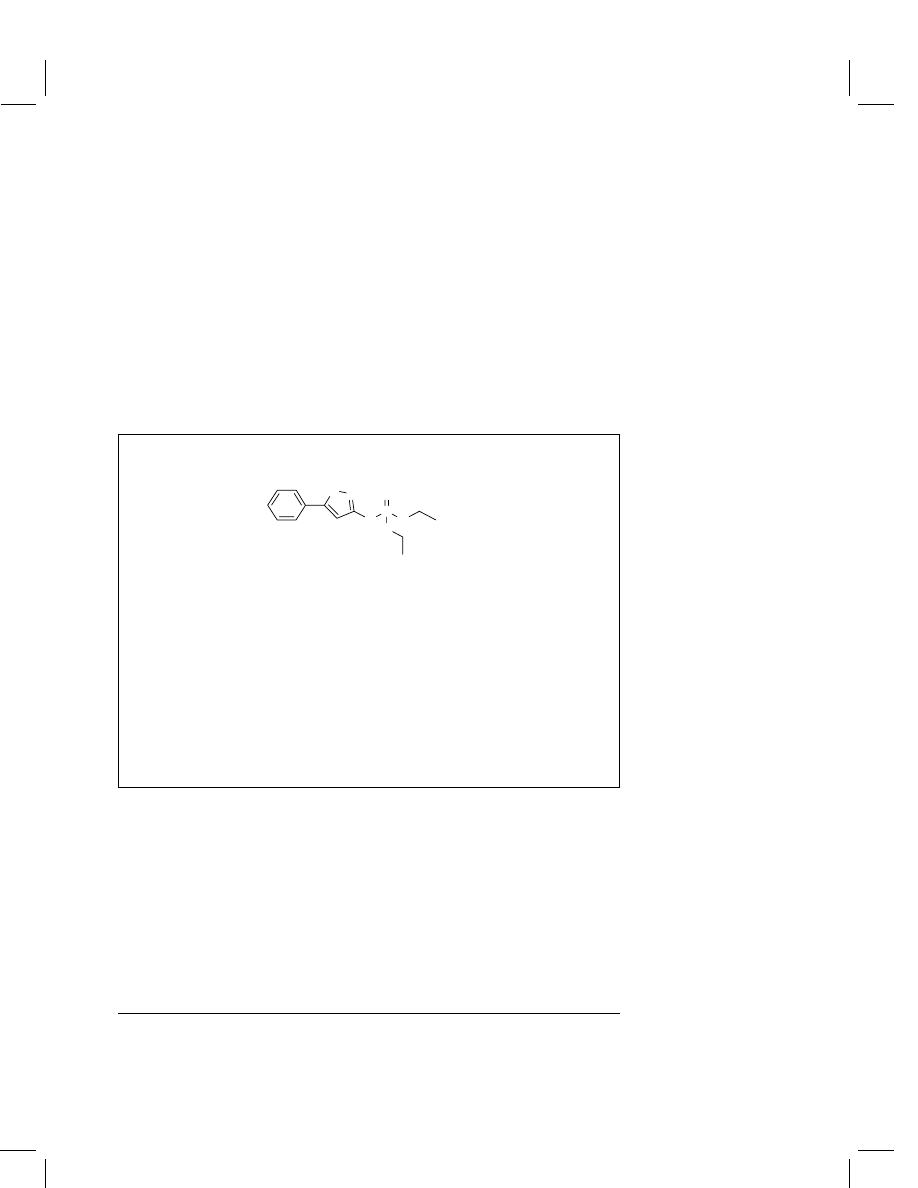
Isoxathion
Materials to be
analyzed
Rice, wheat, fruit, vegetable, potato, sugar cane and tea
Instrumentation
Gas-chromatographic determination for plant materials
1
Introduction
Chemical name
(IUPAC)
O,O-Diethyl-O-5-phenylisoxazol-3-yl phosphorothioate
Structural formula
O
P
O
S
O
N
O
Empirical formula
C
13
H
16
NO
4
PS
Molar mass
313.3
Form
Pale yellow liquid
Boiling point
160
◦
C/0.15 mmHg
Vapor pressure
<0.133 mPa at (25
◦
C)
Log P
3.88 (pH 6.3)
Solubility
Water 1.9 mg L
−1
(25
◦
C)
Readily soluble in organic solvents
Stability
Unstable in alkaline solutions
Use pattern
Isoxathion is used as an insecticide for rice, orange, tea,
various vegetables, soybean, etc.
2
Outline of method
Isoxathion is extracted from plant materials with aqueous acetone. The extracts are
concentrated and partitioned with n-hexane after addition of sodium chloride. The
n-hexane phase is collected and concentrated after dehydration. The extract is par-
titioned with n-hexane and acetonitrile. The acetonitrile phase is collected, concen-
trated, and subjected to Florisil column chromatography. Isoxathion is eluted with
diethyl ether–n-hexane after washing the column with the solvent. Isoxathion in the
eluate is concentrated and dissolved in acetone and injected into a gas chromatograph
for quantitative determination.
Handbook of Residue Analytical Methods for Agrochemicals.
C
2003 John Wiley & Sons Ltd.

1328
Individual compounds
3
Apparatus
Gas chromatograph: HP-6890 equipped with a nitrogen–phosphorus detector
(Hewlett-Packard)
Integrator: HP-3396C integrator (Hewlett-Packard)
Rotary evaporator: Model NE-1 (Tokyo Rikakikai Co., Japan)
Erlenmeyer flask, 500-mL
Round bottom flask, 500-mL
Separatory funnel, 500-mL
Glass chromatography column
4
Reagents
Isoxathion: analytical standard,
>98% purity
Acetone, acetonitrile, n-hexane, diethyl ether, sodium chloride, anhydrous sodium
sulfate: reagent grade (Wako Pure Chemical Inc., Japan)
Florisil for column chromatography, 60–100 mesh (Floridin Co.)
Diatomaceous earth for chemical analysis
5
Analytical procedure
5.1
Extraction
A 50-g amount (in the case of powder tea, 25 g) of each minced and homogenized
plant sample is weighed into a 500-mL flask with a ground stopper and 100 mL of
water are added. After standing for 2 h, 150 mL of acetone are added and the flask is
vigorously shaken with a shaker for 30 min. The mixture is filtered by suction through
a filter paper with a layer of diatomaceous earth 1-cm deep. The residue on the filter
paper is returned to the flask and re-extracted with 100 mL of acetone by shaking for
10 min and the mixture is filtered. The combined filtrate in the round-bottom flask is
concentrated to less than 100 mL under reduced pressure below 40
◦
C.
5.2
Partition of n-hexane and aqueous solution
To the concentrated solution, 200 mL of 5% sodium chloride aqueous solution and
100 ml of n-hexane are added and vigorously shaken in a separatory funnel for 5 min.
After leaving for a while, the n-hexane layer is collected. To the aqueous layer 100 mL
of n-hexane are added and the partition procedure is repeated. The combined n-hexane
layer is dried by passing through a funnel containing 50 g of anhydrous sodium sulfate
and is concentrated under reduced pressure below 40
◦
C.
5.3
Partition of acetonitrile and hexane
To the concentrated sample, 30 mL of acetonitrile and 30 mL of n-hexane are added
and shaken for 5 min. The acetonitrile layer is collected. To the n-hexane layer, 30 mL
of acetonitrile are added and shaken for 5 min and the acetonitrile layer is collected.

Isoxathion
1329
The acetonitrile extracts are combined and concentrated under reduced pressure below
40
◦
C.
5.4
Florisil column chromatography
A glass chromatography column (1.5-cm i.d., 30-cm length) is filled with 10 g of
Florisil using a solution of diethyl ether–n-hexane (3 : 17, v/v) and 5 g of anhydrous
sodium sulfate are placed on the top of the Florisil. The residual sample obtained in
Section 5.3 is dissolved in 10 mL of diethyl ether–n-hexane (3 : 17, v/v) and transferred
on to the column and 100 mL of diethyl ether–n-hexane (3 : 17, v/v) are added as
eluent and discarded. Using 100 mL of diethyl ether–n-hexane (3 : 7, v/v), isoxathion
is eluted. The eluate is collected and concentrated under reduced pressure below
40
◦
C.
5.5
Determination
The concentrated sample is dissolved in 2 mL of acetone and 2 µL of the solution are
injected into a previously conditioned gas chromatograph and the residue concentra-
tion is determined.
Operating conditions
Gas chromatograph
Hewlett-Packard Model 6890 equipped with a
nitrogen–phosphorus flame ionization detector
Capillary column
Capillary column for gas–liquid chromatography
(GLC), DB-1, 0.53-mm i.d.
× 15 m, 1-µm film
thickness (J&W Scientific)
Column temperature
150
◦
C, held for 1 min, increased at 10
◦
C min
−1
to
240
◦
C, held for 5 min
Injection port temperature
200
◦
C
Detector temperature
250
◦
C
Gas flow rates
Helium carrier gas, 4.2 mL min
−1
Hydrogen, 3 mL min
−1
Air, 60 mL min
−1
Injection volume
2 µL
Retention time
11 min
6
Evaluation
6.1
Method
Quantitation is performed by the calibration technique. A fresh calibration curve is
constructed with isoxathion standard solutions. The calibration curve is plotted as the
peak height against the amount of isoxathion injected.

1330
Individual compounds
6.2
Limit of detection
The limit of detection of isoxathion in vegetables by this method is 0.004 mg kg
−1
,
as shown below.
Minimum detectable amount: 0.2 ng
Detection limit
= (0.2 ng × 2 mL)/(2 µL × 50 g) = 0.004 mg kg
−1
Sample volume injected: 2 µL
Final solution volume: 2 mL
Sample weight: 50 g
6.3
Recovery rate in plants
The recovery of isoxathion from vegetables fortified at the 0.1 mg kg
−1
level by this
method is more than 94%.
Shingo Sadakane, Manabu Toujigamori, Takeshi Saito and Yasuhiro Tsujino
Sankyo Co. Ltd, Shiga, Japan
Document Outline
- Front Matter
- Table of Contents
- Volume I
- Volume II
- Recent Advances in Analytical Technology, Immunoassay and Other Nonchromatographic Methods
- Best Practices in the Generation and Analyses of Residues in Environmental Samples
- Compound Class
- Individual Compounds
- Azoxystrobin
- Famoxadone
- Fluthiacet-Methyl
- Flutolanil
- Hymexazol
- Imibenconazole
- Mepanipyrim
- Mepronil
- Tebuconazole
- Acetamiprid
- Alanycarb
- Azinphos-Methyl
- Benfuracarb
- Buprofezin
- Cyfluthrin
- Fenothiocarb
- Fenoxycarb
- Fenpyroximate
- Hexythiazox
- Imidacloprid
- Isoxathion
- Milbemectin
- Pyrimidifen
- Pyriproxyfen
- Index
Wyszukiwarka
Podobne podstrony:
91942 08w
91942 abb
91942 08q
91942 04m
91942 05d
91942 03d
91942 01e
91942 08j
91942 05b
91942 08p
91942 04b
91942 06c
91942 04i
91942 01c
91942 03c
91942 01a
91942 08x
91942 03f
91942 toc
więcej podobnych podstron