadciśnienie tętnicze
wyk1 09 materiał
Niewydolność krążenia 09
09 Tydzień zwykły, 09 środa
więcej podobnych podstron