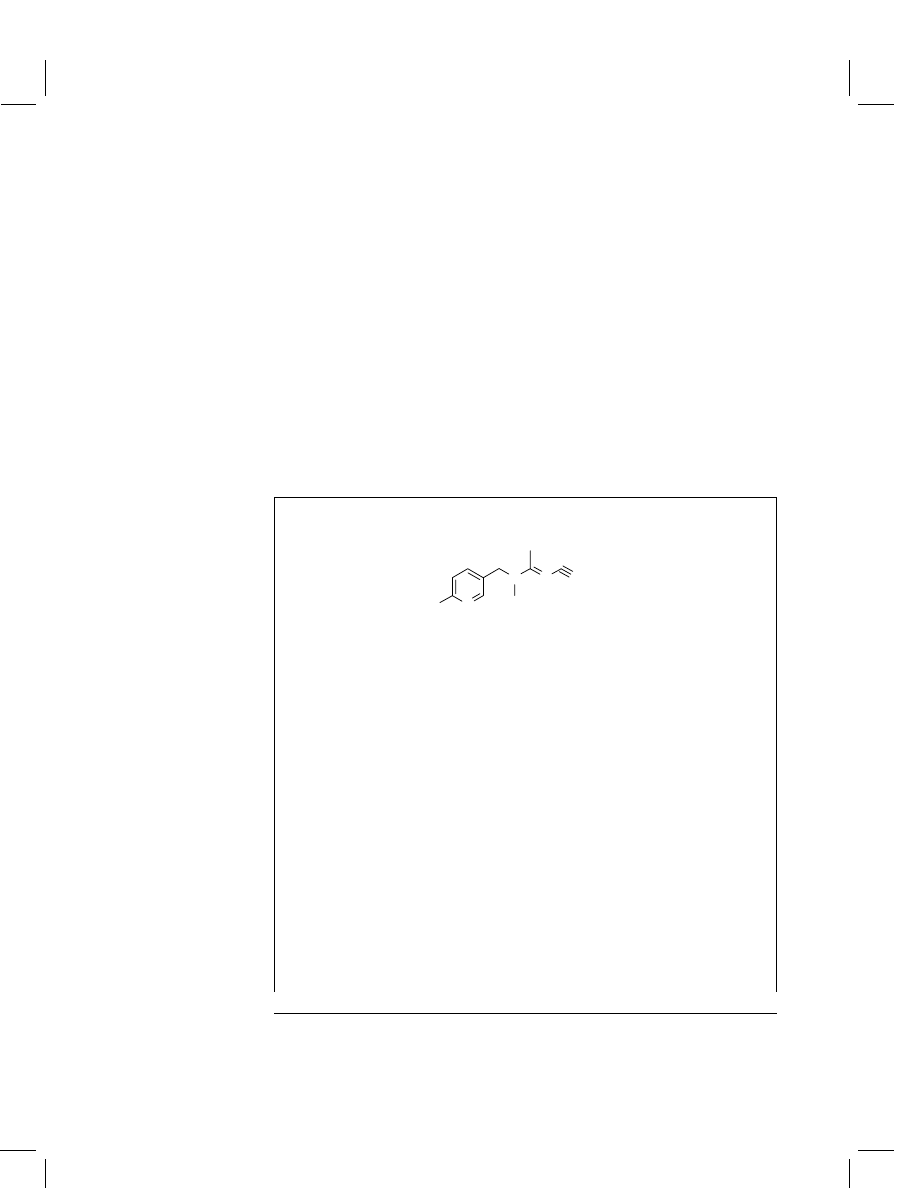
Acetamiprid
Materials to be
analyzed
Cabbage, potato, radish (leaf), radish (root), grape, citrus,
apple, pear, strawberry, cucurbits, green pepper, egg-
plant, tomato, green tea (powder), green tea (leachate)
and soil
Instrumentation
Gas-chromatographic determination for plant materials
High-performance liquid chromatographic determination
for soil
1
Introduction
Chemical name
(IUPAC)
(E )-N
1
-[(6-Chloro-3-pyridyl)methyl-N
2
-cyano-N
1
-methyl-
acetamidine
Structural formula
N
N
N
N
CI
Empirical formula
C
10
H
11
Cl N
4
Molar mass
222.7
Melting point
98.9
◦
C
Vapor pressure
<1×10
−6
Pa at 25
◦
C
Solubility
Water 4.25 g L
−1
at 25
◦
C
Readily soluble in organic solvents such as ace-
tone, acetonitrile, chloroform, dichloromethane and
methanol
Stability
Stable in an acidic to neutral aqueous solution. Unsta-
ble in strongly basic conditions
Stable in most organic solvents
Use pattern
Acetamiprid is a neonicotinoid insecticide with out-
standing systemic activities and a broad insecticidal
spectrum. Acetamiprid controls diverse soil and foliar
insect pests infesting cotton, sugar beet, vegetables,
fruits and other major food crops by both contact and
stomach action
Regulatory position
The residue definition for plant samples is acetamiprid
only. In soil, it includes acetamiprid and three of its
Handbook of Residue Analytical Methods for Agrochemicals.
C
2003 John Wiley & Sons Ltd.
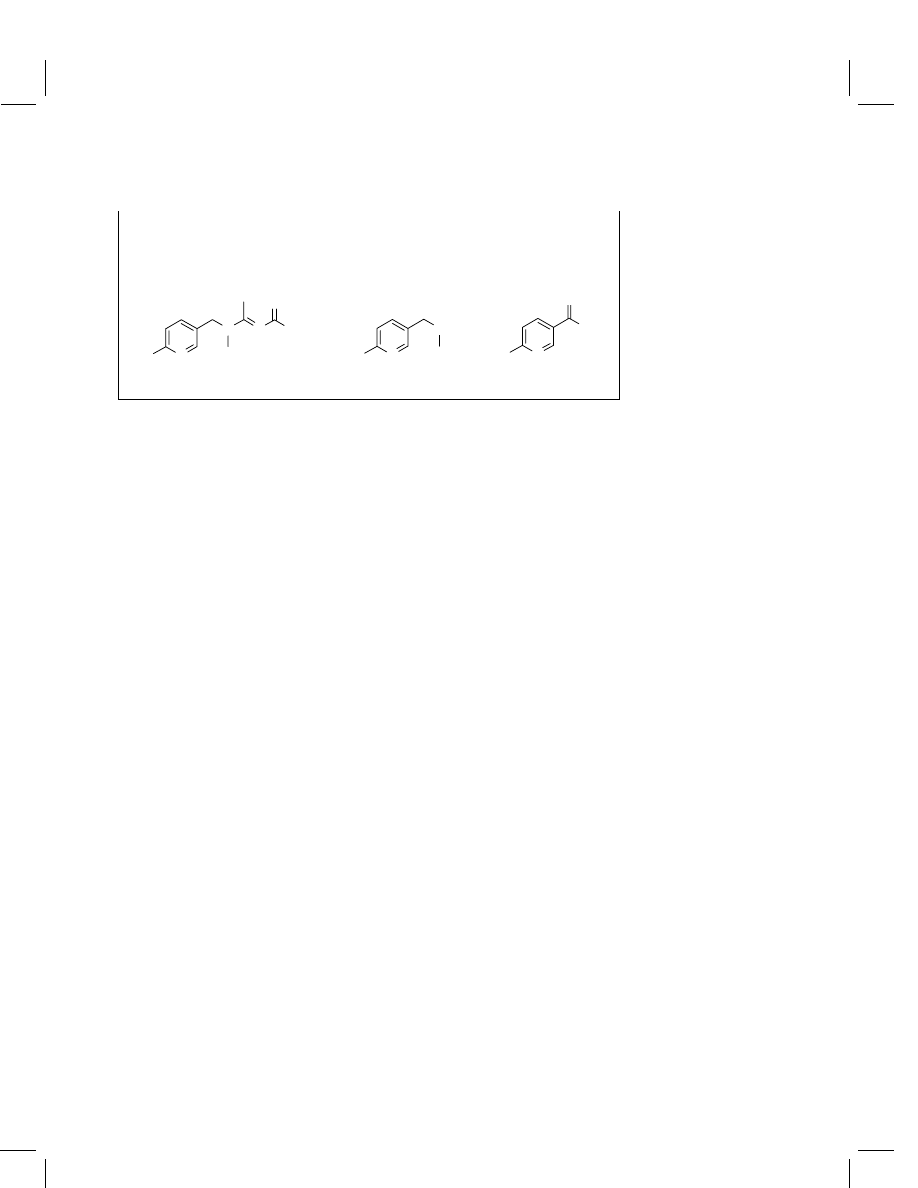
Acetamiprid
1243
metabolites, (E )-N
2
-carbamoyl-N
1
-[(6-chloro-3-
pyridyl)methyl-N
1
-methylacetamidine (IM-1-2),
N-methyl-[(6-chloro-3-pyridyl)methylamine (IM-
1-4) and 6-chloronicotinic acid (IC-0)
IM-1-2
N
N
N
CI
NH
2
O
IM-1-4
N
NH
CI
IC-0
N
OH
CI
O
2
Outline of method
2.1
Plant
Plant materials are homogenized with methanol. Acetamiprid residue is extracted with
dichloromethane by liquid–liquid partitioning. Dichloromethane is removed by rotary
evaporation, and the residue is subjected to a clean-up procedure using Florisil PR
column chromatography. The concentrated eluate is analyzed by gas chromatography
(GC).
2.2
Soil
Soil sample is extracted with a mixture of methanol and 0.1 M ammonium chlo-
ride. Acetamiprid, IM-1-2 and IM-1-4 residues are extracted with dichloromethane
under alkaline conditions. After adding diethylene glycol, dichloromethane in the
extract is removed by rotary evaporation, and the residue is subjected to a cleanup
procedure using Florisil PR column chromatography and then with a packed Extrelut
20 column.
IC-0 residue is cleaned up with a mixture of dichloromethane and acetone by
liquid–liquid partitioning under neutral conditions and then extracted into diethyl
ether under acidic conditions. The diethyl ether in the extract is removed by rotary
evaporation and the residue is dissolved in buffer solution, which is subjected to a
cleanup procedure using a Sep-Pak C
18
Env. column.
The concentrated eluate is subjected to high-performance liquid chromatography
(HPLC) analysis.
3
Apparatus
High-speed blender fitted with leak-proof glass jar and explosion-proof motor
Balances
Macerator (Polytron)
Laboratory mechanical shaker

1244
Individual compounds
Glass tube for column chromatography, 15-mm i.d., 30-mm length
Separatory funnels, 200-mL, 500-mL and 1-L
Filter paper
Erlenmeyer flask, 500-mL
Round-bottom flasks, 300-mL
Rotary vacuum evaporator
Stainless-steel centrifuge tube, 250-mL
Ultracentrifuge
pH meter
Packed column (Extrelut 20)
Packed column (Sep-Pak C
18
)
Packed column (Sep-Pak C
18
Env.)
Gas chromatograph equipped with an electron capture detector
High-performance liquid chromatograph
4
Reagents
Methanol, guaranteed reagent grade
Celite (No. 545)
Sodium chloride, guaranteed reagent grade
n-Hexane, guaranteed reagent grade
Dichloromethane, guaranteed reagent grade
Sodium hydroxide, guaranteed reagent grade
Sodium sulfate, anhydrous, guaranteed reagent grade
Florisil, nonactivated (Florisil PR)
Ammonium chloride, guaranteed reagent grade
Diethylene glycol, guaranteed reagent grade
Hydrochloric acid, guaranteed reagent grade
Acetone, guaranteed reagent grade
Diethyl ether, guaranteed reagent grade
Acetonitrile, guaranteed reagent grade
Disodium hydrogenphosphate, guaranteed reagent grade
Citric acid, anhydrous
Nitrogen, repurified
5
Sampling and preparation
5.1
Green tea
Grind leaves with dry-ice using a high-speed blender.
5.2
Fruits and vegetables
Cut into pieces with a kitchen knife.

Acetamiprid
1245
6
Procedure
6.1
Extraction
6.1.1
Plant material
Homogenize 20 g of a prepared sample with 100 mL of methanol in a macerator
for 3 min and shake for 30 min with a mechanical shaker. In the case of green tea
(powder), soak 4 g of a prepared sample with 16 mL of distilled water for 2 h. Add
100-mL of methanol and shake for 30 min.
Filter the homogenate through a Celite layer (1–2 cm thickness) under reduced
pressure. Wash the cake and vessel twice with 25 mL of methanol and filter the wash-
ings through the same Celite layer. Combine these filtrates and transfer to a 500-mL
separatory funnel. Add 150 mL of 5% sodium chloride solution to the filtrate and
wash twice with 100 mL of hexane for 10 min. Discard the hexane extract. Transfer
the aqueous methanol to another 500-mL separatory funnel. In the case of green tea
(leachate), soak 4 g of the ground sample in 240 mL of boiling water for 5 min. Filter
the mixture through a filter paper and cool to ambient temperature. Remove half of the
filtrate for further analysis (corresponds to 2 g of green tea dried). Add 5 g of sodium
chloride and 120 mL of methanol to the filtrate and then wash twice with 100 mL of
hexane for 10 min. Discard the hexane extract. Transfer the aqueous methanol into
a 500-mL separatory funnel. Extract the solution with two portions of 100 mL of
dichloromethane for 10 min. Collect the dichloromethane in a flask. In the case of
citrus, wash the dichloromethane with 100 mL of 0.05 M sodium hydroxide solution
for 5 min and discard the alkaline solution.
Pass the dichloromethane through a filter paper with anhydrous sodium sulfate and
collect the dichloromethane in a 300-mL round-bottom flask. Add 1-g of Florisil PR
and then evaporate dichloromethane to near dryness on a water-bath at ca 40
◦
C by
rotary evaporation.
6.1.2
Soil
Recovery of acetamiprid, IM-1-2 and IM-1-4. Combine 20 g of the air-dried
soil with 100 mL of a mixed solvent of methanol and 0.1 M ammonium chloride
(4 : 1, v/v) in a 250-mL stainless-steel centrifuge tube, shake the mixture with a
mechanical shaker for 30 min and centrifuge at 8000 r.p.m. for 2 min. Filter the su-
pernatant through a Celite layer (1-cm thick) under reduced pressure into a 500-mL
flask. Add a second 100 mL of mixed solvent to the residue and then extract and filter
in the same manner. Combine the filtrates and add 150 mL of distilled water with
1 g of sodium chloride. Transfer the aqueous methanol solution into a 1-L separatory
funnel and shake the solution with 200 mL of dichloromethane for 5 min. Collect the
dichloromethane in a flask and adjust the pH of aqueous methanol to 13 with sodium
hydroxide. Extract the solution with two portions of 200 mL of dichloromethane for
5 min. Combine the dichloromethane extracts and pass through a filter paper with
anhydrous sodium sulfate. Add 0.5 mL of diethylene glycol and then concentrate
the dichloromethane extract to about 0.5 mL on a water-bath at ca 40
◦
C by rotary
evaporation.

1246
Individual compounds
Recovery of IC-0. Combine 20 g of the air-dried soil with 100 mL of a mixed
solvent of methanol and 0.1 M ammonium chloride (4 : 1, v/v) in a 250-mL stainless-
steel centrifuge tube, shake the mixture with a mechanical shaker for 30 min and
centrifuge at 8000 r.p.m. for 2 min. Filter the supernatant through a Celite layer
(1-cm thick) under reduced pressure into a 500-mL flask. Add 100 mL of mixed
solvent of methanol and 0.5 M sodium hydroxide solution (4 : 1, v/v) to the residue
and then extract and filter in the same manner. Combine the filtrates and concentrate to
approximately 40 mL on a water-bath at ca 40
◦
C by rotary evaporation. Add 10 mL
of distilled water and adjust the pH of the aqueous layer to 7 with hydrochloric
acid. Transfer the aqueous solution into a 200-mL separatory funnel and shake the
solution with 50 mL of mixed solvent of dichloromethane and acetone (1 : 1, v/v)
for 5 min. Discard the mixed solvent and adjust the pH of the aqueous layer to 1.5
with hydrochloric acid. Extract the solution with three portions of 50 mL of diethyl
ether. Combine the diethyl ether extracts and dry over anhydrous sodium sulfate.
Concentrate to dryness on a water-bath at ca 40
◦
C by rotary evaporation.
6.2
Cleanup
6.2.1
Plant materials
Transfer the residue with 1 g of Florisil PR on to the top of the column packed with 9 g
of Florisil PR with the aid of hexane. Rinse the column with 150 mL of hexane-acetone
(4 : 1, v/v). Elute acetamiprid with 120 mL of a mixed solvent of acetone-hexane (1 : 1,
v/v) and concentrate the eluate to near dryness by rotary evaporation at 40
◦
C. Dissolve
the residue with 5 mL of distilled water and apply the solution to the top of the packed
Sep-Pak C
18
column pretreated with 20 mL each of methanol and distilled water.
Elute acetamiprid with 30 mL of a mixed solution of water-acetonitrile (17 : 3, v/v).
Concentrate to dryness on a water-bath at ca 40
◦
C by rotary evaporation. Prepare the
GC-ready sample by dissolving the residue in acetone.
6.2.2
Soil
Cleanup procedures for acetamiprid, IM-1-2 and IM-1-4. Dilute the concentrate
with 10 mL of distilled water and apply the solution to an Extrelut 20 column, equili-
brate for 20 min at ambient temperature and pass 100 mL of dichloromethane through
the column. Collect the eluate and add 0.5 mL of diethylene glycol and then concen-
trate the dichloromethane to about 0.5 mL by rotary evaporation. Prepare the HPLC-
ready sample solution by dissolving the residue in 50% aqueous acetonitrile.
Cleanup procedure for IC-0. Dissolve the residue with 10 mL of pH 5 phosphate
buffer solution and apply the solution to the top the Sep-Pak C
18
Env. column pre-
treated with 10 mL each of methanol and distilled water. Discard the passed solution
and elute IC-0 with 15 mL of a second buffer solution. Add 35 mL of distilled water
and adjust the pH of solution to 1.5 with hydrochloric acid. Extract the solution with
three portions of 50 mL of diethyl ether. Combine the diethyl ether extracts and dry
over anhydrous sodium sulfate. Concentrate to dryness on a water-bath at ca 40
◦
C

Acetamiprid
1247
by rotary evaporation. Prepare the HPLC-ready sample solution by dissolving the
residue in 50% aqueous acetonitrile solution.
6.3
Determination
6.3.1
Plant materials
Inject an aliquot of the GC-ready sample solution into the gas chromatograph.
Operating conditions
Gas chromatograph
Model GC-14B, Shimadzu
Column
5% PEG HT/Chromosorb W HP column, 60–80
mesh, 3.2-mm i.d., 1.0-m length short column
Column temperature
260
◦
C
Injection port temperature
320
◦
C
Detector
Electron capture detector
Detector temperature
320
◦
C
Gas flow rates
Nitrogen carrier gas, column head pressure
1.5 kg cm
−2
Injection volume
2 µL
6.3.2
Soil
Inject an aliquot of the HPLC-ready sample solution into the high-performance liquid
chromatograph.
Operating conditions
High-performance liquid
Model LC-10AD, Shimadzu
chromatograph
Column
Stainless-steel column, 4.6-mm i.d., 150 mm-length
Stationary phase
Inertsil ODS-3
(1) Determination of acetamiprid, IM-1-2 and IM-1-4
.
Mobile phase
(A) 0.1 M ammonium acetate adjusted to pH 8.5 with
aqueous ammonia
(B) Acetonitrile
Gradient rate
0–20 min 85% A–15% B to 70% A–30% B
20–22 min 70% A–30% B to 30% A–70% B
22–30 min 85% A–15% B
Flow rate
1.0 mL min
−1
Column temperature
50
◦
C
Detection
UV detector (SPD-10AV) at 270 nm (IM-1-4) for initial
11 min and then change at 246 nm (acetamiprid, IM-
1-2) for 19 min
Injection volume
25 µL

1248
Individual compounds
(2) Determination of IC-0
Mobile phase
Acetonitrile–1% acetic acid (1 : 4, v/v)
Flow rate
1.0 mL min
−1
Column temperature
50
◦
C
Detection
UV detector (SPD-10AV) at 270 nm (IC-0)
Injection volume
25 µL
7
Evaluation
7.1
Method
7.1.1
Plant materials
Quantification is performed by the calibration technique. Construct a new calibration
curve with acetamiprid standard solutions using acetone for each set of analyses.
Inject 2-µL aliquots of the standard solution containing acetamiprid from 0.04 to 1 ng
in 2µL of acetone. The acetamiprid peak usually appears at a retention time around
4 min. Plot the peak height against the injected amount of acetamiprid.
7.1.2
Soil
Quantification is performed by the calibration technique. Construct a new calibration
curve with the mixed standard solutions of acetamiprid, IM-1-2 and IM-1-4 for each
set of analyses. Inject 25-µL aliquots of the standard solutions containing compounds
from 1 to 10 ng in 25 µL of 50% aqueous acetonitrile. With regard to IC-0, prepare
the calibration curve in the same manner. The retention times are around 8 min for
IM-1-4 and IC-0, 12 min for IM-1-2 and 20 min for acetamiprid. Plot the peak area
against the injected amount of each standard.
7.2
Recoveries, limit of detection and limit of determination
7.2.1
Plant materials
With a fortification level of 0.1 mg kg
−1
, recoveries from untreated plant matrices
ranged from 90 to 104%. The limit of detection (LOD) was 0.005 mg kg
−1
(fruits and
vegetables). With regard to green tea (powder and leachate), the method recoveries
were 95 and 98%, respectively, at the 0.5 mg kg
−1
fortification level. The LOD was
0.05 mg kg
−1
.
7.2.2
Soil
At the fortification levels at 0.1 and 0.2 mg kg
−1
, recoveries of acetamiprid, IM-1-2,
IM-1-4 and IC-0 from soils ranged from 70 and 95%. The LOD for each compound
was 0.01 mg kg
−1
.

Acetamiprid
1249
7.3
Calculation of residues
The amount of acetamiprid and its related compounds (R, mg kg
−1
) in the sample is
calculated by the following equation:
R
= C × V/G
where
C
= concentration of compound in the final solution (µg mL
−1
)
V
= final sample volume (mL)
G
= original sample weight (g)
8
Important points
Since IM-1-4 is a volatile compound, diethylene glycol should be added to the solution
containing IM-1-4 to reduce the amount of sample lost in the concentration step under
reduced pressure.
Further reading
M. Tokieda, K. Iiyoshi, K. Sugioka, and T. Gomyo, J. Pestic. Sci., 22, 129 (1997).
M. Tokieda, T. Tanaka, M. Ozawa, and T. Gomyo, J. Pestic. Sci., 23, 296 (1998).
Shigeji Sugimoto
Nippon Soda Co. Ltd, Tokyo, Japan
Document Outline
- Front Matter
- Table of Contents
- Volume I
- Volume II
- Recent Advances in Analytical Technology, Immunoassay and Other Nonchromatographic Methods
- Best Practices in the Generation and Analyses of Residues in Environmental Samples
- Compound Class
- Individual Compounds
- Azoxystrobin
- Famoxadone
- Fluthiacet-Methyl
- Flutolanil
- Hymexazol
- Imibenconazole
- Mepanipyrim
- Mepronil
- Tebuconazole
- Acetamiprid
- Alanycarb
- Azinphos-Methyl
- Benfuracarb
- Buprofezin
- Cyfluthrin
- Fenothiocarb
- Fenoxycarb
- Fenpyroximate
- Hexythiazox
- Imidacloprid
- Isoxathion
- Milbemectin
- Pyrimidifen
- Pyriproxyfen
- Index
Wyszukiwarka
Podobne podstrony:
91942 08w
91942 abb
91942 08q
91942 04m
91942 05d
91942 03d
91942 01e
91942 05b
91942 08p
91942 04b
91942 08u
91942 06c
91942 01c
91942 03c
91942 01a
91942 08x
91942 03f
91942 toc
więcej podobnych podstron