
10
Cellulose
Prof. Dr. Dieter Klemm
1
, Prof. Dr. Hans-Peter Schmauder
2
,
Prof. Dr. Thomas Heinze
3
1
Institute of Organic and Macromolecular Chemistry, Friedrich Schiller University
of Jena, Humboldtstrasse 10, D-07743 Jena, Germany; Tel.:
49-3641-948-260;
Fax:
49-3641-948-202; E-mail: c9kldi@uni-jena.de
2
Research Centre of Medical Technology and Biotechnology, Geranienweg 7,
D-99947 Bad Langensalza, Germany; Tel.:
49-3603-833-140;
Fax:
49-3603-833-150; E-mail: hpschmauder@fzmb.de
3
Bergische University of Wuppertal, FB 9, Chemistry, Gauss Strasse 20,
D-42097 Wuppertal, Germany; Tel.:
49-202-439-2654; Fax: 49-202-439-2648;
E-mail: theinze@uni-wuppertal.de
1
Introduction and Historical Outline
. . . . . . . . . . . . . . . . . . . . . . . . .
277
2
Occurrence
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
278
2.1
Natural Sources . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
278
2.2
Synthetic Cellulose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
279
3
Structure and Analysis
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
280
3.1
Hydrogen Bonding . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
280
3.2
Crystal Structure
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
281
3.2.1
Cellulose I Polymorph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
281
3.2.2
Further Cellulose Polymorphs
. . . . . . . . . . . . . . . . . . . . . . . . . . .
282
3.3
Morphology . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
283
3.4
Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
284
4
Physiological Function
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
284
5
Biosynthesis
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
285
5.1
Synthesis of Substrates for the Polymerizing Enzyme . . . . . . . . . . . . .
286
5.2
Polymerizing Enzyme System and Enzymology of Biosynthesis . . . . . . .
287
5.3
Genetic Basis of Synthesis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
288
5.4
Regulation of Synthesis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
289
275

5.5
Summarizing Open Questions in Research on Plant Cellulose Synthesis . .
289
6Biodegradation
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
290
6.1
Intracellular Biodegradation . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
290
6.2
Extracellular Biodegradation . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
291
7
Biotechnological Production
. . . . . . . . . . . . . . . . . . . . . . . . . . . . .
293
8
Properties
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
293
8.1
Physical and Material Properties . . . . . . . . . . . . . . . . . . . . . . . . . .
293
8.2
Chemical Properties . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
295
9
Applications of Cellulose and Its Derivatives
. . . . . . . . . . . . . . . . . . .
298
9.1
Technical Applications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
299
9.1.1
Regenerated Cellulose Products
. . . . . . . . . . . . . . . . . . . . . . . . . .
299
9.1.2
Microcrystalline Cellulose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
302
9.1.3
Cellulose Esters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
302
9.1.4
Cellulose Ethers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
304
9.1.5
Oxidized Products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
305
9.2
Other Applications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
306
10
Relevant Patents for Biosynthesis, Biodegradation, and Biological Applications
309
11
Current Problems and Limitations
. . . . . . . . . . . . . . . . . . . . . . . . .
310
12
Outlook and Perspectives
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
311
13
References
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
312
AGU
anhydro glucopyranose unit(s)
Bn
benzyl
CA
cellulose acetate
CMC
carboxymethyl cellulose
Cuam
cuprammonium hydroxide [ Cu(NH
3
)
4
]OH
DMA
N,N-dimethylacetamide
DMF
N,N-dimethylformamide
DMSO
dimethylsulfoxide
DP
degree of polymerization
DS
degree of substitution
EHEC
ethylhydroxyethyl cellulose
HEC
hydroxyethyl cellulose
HPC
hydroxypropyl cellulose
MC
methylcellulose
mesylate
methane sulfonate
MHEC
methylhydroxyethyl cellulose
10 Cellulose
276

MHPC
methylhydroxypropyl cellulose
NMMO
N-methylmorpholine-N-oxide
SAXS
small-angle X-ray scattering
SEC
size-exclusion chromatography
tosylate
p-toluenesulfonate
WAXS
wide-angle X-ray scattering
1
Introduction and Historical Outline
Cellulose constitutes the most abundant,
renewable polymer resource available today
worldwide. It has been estimated that by
photosynthesis, 10
11
± 10
12
tons are synthe-
sized annually in a rather pure form, e.g., in
the seed hairs of the cotton plant, but mostly
are combined with lignin and other polysac-
charides (so-called hemicelluloses) in the
cell wall of woody plants (Kr‰ssig, 1993).
Cellulose is a polymer raw material used
for two general purposes. For many centu-
ries it has served mankind as a construction
material, mainly in the form of intact wood
and textile fibers such as cotton or flax, or in
the form of paper and board. On the other
hand, cellulose is a versatile starting material
for chemical conversions, aiming at the
production
of
artificial,
cellulose-based
threads and films as well as a variety of
stable cellulose derivatives used in many
areas of industry and domestic life (Klemm
et al., 1998a). Empirical knowledge of dying
cellulose fibers, of burning wood, of prepar-
ing charcoal, and of the biodegradation of
cellulose by rotting was acquired already
thousands of years ago.
Cellulose occupies a unique place in the
annals of polymers. As early as 1838, Payen
recognized cellulose as a definitive sub-
stance and coined the name ™cellulose∫
(Payen, 1838). Cellulose as a precursor for
chemical modifications has been used even
before its polymeric nature was recognized
and well understood. Milestones on this
pathway were the discovery of cellulose
nitrate (commonly misnamed nitrocellu-
lose) by Schˆnbein (1846), the preparation
of Schweizer's reagent, i.e., a cuprammoni-
um hydroxide solution representing the first
cellulose solvent (Schweizer, 1856, 1857,
1859) in 1857, and the synthesis of an
organo-soluble cellulose acetate by Sch¸t-
zenberger
in
1865
(Sch¸tzenberger
1865a,b). Partially functionalized cellulose
nitrate mixed with camphor as softener was
one of the first polymeric materials used as a
™plastic∫ and is well known under the trade
name of Celluloid. Cellulose nitrates of
higher N-content have been used extensively
for military purposes. Today, cellulose nitrate
is the only inorganic cellulose ester of
commercial interest (Balser et al., 1986a).
Regenerated cellulose filaments were ob-
tained by spinning cellulose dissolved in
cuprammonium hydroxide in an aqueous
bath. By far the largest part of cellulose-based
artificial fibers have been manufactured for
about the last century by the so-called viscose
process, invented in 1892 by Cross et al.
(1893). This process is practiced today with
an output of about 3 million tons annually
worldwide. It makes use of the formation of
cellulose xanthogenate, i.e., a water-soluble,
less-stable anionic ester, prepared by reac-
tion of cellulose with aqueous sodium
hydroxide and CS
2
and its decomposition
by spinning in an acid bath.
The origin of cellulose chemistry as a
branch of polymer research can be traced
back to the fundamental experiments of H.
Staudinger in the 1920s and 1930s on the
1 Introduction and Historical Outline
277
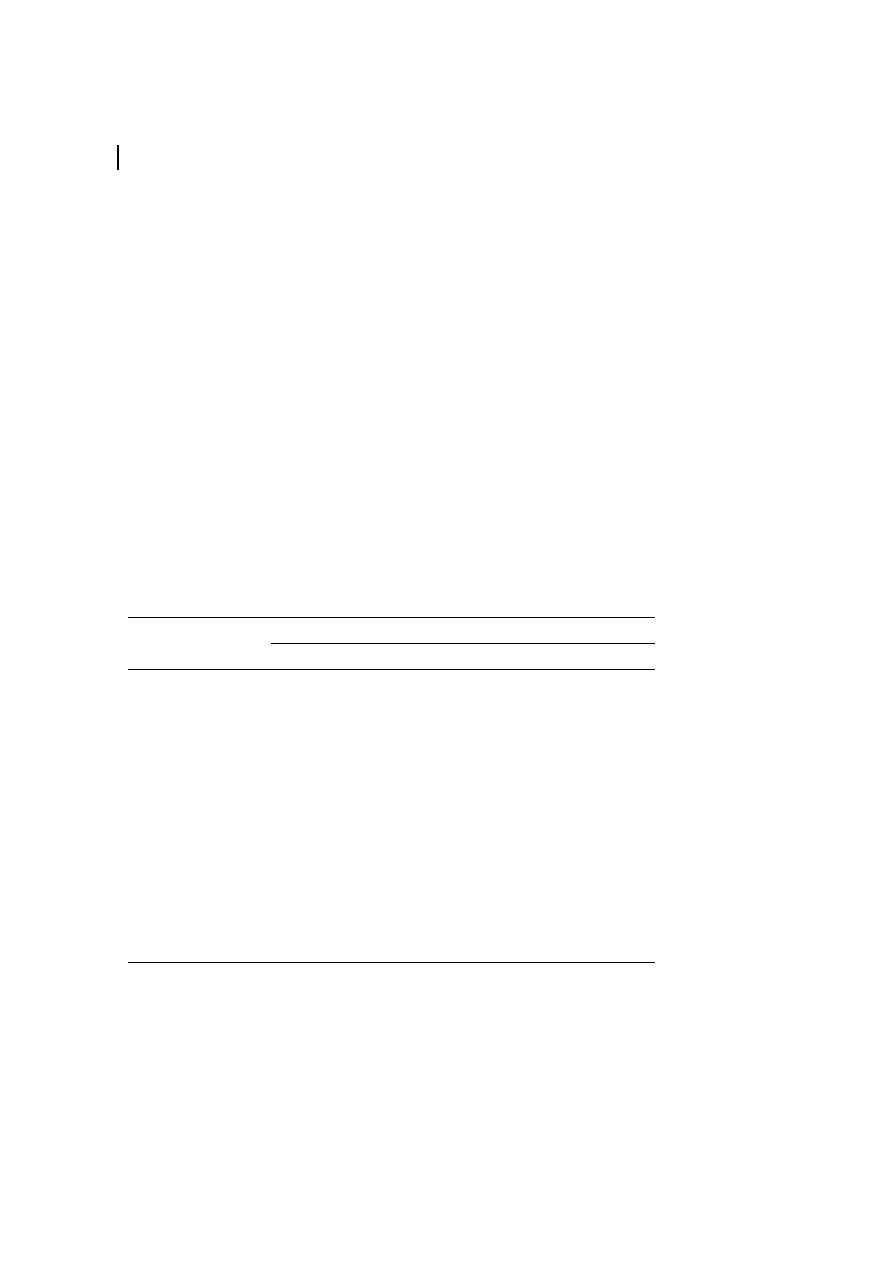
acetylation and deacetylation of cellulose;
these experiments resulted in the concept of
polymer-analogous reactions (Staudinger
and Daumiller, 1937). According to this
concept, functional groups of macromole-
cules ± in the case of cellulose predomi-
nantly hydroxyl groups ± can undergo the
same kind of reactions as the corresponding
low-molecular compounds. Further, it was
observed that the supramolecular structure
of the polymer may play an important role in
determining the rate and final degree of
conversion, as well as the distribution of the
functional groups, which has been well
recognized for cellulose.
2
Occurrence
The main source of cellulose is the occur-
rence of this polysaccharide in different
types of plants often combined with other
biopolymers. Of great scientific importance
is access to cellulose using enzymatic and
chemical methods, respectively, developed
during the last decade.
2.1
Natural Sources
The primary occurrence of cellulose is the
existing lignocellulosic material in forests,
with wood as the most important source.
Other cellulose-containing materials include
agriculture residues, water plants, grasses,
and other plant substances. Besides cellu-
lose, they contain hemicelluloses, lignin,
and a comparably small amount of extrac-
tives (Hon, 1996). Commercial cellulose
production concentrates on harvested sour-
ces such as wood or on naturally highly pure
sources such as cotton (Table 1).
10 Cellulose
278
Tab. 1
Chemical composition of some typical cellulose-containing materials
a
Source
Composition (%)
Cellulose
Hemicellulose
Lignin
Extract
Hardwood
43 ± 47
25 ± 35
16 ± 24
2 ± 8
Softwood
40 ± 44
25 ± 29
25 ± 31
1 ± 5
Bagasse
40
30
20
10
Coir
32 ± 43
10 ± 20
43 ± 49
4
Corn cobs
45
35
15
5
Corn stalks
35
25
35
5
Cotton
95
2
1
0.4
Flax (retted)
71
21
2
6
Flax (unretted)
63
12
3
13
Hemp
70
22
6
2
Henequen
78
4 ± 8
13
4
Istle
73
4 ± 8
17
2
Jute
71
14
13
2
Kenaf
36
21
18
2
Ramie
76
17
1
6
Sisal
73
14
11
2
Sunn
80
10
6
3
Wheat straw
30
50
15
5
a
Adapted from Hon (1996).
As a naturally occurring material, cellu-
lose may contain byproducts leading to
application problems and difficulties in
chemical modification reactions. However,
up-to-date cellulose isolation and purifica-
tion yield materials of high purity and
variability. Table 2 gives some examples of
such cellulose materials. The values of the
degree of polymerization (DP ) (molecular
weight
DP î 162 g mol
1
), included in Ta-
ble 2, manifest the huge variety in molecular
weight available.
A rather new approach to pure cellulose
gaining increasing interest is the lab-scale
production of the polymer by acetic acid-
producing bacteria, such as Gluconacetobact-
er xylinum and Acanthamoeba castellani
(Tarchevsky and Marchenko, 1991). Algae
form another origin of cellulose, e.g., Valonia
ventricosa and Chaetamorpha melagonicum.
The cellulose obtained is highly crystalline
and is useful for studying polymorphs of the
polymer (see Section 3.2.1) ( Vander Hart
and Atalla, 1984; Isogai et al., 1989; Sugiya-
ma et al., 1990; Yamamoto et al., 1989).
Cellulose of the Valonia type is found in
fungal cell walls as well. In addition, there
are several celluloses of animal origin, of
which tunicin, a cell wall component of
ascidians, has been extensively studied.
2.2
Synthetic Cellulose
The chemosynthesis of functionalized cellu-
lose by ring-opening polymerization of 3,6-
di-O-benzyl-
a-d-glucopyranose 1,2,4-ortho-
pivalate (Nakatsubo et al., 1996), or by step-
wise reactions of selectively protected
b-d-
glucose as, e.g., 1-allyl-2,6-di-O-acetyl-3-benzyl-
4-O-(p-methoxybenzyl)-
b-d-glucopyranoside
(Nishimura et al., 1993) has been experi-
mentally realized. Up to now, complete
deprotection of the polymers may yield
cellulose with a rather low DP in the range
from 9 to 55, dependent upon the applied
protecting groups. The non-biosynthetic
preparation of cellulose of a molecular
weight of 6300 g mol
1
was described in-
volving an enzymatic polymerization using
b-d-cellobiosyl fluoride as a substrate for
purified cellulase, in a mixture of acetonitrile
and acetate buffer at pH 5 (Kobayashi et al.,
1991, 1994). Whether the cellulose I or
cellulose II polymorph (see Section 3.2.1 and
3.2.2) is formed depends on the solvent
composition and the purity of the cellulase
(Lee et al., 1994). This approach has inter-
esting potential for the control of molecular
weight and dispersity of the cellulose
formed. Further, the enzyme-controlled ster-
eospecifity and regioselectivity may mini-
mize or even avoid the laborious, multi-step
2 Occurrence
279
Tab. 2
Carbohydrate composition and degree of polymerization (DP ) of some cellulose samples
a
Sample
Producer
Carbohydrate composition (%)
DP
Glucose
Mannose
Xylose
Avicel
Fluka
100
±
±
280
Sulfate pulp V-60
Buckeye
b
95.3
1.6
3.1
800
Sulfate pulp A-6
Buckeye
96.0
1.8
2.2
2000
Sulfite pulp 5-V-5
Borregaard
c
95.5
2.0
2.5
800
Linters
Buckeye
100
±
±
1470
Linters
Buckeye
100
±
±
2000
a
Adapted from Heinze (1998a)
b
Buckeye Cellulose Corp., 1001 Tillman Street, Memphis/Tennessee
38108 ± 0407, USA.
c
Borregaard ChemCell, P.O. Box 162, N-1701 Sarpsborg, Norway.
chemical
protection-deprotection
proce-
dures that generally are required in polysac-
charide chemistry.
3
Structure and Analysis
Cellulose is a polydisperse linear homopol-
ymer, consisting of regio- and enantioselec-
tively
b-1,4-glycosidic linked d-glucopyra-
nose units (so-called anhydroglucose units
[ AGU]) (Figure 1). It has been shown by
1
H-
NMR spectroscopy that the
b-d-glucopyra-
nose adopts the
4
C
1
chain conformation, the
lowest free energy conformation of the
molecule (Kr‰ssig, 1993). As a consequence,
the hydroxyl groups are positioned in the
ring plane (equatorial), while the hydrogen
atoms are in the vertical position (axial). The
polymer contains free hydroxyl groups at the
C-2, C-3, and C-6 atoms. Based on the OH
groups and the oxygen atoms of both the
pyranose ring and the glycosidic bond,
ordered hydrogen bond systems form vari-
ous types of supramolecular semi-crystalline
structures.
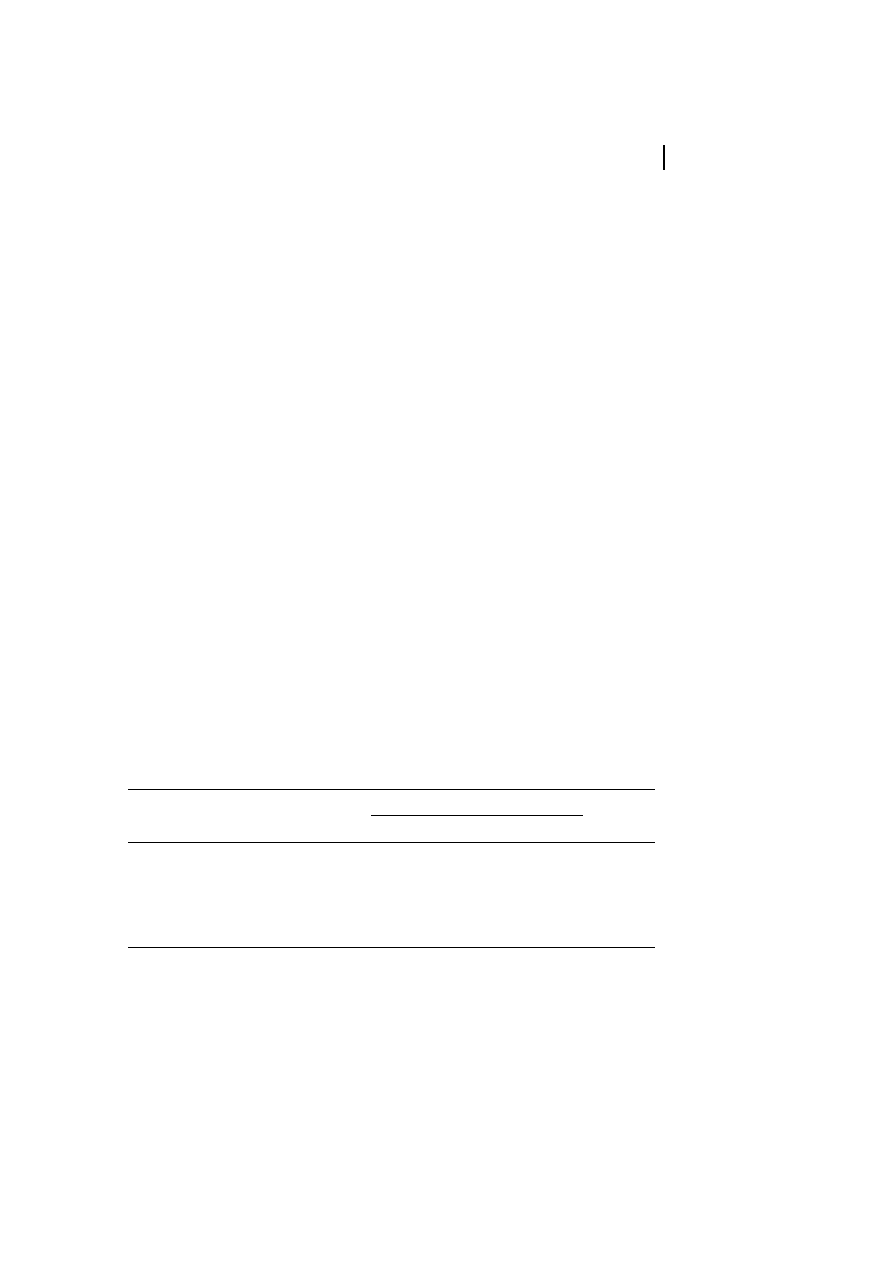
3.1
Hydrogen Bonding
Both intra- and intermolecular hydrogen
bonding occurs in cellulose. The detailed
structure of this hydrogen-bond network is
still a subject of discussion. The presence of
intramolecular hydrogen bonds is of high
relevance with regard to the single-chain
conformation and stiffness. The existence of
hydrogen bonds between O-3-H and O-5
'
(2.75 ä, means of neighboring AGU ) of the
adjacent glucopyranose unit and O-2-H and
O-6
' (2.87 ä) in native crystalline cellulose
(cellulose I, Figure 2) can be concluded from
X-ray diffraction and NMR- and IR spectro-
scopical data (Liang and Marchessault, 1959;
Marchessault and Liang, 1960; Gardner and
Blackwell, 1974; Sarko and Muggli, 1974).
In cellulose II crystallites, the hydrogen
bonds are essentially the same as those
proposed for cellulose I, considering the O-3-
H and O-5
' (2.69 ä) hydrogen bond. The
conformation of the C-6 hydroxymethyl
group differs in each chain since the chains
are oriented anti-parallel in the unit cell, i.e.,
the CH
2
OH groups of the respective chains
are not equivalent (Kolpak and Blackwell,
1976). Because one of the chains has one
intramolecular hydrogen bond per AGU
while the other chain has two, a more
complex hydrogen-bonding network occurs
(Stipanovic and Sarko, 1976).
10 Cellulose
280
Fig. 1
Molecular structure of cellulose.
Fig. 2
Hydrogen bond system of cellulose I.
The intermolecular hydrogen bonding in
cellulose is responsible for the sheet-like
nature of the native polymer. Today, inter-
molecular hydrogen bonding between only
the OH group at the C-6
' and C-3' ('' means of
the neighboring chain) positions of cellulose
molecules adjacently located in the same
lattice plane (020 planes) is assumed (Black-
well et al., 1977).
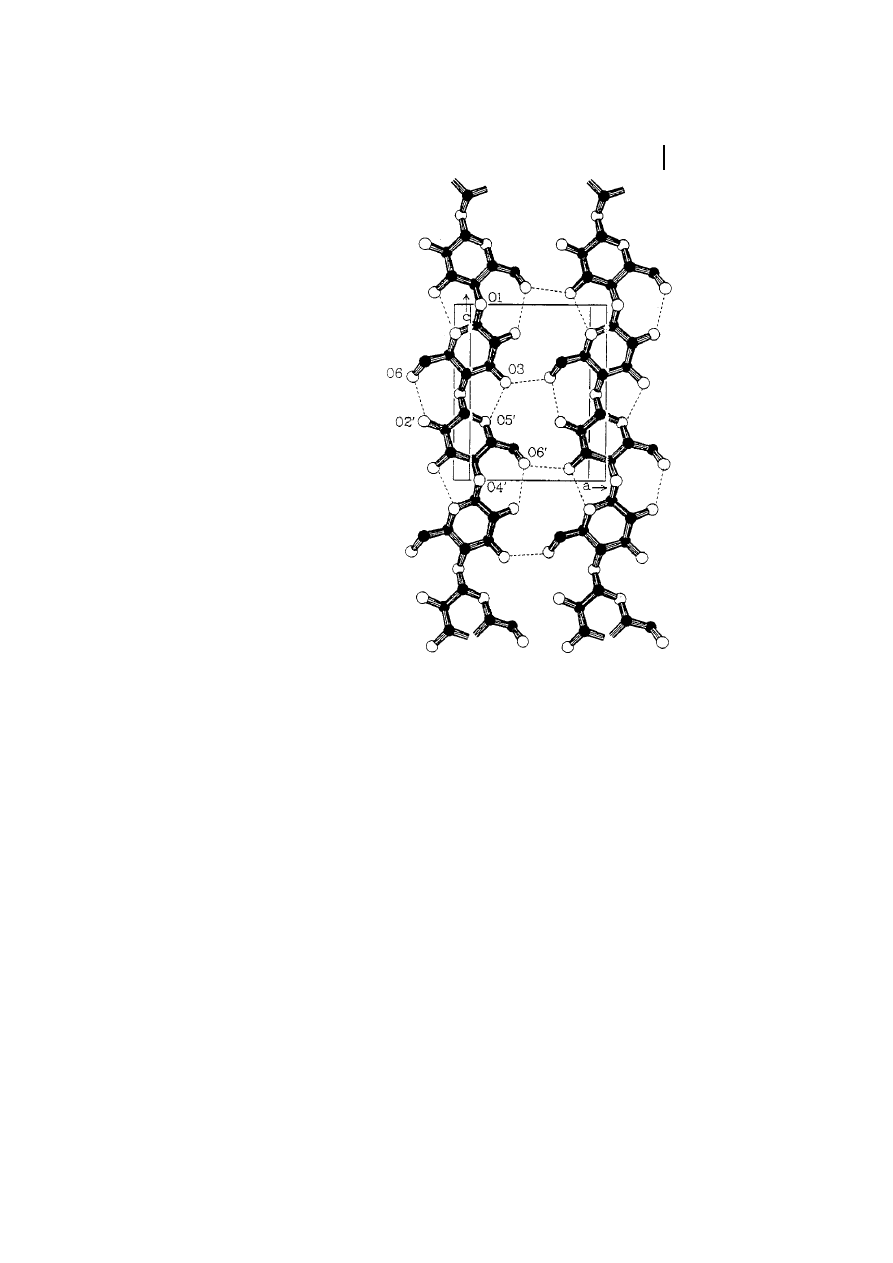
The intermolecular hydrogen bonding in
cellulose II is significantly more complex
compared to that of cellulose I. The anti-
parallel chain model enables the formation
of not only interchain but also of interplane
hydrogen bonds. The most widely accepted
representation of the bonding situation has
been given by Kolpak and Blackwell (1976,
1978), as shown in Figure 3. It should be
pointed out again that the hydrogen bonding
of cellulose has been interpreted in many
ways, as nicely discussed by Gilbert and
Kadla (1998).
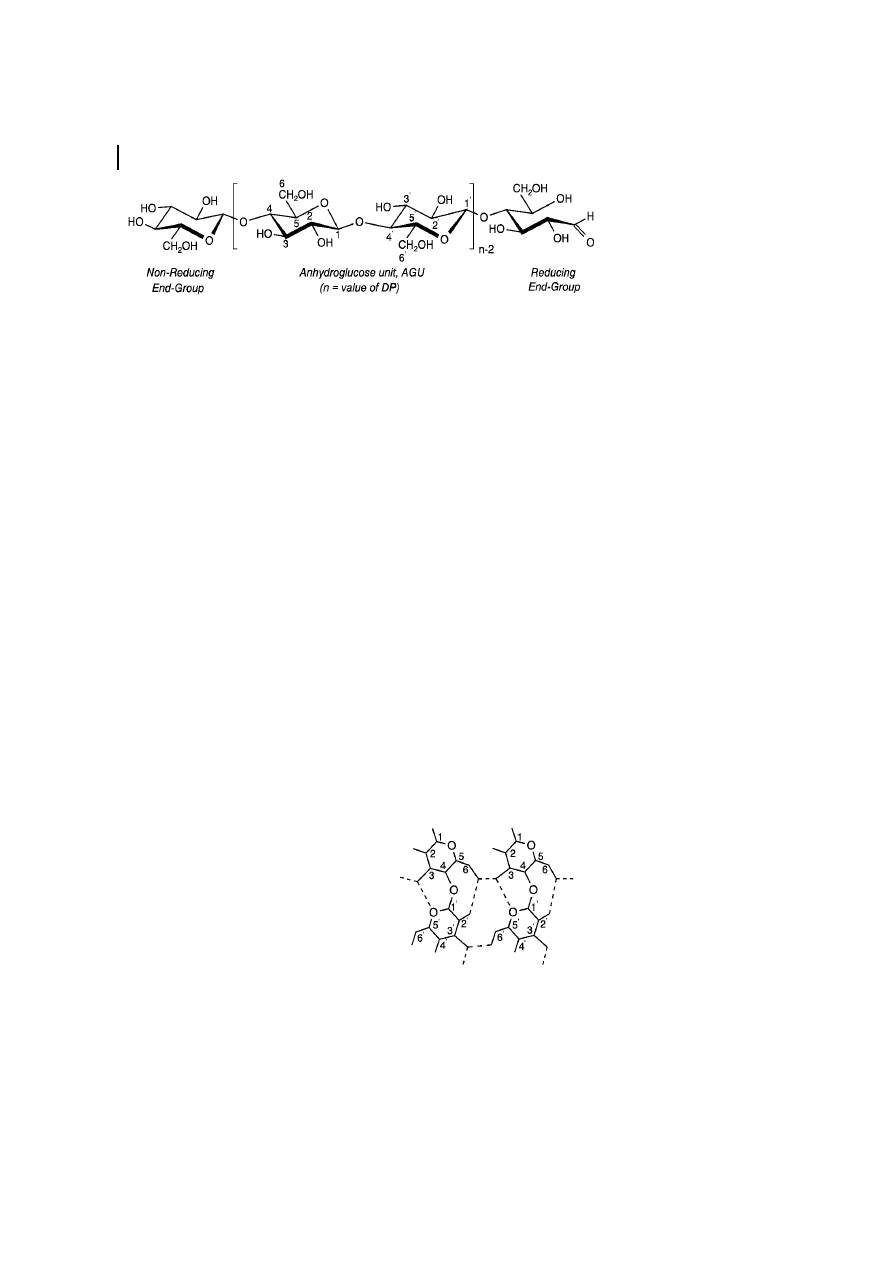
3.2
Crystal Structure
The order of the macromolecules in a
cellulose fiber is not uniform throughout
the whole structure. There exist regions of
low order (so-called amorphous regions) as
well as of very high crystalline order. The
experimental evidence available today is
adequately interpreted by a two-phase mod-
el, the fringed fibril model, assuming low-
order (amorphous) and high-order (crystal-
line) regions and neglecting the rather small
amount of matter with an intermediate state
of order (Hearle, 1958).
The relative amount of polymer within the
highly ordered regions is usually assessed
from wide-angle X-ray scattering ( WAXS )
patterns or from the evaluation of a
13
C CP-
MAS NMR spectrum. The degree of crystal-
linity of cellulose (usually in the range of
40% to 60%) covers a wide range and
depends on the origin and pretreatment of
the sample (Fink and Walenta, 1994).
3.2.1
Cellulose I Polymorph
Cellulose exists in several crystal modifica-
tions, differing in unit cell dimensions and,
possibly, in chain polarity. For crystalline
native cellulose, i.e., cellulose I, Meyer,
Mark, and Misch (Meyer and Mark, 1929;
Meyer and Misch, 1937) proposed a unit cell
of the crystal lattice, already 60 years ago,
that is still applicable for practical purposes
today (Figure 4). This model assumes a
monoclinic unit cell with the space group
3 Structure and Analysis
281
Fig. 3
Most probable bond pattern of cellulose I
(Kolpak and Blackwell, 1976, 1978).
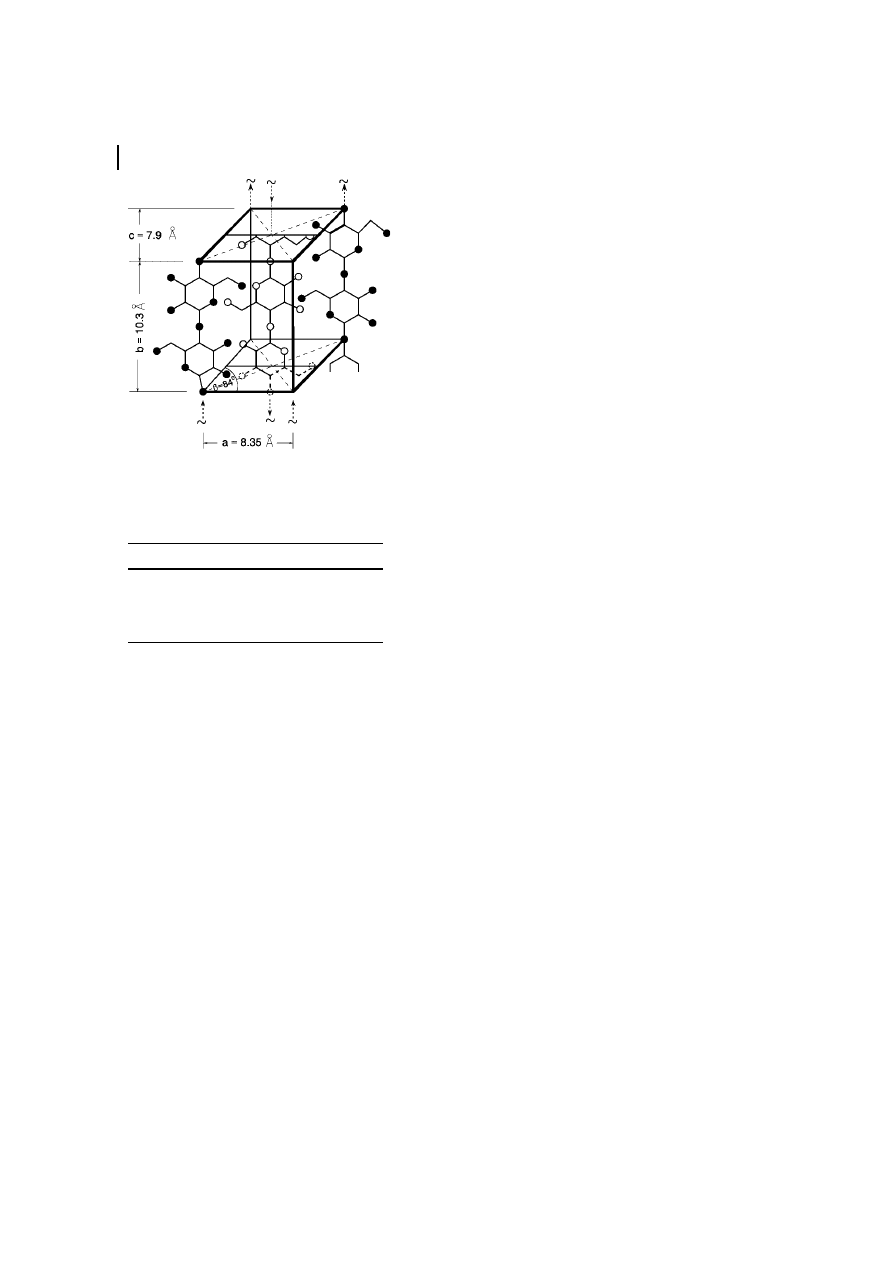
P2
1
and two anti-parallel cellobiose chain
segments running in opposite directions
along the fiber axis. The dimensions of this
cell are given in Table 3.
Numerous authors have suggested that
the unit cell of native cellulose may depend
on the source. Honjo and Watanabe (1958)
concluded from low temperature electron
diffractograms a doubling of the size of the
unit cell. Sarko and Muggli (1974) agree to
this eight-chain unit cell but state that the
Meyer-Misch model also adequately repre-
sents most of the crystallographic evidence
of native crystalline cellulose. Based on the
more refined WAXS technique, Gardner and
Blackwell (1974) proposed for cellulose
(from Valonia alga) a monoclinic lattice with
two parallel running chains and assumed
this to be valid for cellulose I in general.
Sarko and Muggli (1974) proposed from a
combined packing and X-ray intensity anal-
ysis a triclinic lattice cell with two cellulose
chain segments running parallel along the
fiber axis.
Atalla and Vanderhart (1984) showed that
native cellulose consists of two different
crystal structures, cellulose I
a
and I
b
, using
high-resolution, solid-state
13
C NMR spec-
troscopical studies. There are differences in
the resonances of the C-1 atoms. A singlet
for cellulose I
a
and a doublet for cellulose I
b
appears at about 106 ppm. This rather small
difference indicates a different hydrogen-
bonding pattern of the glycosidic linkages.
Bacterial cellulose and Valonia cellulose
(from alga) contain a large amount of I
a
modification, while in ramie, cotton, and
wood cellulose, the I
b
phase is the dominat-
ing modification. The I
a
modification is
described as a triclinic P-1 structure, with
one cellulose chain per unit cell, whereas the
I
b
phase is assumed to be a monoclinic unit
cell of the Meyer-Misch type (space group P-
2
1
with two chains per unit cell) as concluded
from electron diffraction experiments (Su-
gijama et al., 1991). According to Yamamoto
and Horii (1993), the I
a
phase is metastabile
and can be transformed (not completely,
however) into the thermodynamically more
stable I
b
phase by annealing at 260
8C to
280
8C.
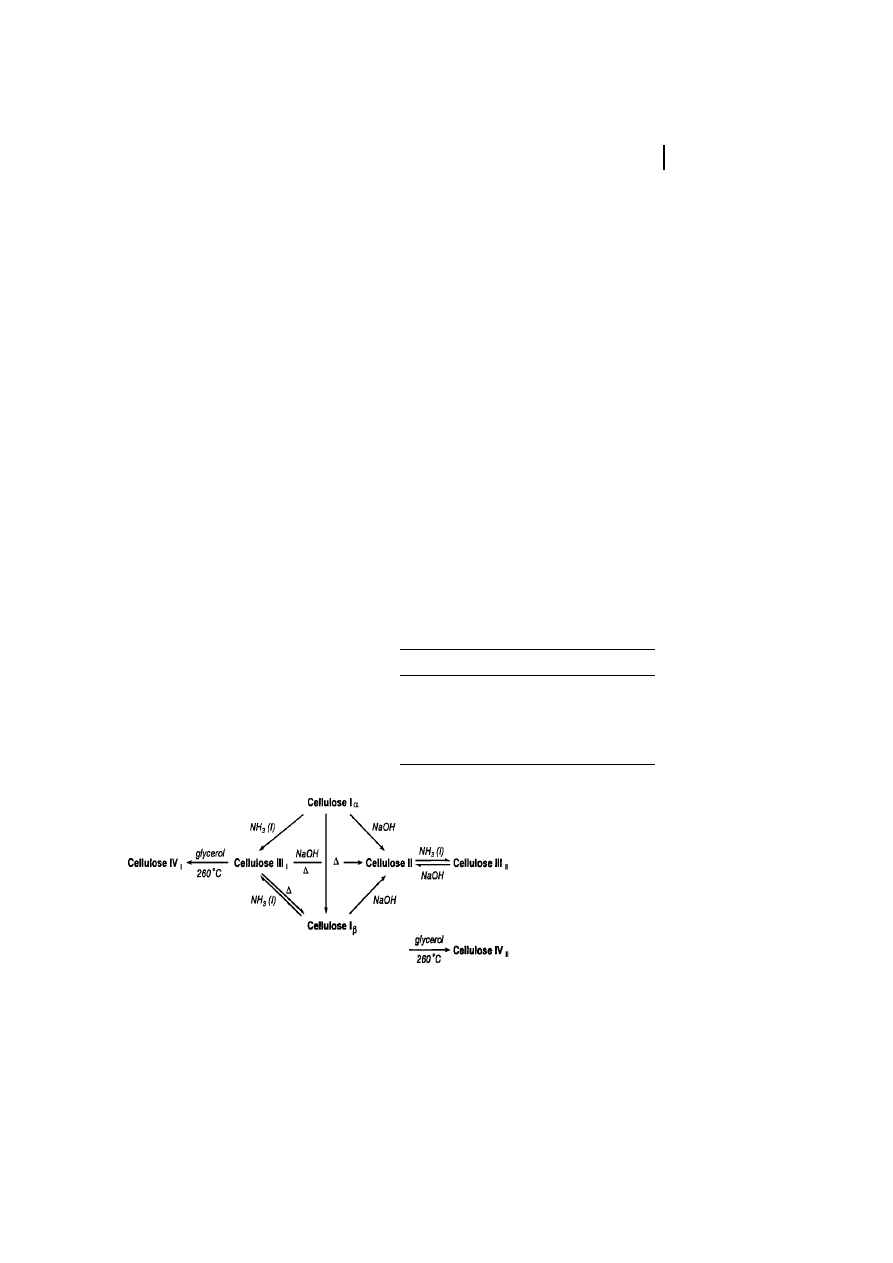
3.2.2
Further Cellulose Polymorphs
Besides cellulose I, cellulose II is the most
important crystalline form of cellulose from
a technical and commercial point of view.
Cellulose II can be prepared by precipitating
dissolved cellulose into an aqueous medi-
um; this is the typical process for the
technical spinning of man-made cellulose
10 Cellulose
282
Fig. 4
Unit cell of cellulose I according to the
Meyer-Misch model.
Tab. 3
Unit cell dimensions of various cellulose
allomorphs
a
a-axis (ä) b-axis (ä) c-axis (ä)
g-axis (ä) Polymorph
7.85
8.17
10.34
96.4
Cellulose I
9.08
7.92
10.34
117.3
Cellulose II
9.9
7.74
10.3
122
Cellulose III
7.9
8.11
10.3
90
Cellulose IV
a
As accomplished by Kr‰ssig (1993).
fibers. It is also obtained by the so-called
mercerization process, i.e., by soaking cellu-
lose in aqueous NaOH (17% to 20%, w/v)
followed by decomposition of the intermedi-
ate by neutralization or washing out the
NaOH. Mercerization is used to activate the
polymer prior to the production of technical
cellulose ether. The process of transforma-
tion of cellulose I to cellulose II is generally
considered to be irreversible.
Cellulose II is formed naturally by a
mutant strain of Gluconacetobacter xylinum
(Kuga et al., 1993) and occurs in the alga
Halicystis (Sisson, 1938), which were both
very useful to provide an insight into the
crystal structure of cellulose II.
The crystalline modification of cellulose
III is obtained by treating native cellulose
with liquid ammonia (below ±30
8C) or an
organic amine such as ethylene diamine,
followed by washing with alcohol. Small
differences in lattice dimensions exist be-
tween the two submodifications cellulose
III
I
and III
II
. As the fourth modification
reported so far, cellulose IV is formed upon
treatment of the other modification of
cellulose in a suitable liquid at high temper-
ature and under tension (Figure 5, see
Table 3).
3.3
Morphology
Cellulose morphology represents a well-
organized architecture of fibrillar elements.
It has been considered that the elementary
fibril of native cellulose is the smallest
morphological unit with a diameter of about
3.5 nm (M¸hlethaler, 1965; Heyn, 1966;
Fengel and Wegener, 1989). Recent electron
microscopic and WAXS data indicate that
the diameter may differ in the range of 3 to
35 nm depending on the cellulose source
(Table 4, Chanzy et al., 1986; Fink et al.,
1990). The so-called microfibril is described
as the lowest well-defined morphological
entity, although it consists of non-uniform
subunits (Fink et al., 1990). The length of
the microfibril can reach micrometers,
which in turn forms the macrofibrils with
a diameter in the range of micrometers
(Fengel and Wegner, 1989; Kr‰ssig, 1993).
Micro- and macrofibrils represent the
construction units of the cellulose fiber
3 Structure and Analysis
283
Fig. 5
Transformation of cellulose into its various polymorphs.
Tab. 4
Range of microfibril diameter of various
cellulose samples
a
Sample
Microfibril diameter (nm)
Bacterial cellulose
4 ± 7
Cotton linters
7 ± 9
Ramie
10 ± 15
Dissolving pulp
10 ± 30
Valonia cellulose
10 ± 35
a
Source: Fink et al. (1990).
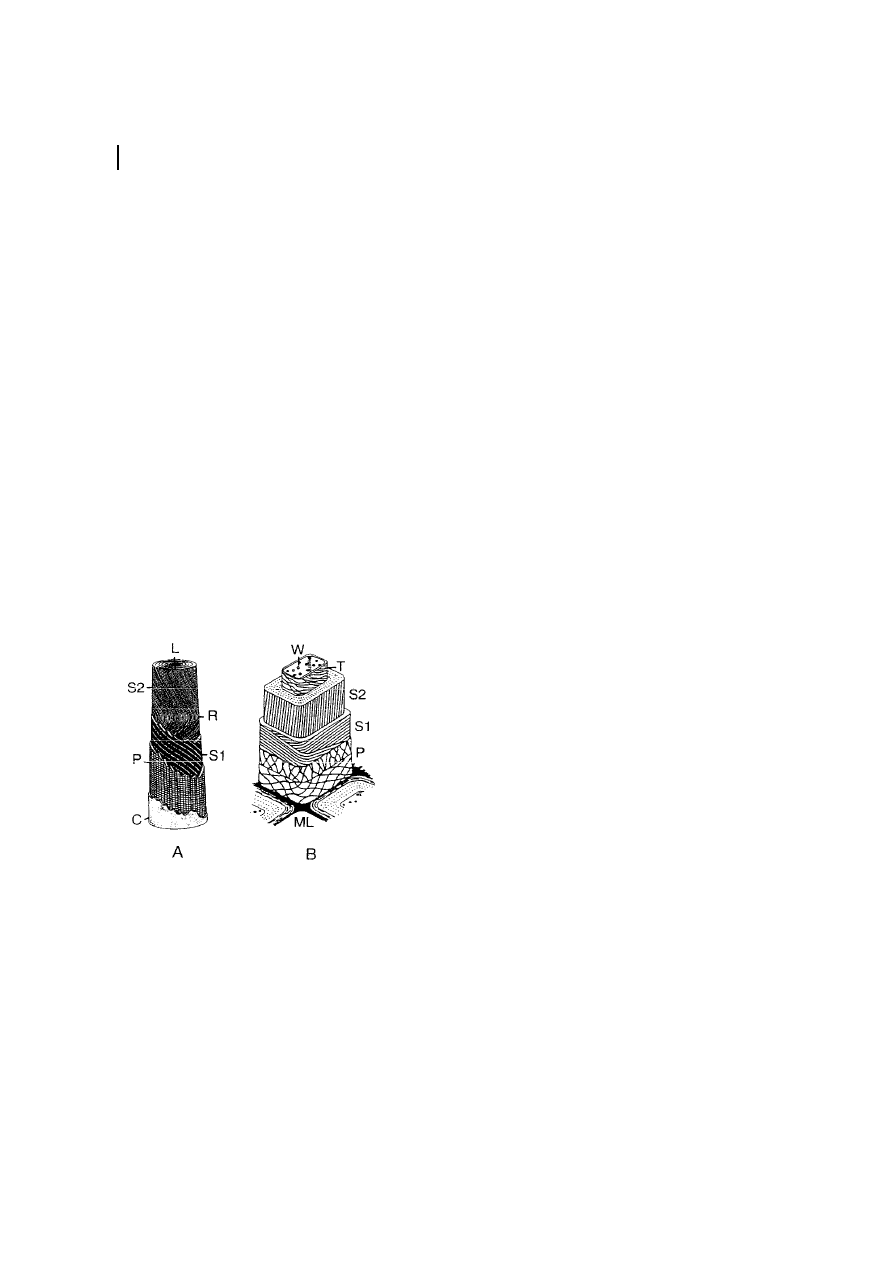
cell-wall architecture, which is characterized
by layers differing in fibril texture (Figure 6).
The fibers consist of different layers, with the
fibril position giving different densities and
textures, as shown for a cotton fiber and a
delignified spruce pulp fiber. The primary
wall (P ) fibrils have a diameter of about
10 nm and are positioned crosswise to a layer
with a thickness of about 50 nm. The
secondary cell wall (S ) consists of two layers,
S1 and S2, with a thickness of about 100 nm
(cotton) to 300 nm (spruce pulp). The S1 and
S2 layers contain most of the cellulose mass.
The fibrils are aligned parallelly and packed
densely in a flat helix. The inner layer closest
to the fiber lumen ( W) is the tertiary layer
(T ), which is rather thin and has fibrils
aligned in a flat helix as well (Kr‰ssig, 1993).
3.4
Analysis
Qualitatively, cellulose can be determined by
X-ray spectroscopy, by color reactions with
KI or ZnCl
2
, by reacting with a solution of
iodine in sulfuric acid or phosphoric acid,
and with Congo red and others (Blazej et al.,
1979). However, these color reactions are not
specific.
For a quantitative determination, the
Cross-Bevan method and the K¸rschner-
Hoffer method are quite appropriate (Blazej
et al., 1979). For these methods, the acces-
sory components are removed by a treatment
with gaseous hydrogen chloride and subse-
quent heating with aqueous sodium sulfite
or with nitric acid/ethanol after pretreat-
ment with aqueous potassium hydroxide,
respectively (K¸rschner and Popik, 1962).
In the analysis of cellulose and its deriv-
atives, instrumental methods are employed
for assessing the size and chemical structure
of the macromolecules within the entity of a
given sample. Moreover, instrumental de-
tection methods also are required for the
monitoring of structurally relevant parame-
ters during continuous fractionation of the
polymer or chromatographic separation of
its fragments. The spectroscopic methods
preferentially used are UV/visible- (Fengel
and Wegener, 1989), IR- (Grˆbe, 1989), and
NMR-spectroscopy (Nehls et al., 1994). To-
day, predominately HPLC and gas chroma-
tography are used to analyze the fragments
obtained by acid or enzyme degradation
(Mischnick, 1995; Heinze et al., 1994;
Heinze, 1998b; Saake et al., 2000).
4
Physiological Function
Cellulose is the main component of plant
cell walls. Protection of cells and formation
of structures are as such the main functions
of cellulose during plant life. The defined
cell shapes and position of cells to each other
are the basis for plant morphology. There-
fore, cellulose is essential for plant life as we
know it. Even structures based on old cell
10 Cellulose
284
Fig. 6
Scheme of the ™morphological architecture∫
of a cotton fiber (a) and a delignified spruce wood
fiber (b) according to Kr‰ssig (1993). C
cuticle
(rich in pectins and waxes); L
lumen; ML middle
lamella (mainly lignin); P
primary wall; R rever-
sal of the fibril spiral; S1
secondary wall (™winding
layer∫); S2
secondary wall (main body); T terti-
ary wall; W
wart layer.

walls from already dead cells are crucial
functional units of higher plants (xylem).
The main building blocks of the primary
cell wall of plants consist of different
components, e.g., pectins, hemicelluloses,
celluloses, and proteins. In the primary cell
wall, the non-cellulosic components domi-
nate; on this basis, the mechanical stability
of the primary cell wall is low. While in later
on formed structures of the cell wall and
units important for plant morphogenesis the
fibrillar structure of cellulose stabilizes the
plant organism. The later on formed struc-
tures of the cell wall and cell units important
for plant morphogenesis were stabilized
by the fibrillar character of cellulose The
primary cell wall swells and forms jelly-like
structures. (To the groups of hemicelluloses
belong glucanes of the (1
! 3)-b as well as
(1
! 4)-b-, gluco-, and galactomannanes and
mainly xylanes; Sitte et al., 1991) The cellu-
lose molecules in the primary cell walls have
high degrees of polymerization between
2000 and 15,000 anhydroglucose units in
long, non-branched molecules. The cellulose
chains are twisted along the axis of the
glucan chains (180
8) and stabilized by hydro-
gen bonds between the chains. As a result,
the rings of the pyranoses lie approximately
in the same level, forming ligaments. The
smaller chains have lengths around 8
mm.
These chains associate to elementary fibrils
having a diameter of about 5 ± 30 nm. In the
secondary cell walls, the fibrils associate to
microfibrils with diameters of about 5 ±
30 nm. These microfibrils have an organ-
ization in crystal lattices, bringing a high
stability into the cell walls of plants. These
associated cellulose fibrils bring the main
contribution
to
the
high
mechanical
strength of the plant cell walls. The tensile
strength of plant cell walls has a basis not
only in the association of the chains by
hydrogen bonds but also in sticking together
with other components of the primary cell
wall, such as proteins, pectines, and hemi-
celluloses. For further increasing the stabil-
ity of plants, lignin is incorporated into the
plant architecture. The portion and distribu-
tion of the different components of cell walls
define the final properties of the plant parts
and tissues. Examples of the role and
structure of hemicelluloses in the plant cell
wall system are given by Henriksson et al.
(2000).
Rose et al. (1997) have found that specific
proteins, so-called expansins, are able to
induce the extension of isolated plant cell
walls in vitro and to disrupt the non-covalent
interactions between hemicelluloses and
cellulose microfibrils. Because the primary
cell wall acts as the main factor for cell
enlargement, this process may be an integral
part to plant cell expansion. Using expan-
sins, the role of the different components
within this primary cell wall can be studied.
Some of these reactions and substance
formations will be regulated by ethylene
and other phytohormones.
The microtubule arrays are of high im-
portance because of their involvement in the
orientation of cellulose microfibrils. The
plant interphase tubulins play an important
role in these processes and have influences
on structuring the microfibrils within the
cell-wall system (Moore et al., 1997).
5
Biosynthesis
The biosynthesis of cellulose is not yet
completely elucidated. Moreover, contrary
results have been described and discussed in
many papers. During the last few years, the
numbers of patents in this field has in-
creased because of the interesting possibility
to increase the cellulose content of plants
and to construct new and more efficient
plants. Because of the ability of some
5 Biosynthesis
285

bacterial strains to form cellulose and be-
cause of the similarity of the biosynthetic
apparatus in some aspects, much of the
research was done using these bacteria.
Results obtained with the bacterium Gluco-
nacetobacter xylinum as a model organism
are discussed in Volume 5 of Biopolymers,
Chapter 3. Table 5 contains a summarization
of different relevant publications, which will
not be discussed in detail, while Table 6
shows a selection of interesting patents.
5.1
Synthesis of Substrates for the Polymerizing
Enzyme
The only substrate for cellulose biosynthesis
is UDP-glucose. The biosynthesis of this
energy-rich compound follows the normal
biosynthetic pathways in the cells, starting
from glucose (Figure 7). The enzyme cellu-
lose synthase accepts only UDP-glucose as a
substrate; moreover, it was noticed that by
feeding modified glucoses to bacteria (Glu-
conacetobacter xylinum), as well as to plant
cells or cell extracts, no significant formation
of modified celluloses could be detected
(Schmauder, unpublished; Brown, personal
communication).
Another possible source for UDP-glucose
could be sucrose synthase, an enzyme
associated with the plasma membrane,
e.g., of cotton fibers. Because of this location,
a direct channeling of the substrate UDP-
glucose to the polymerizing enzyme is
possible. But the regulation, control, and
targeting of this process is unknown in wide
areas. Other possible sources for the stabi-
lizing and transport of the substrate are
annexin-like molecules, which are able to
bind UDP-glucose, e.g., a 170-kDa polypep-
tide was co-purified with the cellulose
synthase. This protein shows some homol-
ogies to the yeast
b-1,3-glucan synthase (see
also Brown, 1999; Delmer, 1997, 1999a,b,
2000a,b).
10 Cellulose
286
Tab. 5
Selected papers for biosynthesis and structure of cellulose
Topic of the paper
Authors/Applicants
Role of callose synthase and other (1,3)-
b-glucan synthase in cellulose
biosynthesis; enhancing of cellulose synthesis by cellobioses
Him et al., 2000
Effect of retardants on cellulose biosynthesis in cotton; effects on fibers and
seedlings
Akhunov et al., 2000
Review about genes and proteins involved in cellulose synthesis in plants; role
of the sucrose synthase for substrate formation; orientation of the microfibril
deposition; role of membrane-associated cellulase in biosynthesis process
Delmer et al., 2000
Cellulose structure elucidation using atomic force microscopy
Baker et al., 2000
Estimation of the relations between Cellulose I
a
and I
b
in wood; application of
13
C-NMR
Newman, 1999
Supramolecular architecture; fibril formation and its regulation
Kataoka and Kondo,
1999a,b
Cellulose biosynthesis as a binding factor for CO
2
Hayashi et al., 1998b
Reviews on cellulose biosynthesis; comparison of synthesis by microorganisms
and by plants
Brown, 1996; Brown
et al., 1996; Kudlicka
and Brown, 1996
General reviews concerning cellulose biosynthesis by bacteria, fungi, and plants
Blanton and Haigler,
1996

Summarizing those effects, Brett (2000)
states that UDP-glucose on the one hand or
sucrose on the other, as well as further
soluble intermediates from these pathways,
could serve as possible precursors.
5.2
Polymerizing Enzyme System and Enzymology
of Biosynthesis
Different data are described for the cellulose
synthase as the active enzyme system in
cellulose formation. Carpita and Vergara
(1998) discuss the polypeptide formed as a
result of the CelA gene family (cotton), with
a size of about 110 kD, and the existence of
eight transmembrane domains.
Brown and Saxena (2000) and Delmer
(1999b) describe that the cellulose-synthase
complex has a rosette structure character-
ized by ultrastructural investigations. This
structure is highly symmetrically arranged
(about six-fold) and contains transmem-
brane particle subunits. From these sub-
units, the crystalline cellulose I will be
generated. In this review, the historical data
for this finding are discussed in detail. The
catalytic subunit is a transmembrane protein
with some transmembrane regions.
Brown and Saxena (2000) discuss four
different models for cellulose synthesis:
1) The most acceptable model, model 1,
works with the assumption that the
5 Biosynthesis
287
Tab. 6
Selected patents for biosynthetic pathways
Topic of the patent
Authors/Applicants
Overexpression of cellulose synthase genes for modulating expression of
enzymes involved in synthesis of plant cell walls
Taylor and Turner, 2000
Polynucleotides encoding cellulose synthase for acceleration of plant growth
and up-regulation of cellulose synthase level; modifying of lignin biosynthesis
Carraway et al., 2000
Cellulose synthase gene from poplar; application for altering cellulose and
lignin composition
Chiang et al., 2000
New genes encodes maize cellulose synthase polypeptides; modulation of
expression of cellulose synthase in plants; production of transgenic plants
expressing the new protein
Bowen et al., 2000
Genes for cellulose synthase; application of these genes for improving plant
stalk quality; increase of cellulose in stalks etc.
Dhugga et al., 2000
Transgenic plant expressing cell-wall modulating proteins as a basis for, e.g.,
altered morphology, increased growth, modified fiber lengths, increased
cellulose and starch content
Shani et al., 1999
Isolated genes encoding polypeptides involved in cellulose biosynthesis,
transgenic plants, expressed in sense or anti-sense orientation, ribozxymes, co-
suppression, gene-targeting molecules
Arioli et al., 1999
Fig. 7
Intracellular activation of glucose as the precursor for cellulose biosynthesis. 1: Glucokinase; 2:
Phosphoglucomutase; 3: UDP-glucose-pyrophosphorylase; UDP
uridine 5'-diphopsphate; glc glucose;
glc-6-P
glucose-6-phosphate; glc-1-P glucose-1-phosphate; P
i
inorganic phosphate.
Wyszukiwarka
Podobne podstrony:
avr32 gnu toolchain 3 3 0 275 readme
287
275 Prawo pocztowe
275
historyczne bitwy benewent 275 p n e LADGYMSEYNNEPIY3VSFFG6UIYNXY4OJMUBNTVQY
287
274 i 275, AP
21 269 287 Effect of Niobium and Vanadium as an Alloying Elements in Tool Steels
umiej eatno 9c e6+przeprowadzania+negocjacji+ 287+stron 29 RZHUF5Z626YT2ZRETOK5P56RYXLPOSGX3EZB34Q
07 44 287
96.62.287, DZIENNIK USTAW Z 1996 R
275
286 287
287
275 Manuskrypt przetrwania
275
więcej podobnych podstron