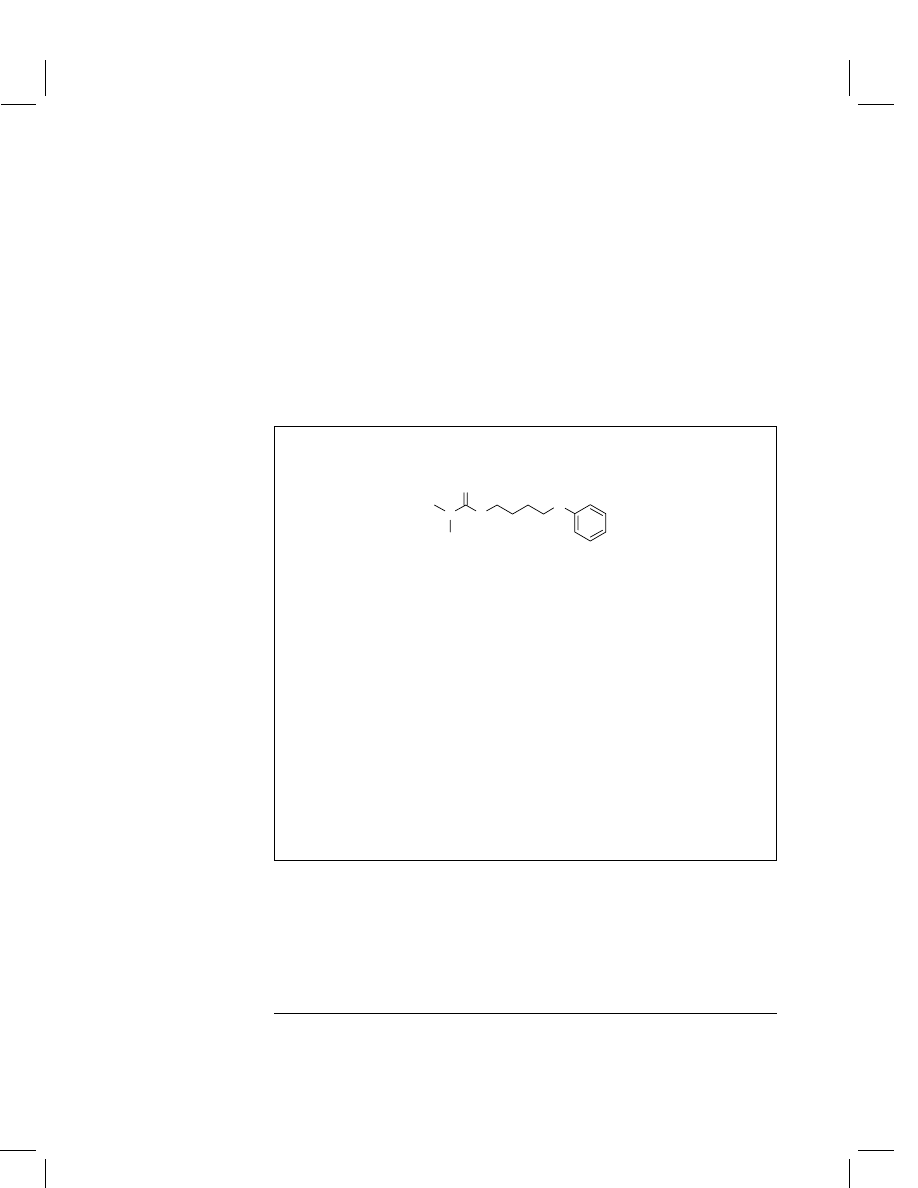
Fenothiocarb
Materials to be
analyzed
Mandarin oranges (juice, pulp, rind), leaves of mandarin
orange tree, soil
Instrumentation
Gas-chromatographic determination
1
Introduction
Chemical name
(IUPAC)
(S)-4-Phenoxybutyl dimethylthiocarbamate
Structural formula
O
S
N
O
Empirical formula
C
13
H
19
NO
2
S
Molar mass
253.4
Melting point
40–41
◦
C
Boiling point
155
◦
C at 0.02 mmHg
Vapor pressure
0.166 mPa at 23
◦
C
Solubility
In water 30 mg L
−1
(20
◦
C). In cyclohexanone 3800,
acetonitrile
3120,
acetone
2530,
xylene
2464,
methanol 1426, kerosene 80, hexane 66 g L
−1
(20
◦
C).
Stability
Slowly decomposed by sunlight. Stable to hydrolysis
for 5 days (pH 5–9, 40
◦
C);
<1% decomposition after
60 days at 55
◦
C.
Use pattern
Fenothiocarb is a nonsystemic acaricide used to con-
trol the eggs and larval stages of Panonychus spp.
Regulatory position
The residue definition is for the parent, fenothiocarb
only.
2
Outline of method
Plant substances (juice, pulp, rind, and leaf) are homogenized with anhydrous sodium
sulfate and methanol and fenothiocarb residue is extracted with acidic methanol [0.5 N
hydrochloric acid–methanol (1 : 3, v/v)] by refluxing for soil. Fenothiocarb in the
Handbook of Residue Analytical Methods for Agrochemicals.
C
2003 John Wiley & Sons Ltd.

Fenothiocarb
1289
extract of plant substances is extracted with hexane. Since oily plant substances af-
fect the subsequent cleanup process, fenothiocarb in the hexane extract is partitioned
into acetonitrile. The concentrated acetonitrile extracts are purified by silica gel col-
umn chromatography with benzene. Fenothiocarb in the soil extract is dissolved in
dichloromethane and purified by silica gel column chromatography with benzene.
Fenothiocarb is determined by gas chromatography using a sulfur-specific flame
photometric detector.
3
Apparatus
Blender (kitchen type)
Juicer mixer (automatic juicer)
High-speed blender (Waring blender, or equivalent)
Bell jar-type filtering apparatus
Buchner funnel, 11-cm i.d.
Rotary vacuum evaporator, 40
◦
C bath temperature
Water-bath, electrically heated, temperature 70
◦
C
Condenser
Round-bottom flasks, 500- and 300-mL
Separatory funnels, 1000-, 300-, and 200-mL
Glass funnel, 10-cm i.d.
Glass chromatography column, 1.5-cm i
.d. × 30 cm with a stopcock
Silica gel column: place a cotton wool plug at the bottom of a glass chromatography
column. Pack 10 g of silica gel slurried with benzene into the glass column. Make
an anhydrous sodium sulfate layer about 1-cm above and below the silica gel bed.
Gas chromatograph equipped with a sulfur-specific flame photometric detector
Microsyringe, 10-µL
4
Reagents
Acetone, acetonitrile, benzene, dichloromethane, n-hexane, and methanol, pesticide
residue analysis grade
Distilled water, HPLC grade
Anhydrous sodium sulfate, hydrochloric acid (36%), sodium chloride, special grade
Silica gel, Wakogel C-100, adjust water content to 6.5% with distilled water (Wako
Pure Chemical Industries, Ltd)
Filter aid, Celite 545 (Johns-Manville Products Corporation)
Filter paper, 11-cm i.d.
Fenothiocarb, analytical grade (Ihara Chemical Industries Co., Ltd)
Fenothiocarb standard solutions: 0.2, 0.5, 1, 2, and 3 µg mL
−1
in acetone
5
Sampling and sample preparation
Collect 2 kg of orange fruits randomly and use 1 kg for juice and the other 1 kg for
pulp and rind. Prepare the juice by crushing oranges directly with a juicer mixer.

1290
Individual compounds
Homogenize pulp with a blender and rind by chopping with a knife. Collect 50 g of
leaf randomly and homogenize by chopping with a knife. Soil, collected from the
top10-cm surface layer, is homogenized and passed through a 5-mm sieve.
6
Procedure
6.1
Extraction
6.1.1
Orange juice and pulp
Homogenize 50 g of the sample for 5 min with 75 mL of methanol and 10 g of
anhydrous sodium sulfate. Add 10 g of Cellite-545 to the homogenate and mix well,
and filter the mixture through a filter paper in a Buchner funnel into a 300-mL round-
bottom flask. Rinse the residue in a blender with 50 mL of a mixture of water and
methanol (1 : 1, v/v). Combine the filtrates.
6.1.2
Orange rind
Homogenize 25 g of the sample for 5 min with 75 mL of methanol, 25 mL of water
and 10 g of anhydrous sodium sulfate. Conduct the subsequent procedures in a similar
manner as described for juice and pulp.
6.1.3
Orange leaves
Homogenize 5 g of the sample for 5 min with 45 mL of methanol, 25 mL of water
and 5 g of anhydrous sodium sulfate. Conduct the subsequent procedures in a similar
manner as described for juice and pulp. Rinse the residue with 30 mL of a mixture of
water and methanol (1 : 1, v/v).
6.1.4
Soil
Weigh 40 g (dry soil weight) of the sample into a 300-mL round-bottom flask, add
120 mL of acid–methanol solution [a mixture of 0.5 N hydrochloric acid and methanol
(1 : 3, v/v)], and reflux the sample at 70
◦
C for 4 h after attaching a condenser.
Add 15 g of Cellite-545 to the sample and mix well, then filter the mixture through a
filter paper in a Buchner funnel into a 500-mL flask. Rinse the 300-mL round-bottom
flask and the residue with 100 mL of methanol.
6.2
Cleanup
6.2.1
Orange juice, pulp and rind
Transfer the sample extract (from Section 6.1) into a 300-mL separatory funnel,
add 50 mL of water and extract the sample with 50 mL of n-hexane three times.
Separate and dry the n-hexane layer with anhydrous sodium sulfate (plug the funnel
with absorbent cotton and 50 g of anhydrous sodium sulfate), and collect the dried
extract in a 300-mL of separatory funnel. Add 50 mL of acetonitrile to the separatory
funnel and mix well for partitioning with the n-hexane extract three times. Collect the

Fenothiocarb
1291
acetonitrile layer in a 300-mL round-bottom flask. Evaporate the acetonitrile under
reduced pressure. Dissolve the residue in 3 mL of benzene.
Adsorb the benzene solution on the top of the silica gel column (10 g of silica
gel) and elute with benzene. Discard the first 50 mL of eluate. Collect the subsequent
fractions of 150 mL in a 300-mL round-bottom flask and evaporate the solvent un-
der reduced pressure. Dissolve the residue in an appropriate volume of acetone for
analysis.
6.2.2
Orange leaves
Transfer the sample extract (from Section 6.1) into a 200-mL separatory funnel, add
30 mL of water and extract the sample extract three times with 30 mL of n-hexane.
Collect and dry the n-hexane layer with anhydrous sodium sulfate in a funnel in a
similar manner as described for the juice, pulp and rind, and evaporate the solvent
under reduced pressure. Dissolve the residue in 3 mL of benzene and clean up the
sample by silica gel column chromatography in a similar manner as described for
juice, pulp and rind.
6.2.3
Soil
Transfer the soil extract (from Section 6.1) into a 1000-mL separatory funnel, add
200 mL of water and 10 mL of saturated sodium chloride solution, and extract the
sample with 100 mL of dichloromethane three times. Dry the dichloromethane ex-
tract with anhydrous sodium sulfate in a funnel in a similar manner as described for
juice, pulp and rind, and collect the dried solution in a 500-mL round-bottom flask.
Evaporate the dichloromethane under reduced pressure. Dissolve the residue in 3 mL
of benzene.
Cleanup the sample by silica gel column chromatography in a similar manner as
described for juice, pulp and rind.
6.3
Gas-chromatographic determination
Inject an aliquot (V
i
) of the solution prepared from Section 6.2 (V
End
) into the gas
chromatograph
Operating conditions
Gas chromatograph (Hitachi 163)
Column
Glass, 3-mm i
.d. × 1.0-m length, packed with 1% FFAP
on Chromosorb W HP, 100–120 mesh
Column temperature
215
◦
C
Injection port temperature
240
◦
C
Detector
Flame photometric detector fitted with a 394-nm sulfur-
specific filter, temperature 150
◦
C
Gas flow rates
Nitrogen carrier gas, 50 mL min
−1
Hydrogen, 50 mL min
−1
Oxygen, 15 mL min
−1
Attenuation
32
× 100
Chart speed
10 mm min
−1

1292
Individual compounds
Injection volume
1–4 µL
Retention time
3.4 min
Minimum detectable amount
0.2 ng
7
Evaluation
7.1
Method
Quantitation is performed by the calibration technique. Construct a fresh calibration
curve with fenothiocarb standard solutions for each set of analyses.
Inject 1 µL of each fenothiocarb standard solution into the gas chromatograph.
Using log–log paper, plot the peak heights in millimeters against the injected amount
of fenothiocarb in nanograms.
Also inject 1–4-µL aliquots of the sample solutions. From the peak heights of the
peaks obtained for these solutions, read the appropriate amounts of fenothiocarb from
the calibration curve.
7.2
Recoveries, limit of detection and limit of determination
7.2.1
Edible part (juice, pulp)
The recoveries from control samples fortified with fenothiocarb at levels of 0.002–
0.10 mg kg
−1
ranged from 81 to 106% and from 87 to 90%, respectively.
The limit of detection was 0.001 mg kg
−1
and the limit of determination was
0.002 mg kg
−1
.
7.2.2
Inedible part (rind, leaf)
The recoveries from control samples fortified with fenothiocarb at levels of 0.02–
1.0 mg kg
−1
ranged from 88 to 98% and from 80 to 94%, respectively.
The limit of detection was 0.01 mg kg
−1
and the limit of determination was
0.02 mg kg
−1
.
7.2.3
Soil
The recoveries from control samples fortified with fenothiocarb at levels of 0.2–
0.4 mg kg
−1
ranged from 86 to 100% and from 83 to 94%, respectively.
The limit of detection was 0.05 mg kg
−1
.
7.3
Calculation of residues
The residue R, expressed in mg kg
−1
fenothiocarb, is calculated from the following
equation:
R
= (W
A
× V
End
)
/(V
i
× G)

Fenothiocarb
1293
where
G
= sample weight (g)
V
End
= terminal volume of sample solution from Section 6.2 (mL)
V
i
= portion of volume V
End
injected into gas chromatograph (µL)
W
A
= amount of fenochiocarb for V
i
read from calibration curve (ng)
8
Important points
1. Solvent for extraction of fenothiocarb in plant substances. Based on the exami-
nation using the field sample, methanol was selected as the solvent for extracting
fenothiocarb in orange. The extraction efficiency of fenothiocarb was higher with
acetone or methanol and was slightly lower with acetonitrile and dichloromethane.
For the ability to extract fenothiocarb and metabolites
1
simultaneously,
2
methanol
was superior to acetone.
2. Extraction method of fenothiocarb in soil.
3
The extraction efficient of fenothiocarb
in soil was evaluated using diluvial soil (orange field) and volcanic ash soil allowed
to stand in a greenhouse immediately after and for 5 days and 2 months under an
upland field condition after addition of fenothiocarb at a level of 10 mg kg
−1
.
The extraction recovery of fenothiocarb in the sample immediately and 5 days after
addition showed no marked difference but showed a substantial difference after
2 months, suggesting the extraction of fenothiocarb remaining in soil for a long
time. The optimum time of refluxing for extraction showing the highest recovery
of fenothiocarb was about 1 h for diluvial soil and about 4 h for volcanic ash soil.
3. Water used to adjust water content in silica gel should be sprayed uniformly. The
water content is measured by heating at 105
◦
C. If it is not uniformly sprayed, the
position of the first elution of fenothiocarb hardly changes, but the fraction will
be collected over an extended period.
4. A large amount of oily substance in the plant extract can vary the elution volume
of fenothiocarb in silica gel column chromatography.
5. Packing materials for column chromatography. Adequate cleanup can be achieved
with alumina instead of silica gel. Activated carbon is not suitable for sample
cleanup of ripe orange and leaf.
6. Sample storage stability. The level of fenothiocarb (0.4 mg kg
−1
) in soils stored in
the dark at 3
◦
C decreased to 94–97% after 40 days and to 68–82% after 120 days.
References
1. T. Unai, M. Tamaru, and C. Tomizawa, J. Pestic. Sci., 11, 347 (1986).
2. Y. Fukai, T. Oishi, Y. Asano and K. Ishikawa, ‘An analytical method of fenothiocarb and its
3 metabolites in mandarin oranges (juice, pulp, rind),’ in “Abstracts of the 9th Annual Meeting
of the Pesticide Science Society of Japan,” p. 150 (1984).
3. A. Yagi, Y. Asano, and K. Ishikawa, ‘Extraction method for fenothiocarb in soils,’ in “Abstracts
of the 5th Annual Meeting of the Pesticide Residue Analysis Society,” pp. 35–38 (1981) (in
Japanese).
Akira Yagi, Mitsumasa Ikeda and Yoshihiro Saito
Kumiai Chemical Industry Co. Ltd, Shizuoka, Japan
Document Outline
- Front Matter
- Table of Contents
- Volume I
- Volume II
- Recent Advances in Analytical Technology, Immunoassay and Other Nonchromatographic Methods
- Best Practices in the Generation and Analyses of Residues in Environmental Samples
- Compound Class
- Individual Compounds
- Azoxystrobin
- Famoxadone
- Fluthiacet-Methyl
- Flutolanil
- Hymexazol
- Imibenconazole
- Mepanipyrim
- Mepronil
- Tebuconazole
- Acetamiprid
- Alanycarb
- Azinphos-Methyl
- Benfuracarb
- Buprofezin
- Cyfluthrin
- Fenothiocarb
- Fenoxycarb
- Fenpyroximate
- Hexythiazox
- Imidacloprid
- Isoxathion
- Milbemectin
- Pyrimidifen
- Pyriproxyfen
- Index
Wyszukiwarka
Podobne podstrony:
91942 08w
91942 abb
91942 08q
91942 04m
91942 05d
91942 03d
91942 01e
91942 08j
91942 05b
91942 04b
91942 08u
91942 06c
91942 04i
91942 01c
91942 03c
91942 01a
91942 08x
91942 03f
91942 toc
więcej podobnych podstron