BOILING
AND
CONDENSATION
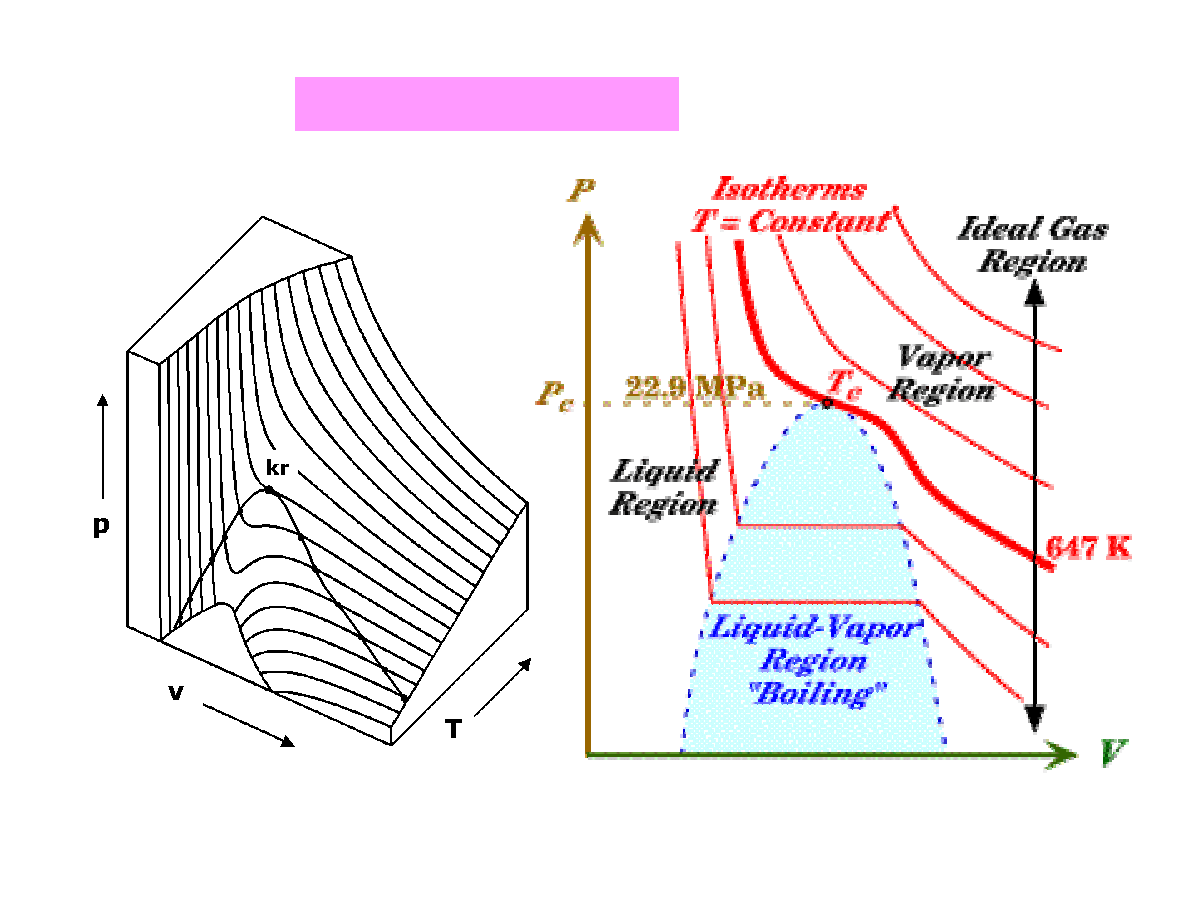
Isotherms of real gas
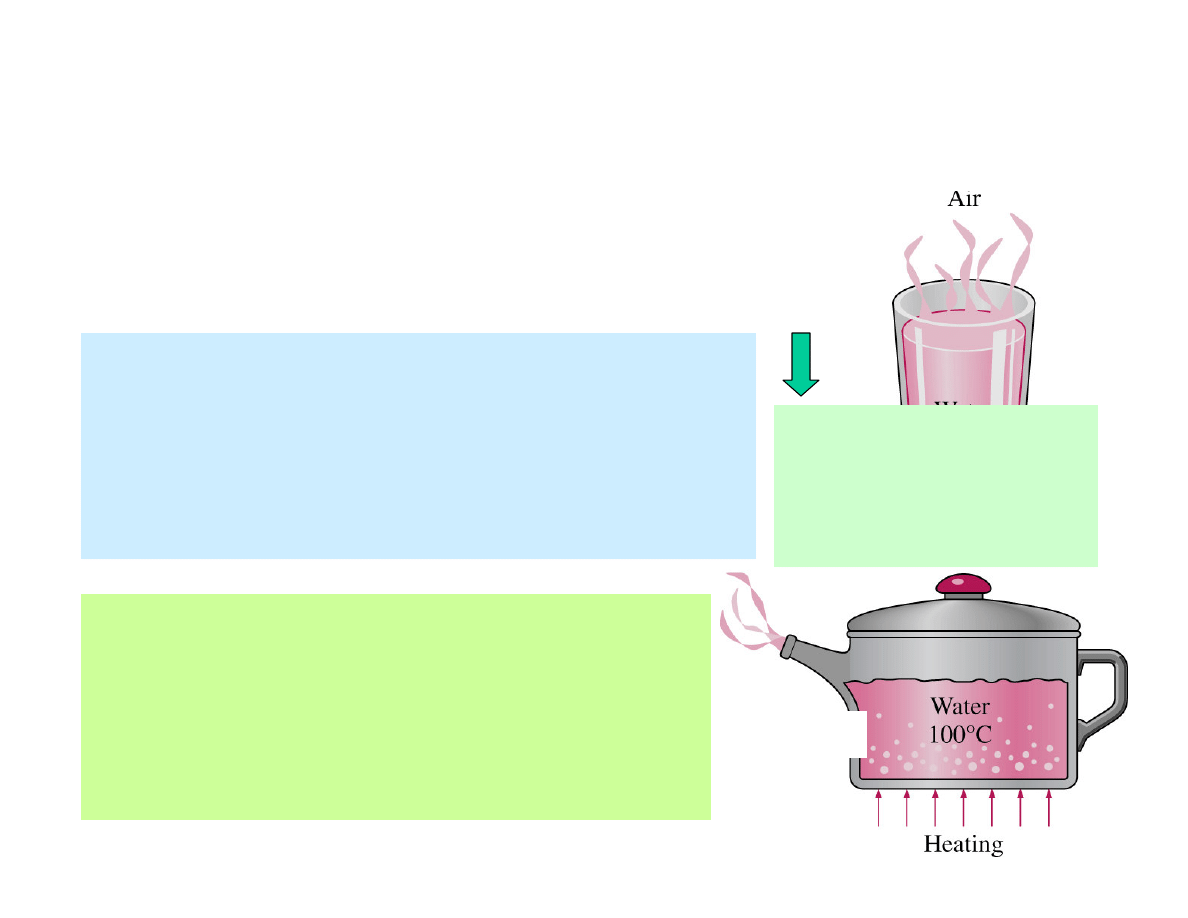
Evaporation
Boiling
Boiling
and
evaporation
- the liquid-to-vapour
phase change
processes that occur at a solid-
liquid interface when the surface is heated
above the
saturation temperature T
sat
of the
liquid →
→
→
→ convection heat transfer.
Evaporation
occurs when the vapour
pressure
is less than the saturation
pressure
of the liquid at a given
temperature, and it involves no
bubble formation or bubble motion.
Boiling
occurs when a liquid is
brought into contact with a surface
maintained at a temperature T
S
sufficiently above the saturation
temperature T
sat
of the liquid.
Refrigerators
Steam power plants
Electronic system
cooling
Water
20
0
C

Boiling →
→
→
→ convection heat transfer →
→
→
→ the boiling heat flux
from a solid surface to the fluid – Newton`s law of cooling:
excess
sat
S
boiling
T
h
T
T
h
q
∆
=
−
=
•
)
(
)
/
(
2
m
W
where ∆
∆
∆
∆T
excess
= T
S
– T
sat
is called the excess temperature,
which represents the excess of the surface above the
saturation temperature of the fluid
Two phases (liquid and vapour) involves:
- two sets of thermophysical properties (density
ρ
ρ
ρ
ρ
, dynamic
viscosity
µ
µ
µ
µ
, conductivity
Λ
Λ
Λ
Λ
, and the specific heat
C
p
)
- the latent heat of vaporisation
h
fg
- the surface tension
σ
σ
σ
σ
Parameter
h
fg
represents the energy absorbed as a unit mass of
liquid vaporizes at a specified temperature or pressure.

Surface tension
→
→
→
→ bubbles →
→
→
→ thermodynamic non-
equilibrium
conditions →
→
→
→ different temperature in the
bubble than in liquid.
The pressure difference between the liquid and the
vapour is balanced by the surface tension at the
interface →
→
→
→ the driving force for heat transfer between
two phases.
When the liquid is at a higher T than the bubble, heat will
be transferred from the liquid to the bubble →
→
→
→ the bubbles
grow and rise to the top under influence of buoyancy.
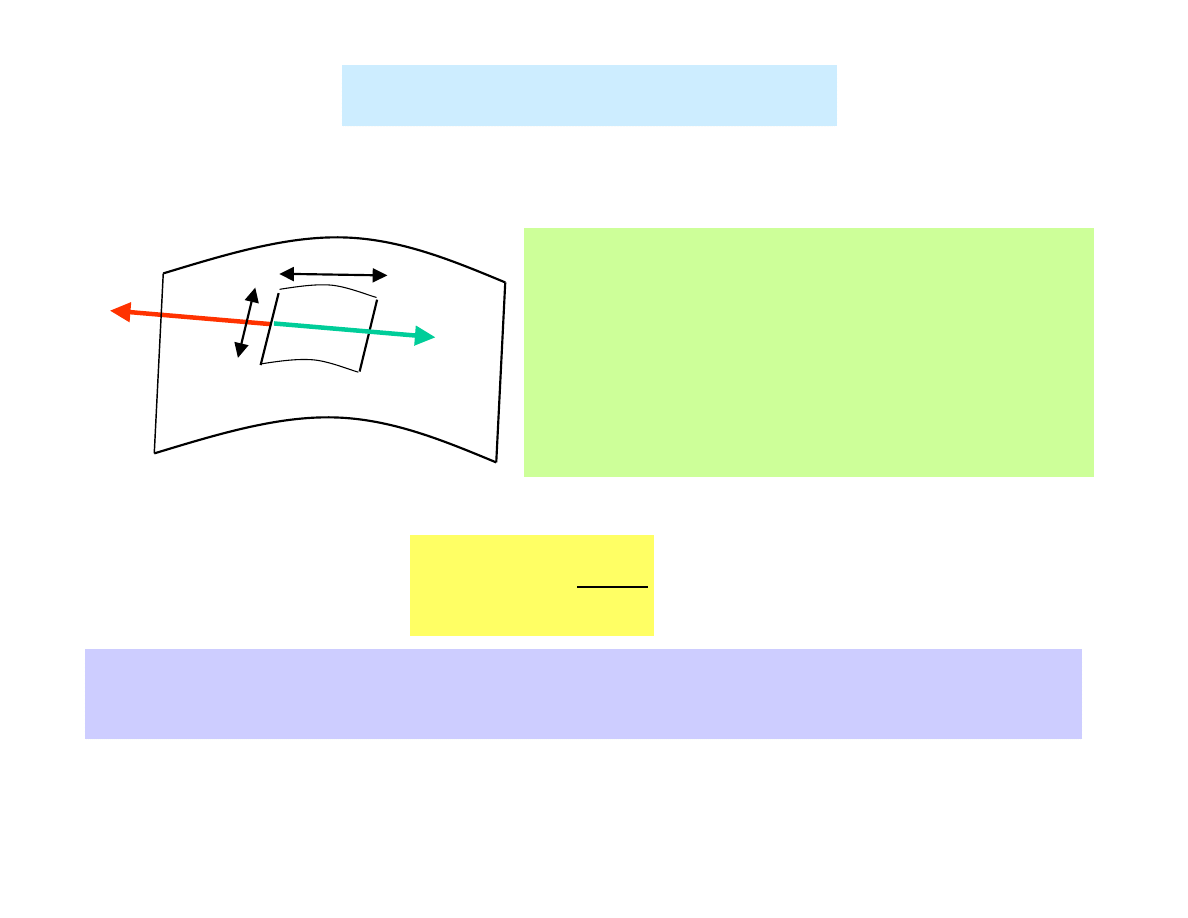
Surface tension
Surface tension in liquids →
→
→
→ in an elastic membrane (2D
effect) →
→
→
→ analogy to tension in an elastic spring (1D effect)
∆
∆
∆
∆l
∆
∆
∆
∆x
T
r
∆
T
r
∆
−
Any line element of the surface of
the „membrane” is in equilibrium
due to equal and opposite forces
exerted perpendicular to ∆
∆
∆
∆l by the
parts of the „membrane” on either
side.
The surface tension σ
σ
σ
σ →
→
→
→ the magnitude of the tensile force
per unit length:
l
|
T
|
limit
0
l
∆
∆
=
→
∆
r
σ
)
/
(
m
N
Note
: The surface tension in a liquid does not change as the
liquid surface is „stretched”.
Effects related to surface tension: liquid drop formation
(surface energy minimum condition) or soup bubbles.
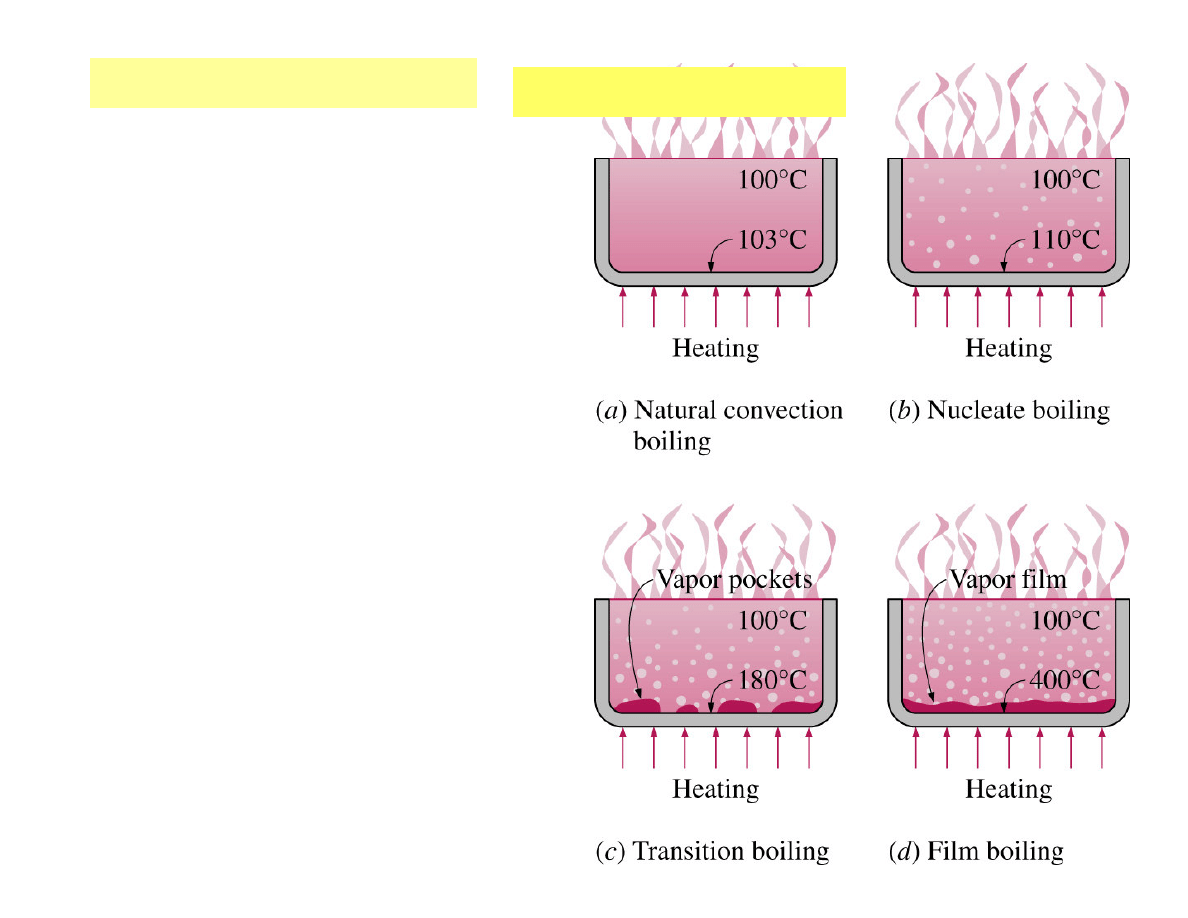
Natural convection boiling
regime
- the fluid motion is governed by
natural convection currents, and
heat transfer from the heating
surface to the fluid is by natural
convection.
Nucleate boiling
regime - bubbles
form at various preferential sites
on the heating surface, and rise
to the top.
Transition boiling
regime - part
of the surface is covered by a
vapor film.
Film boiling
regime - the heater
surface is completely covered by
a continuous stable vapor film,
and heat transfer is by combined
convection and radiation.
Mechanisms of boiling
→
→
→
→ pool boiling
Pioneering work by
S. Nukiyama (1934)
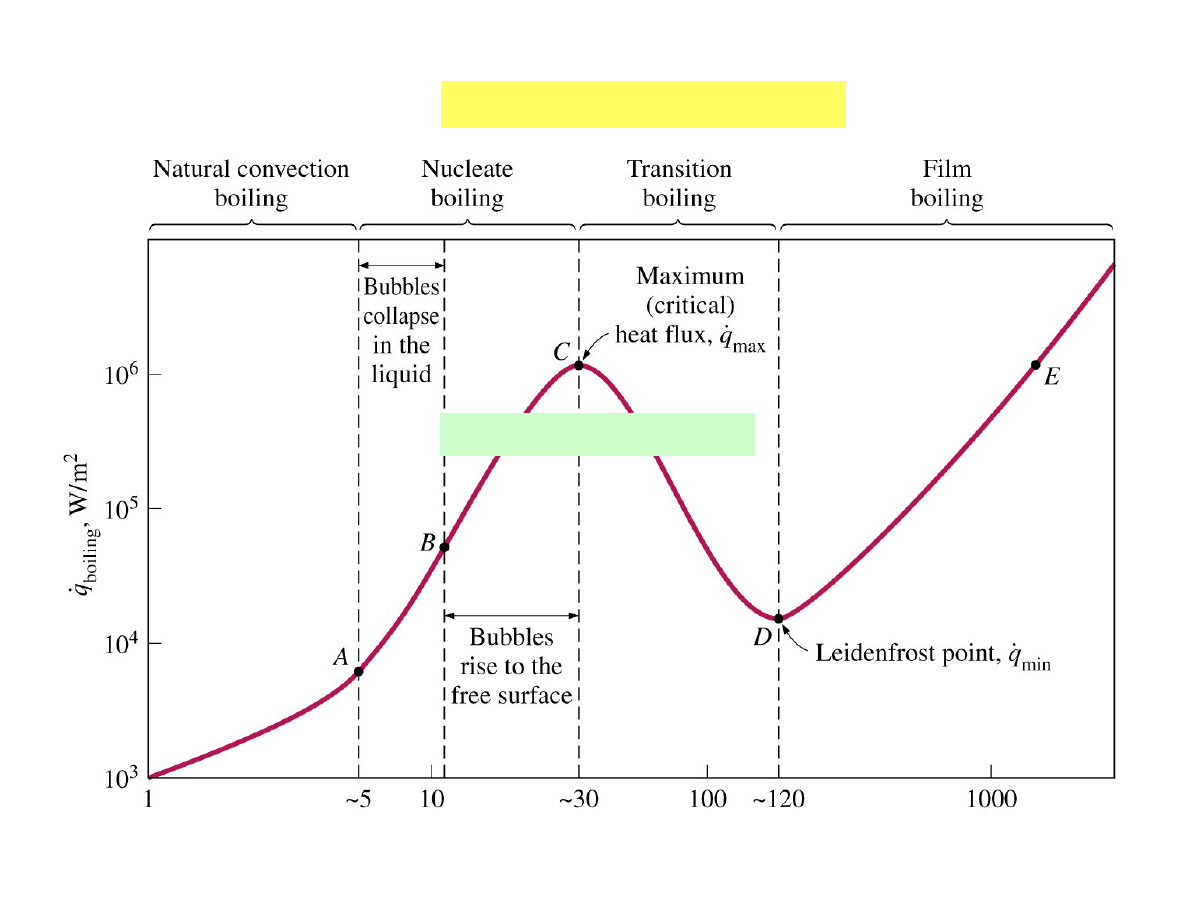
∆
∆
∆
∆T
excess
=T
S
-T
sat
,
0
C
The boiling curve
water
The burnout point C
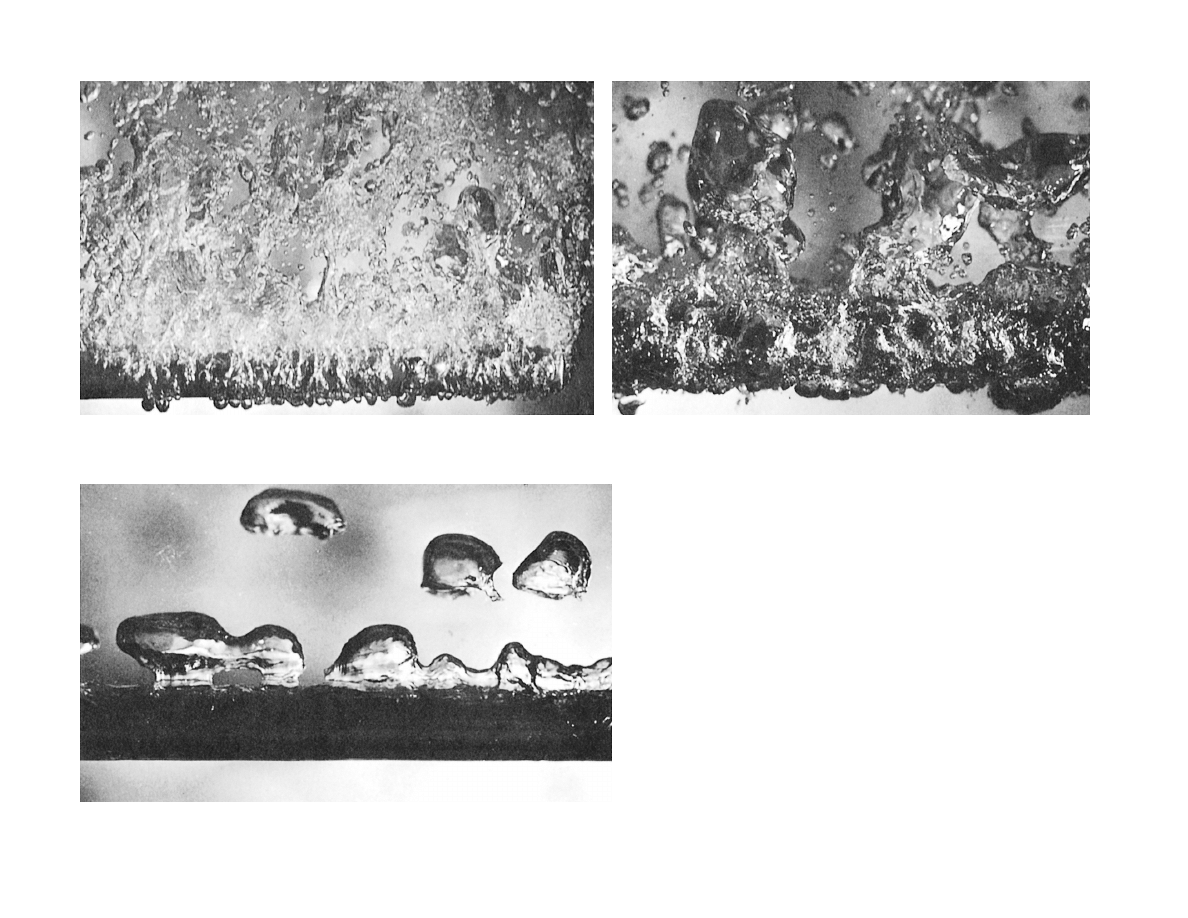
Boiling regimes (methanol)
on a horizontal 1 cm-diameter
steam-heated copper tube
(b) Transition boiling
(a) Nucleate boiling
(c) Film boiling
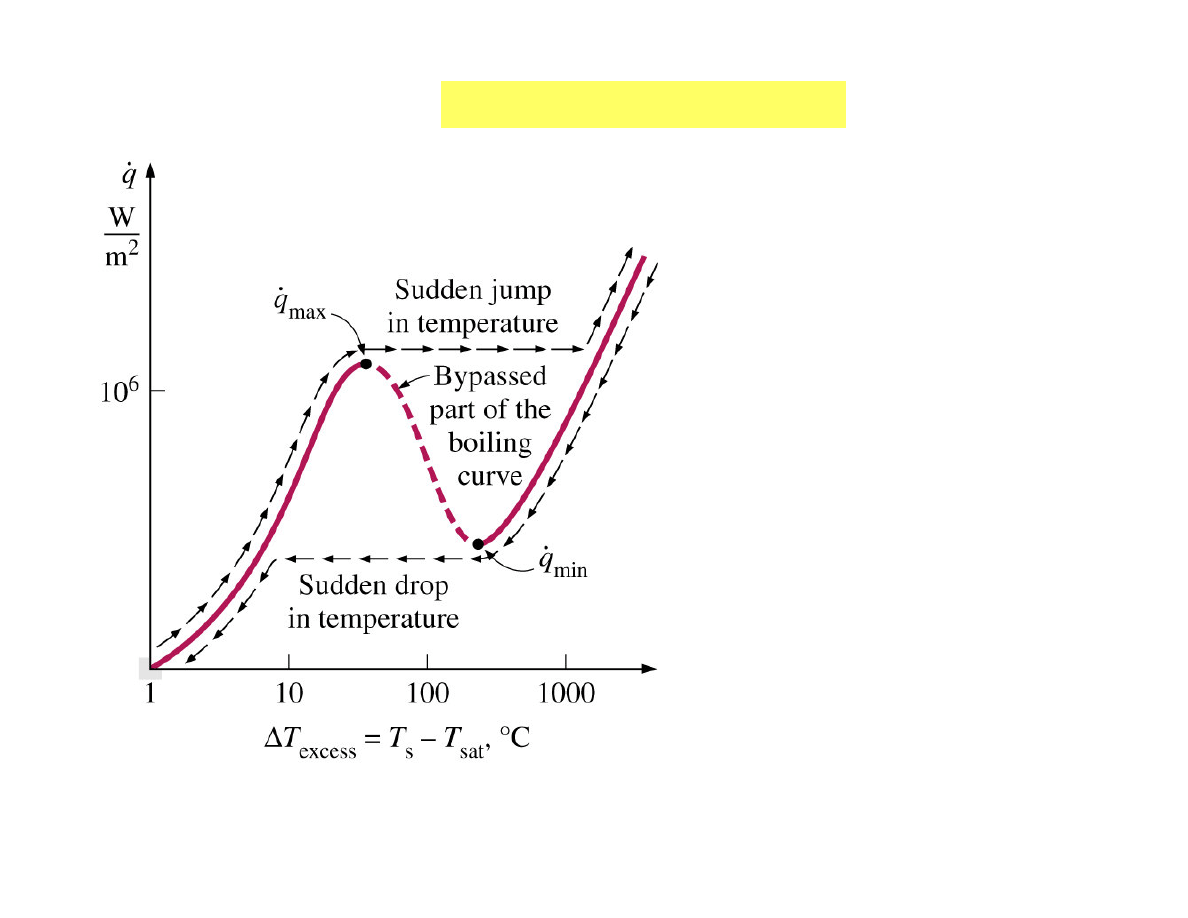
The actual boiling curve
obtained with platinum
wire in water as the heat
flux is increased and then
decreased.
The boiling curve
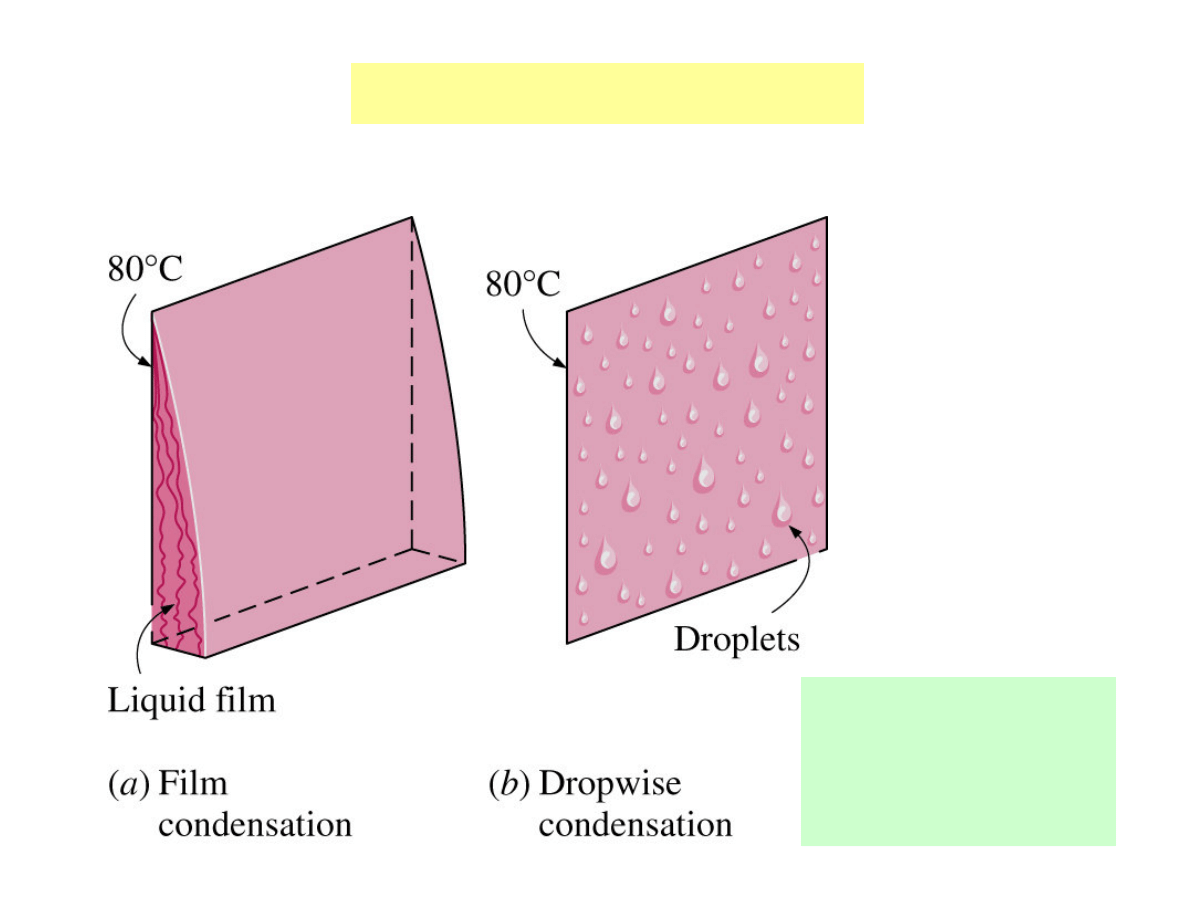
Condensation
Temperature of a vapour - reduced below T
sat
Larger values of
heat transfer rate
→
→
→
→ preferred mode
of condensation
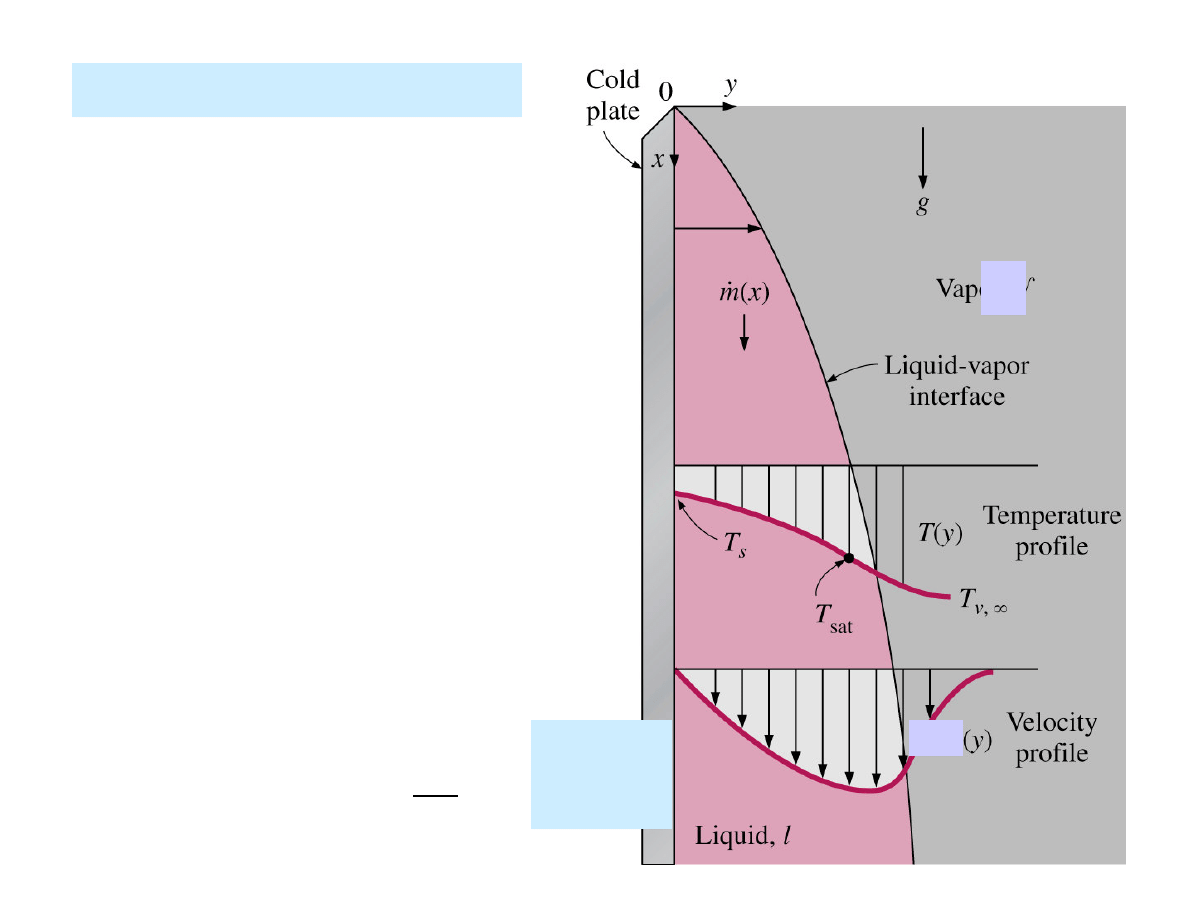
Condensation on a plate
• Influence of gravity.
• T
s
must be below T
sat
of the
vapour for condensation to occur.
• Temperature of the condensate is
T
sat
at the interface and decreases
gradually to T
s
at the wall.
• The velocity of the condensate at
the wall is zero because of the „no-
slip” condition.
• Velocity maximum at the liquid –
vapour interface.
v
v(y)
3
/
1
2
3
/
1
Re
47
.
1
Λ
≅
−
l
l
vert
v
g
h
Heat transfer coefficient:
l
v
ρ
ρ
〈〈
〈
〈
30
Re
0
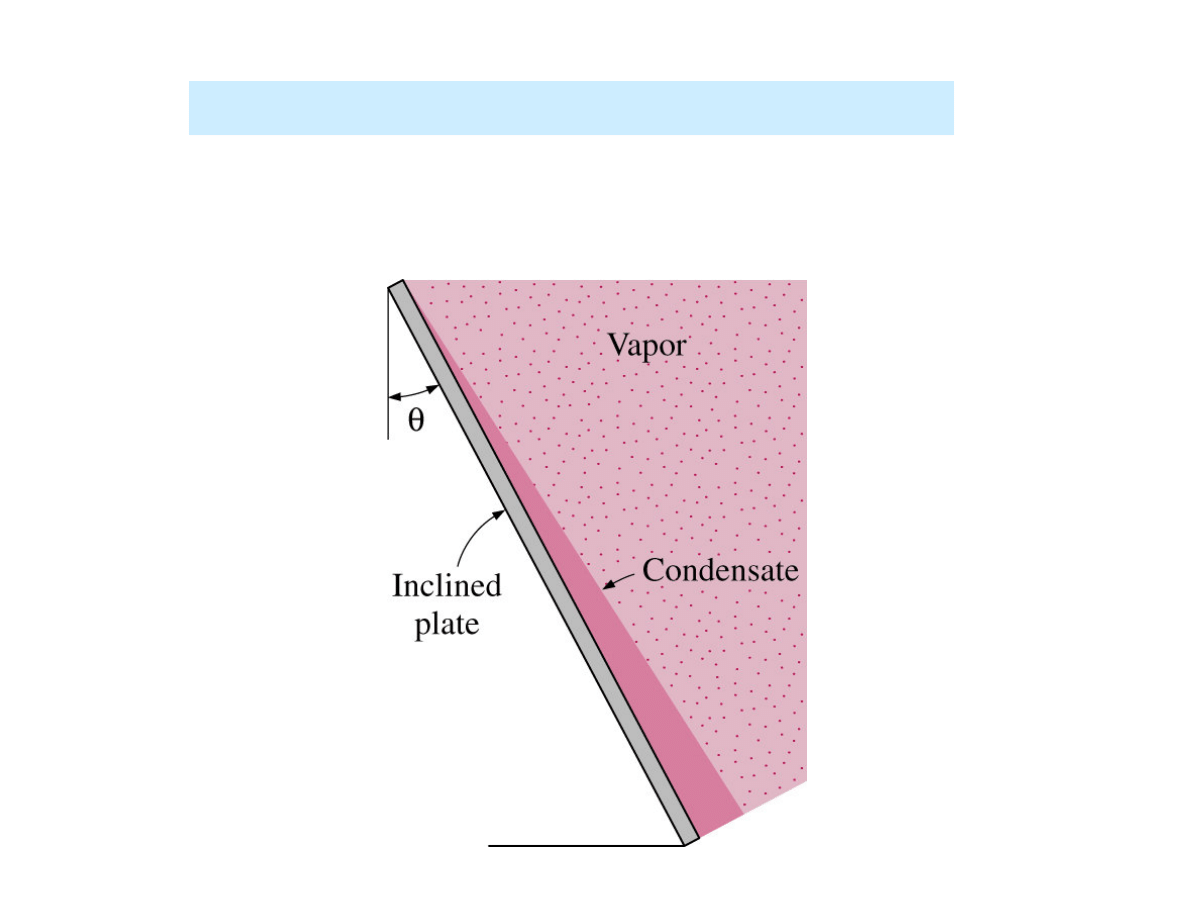
Film condensation on an inclined plate
4
/
1
)
(cos
θ
vert
inclined
h
h
=
for laminar flow of condensate
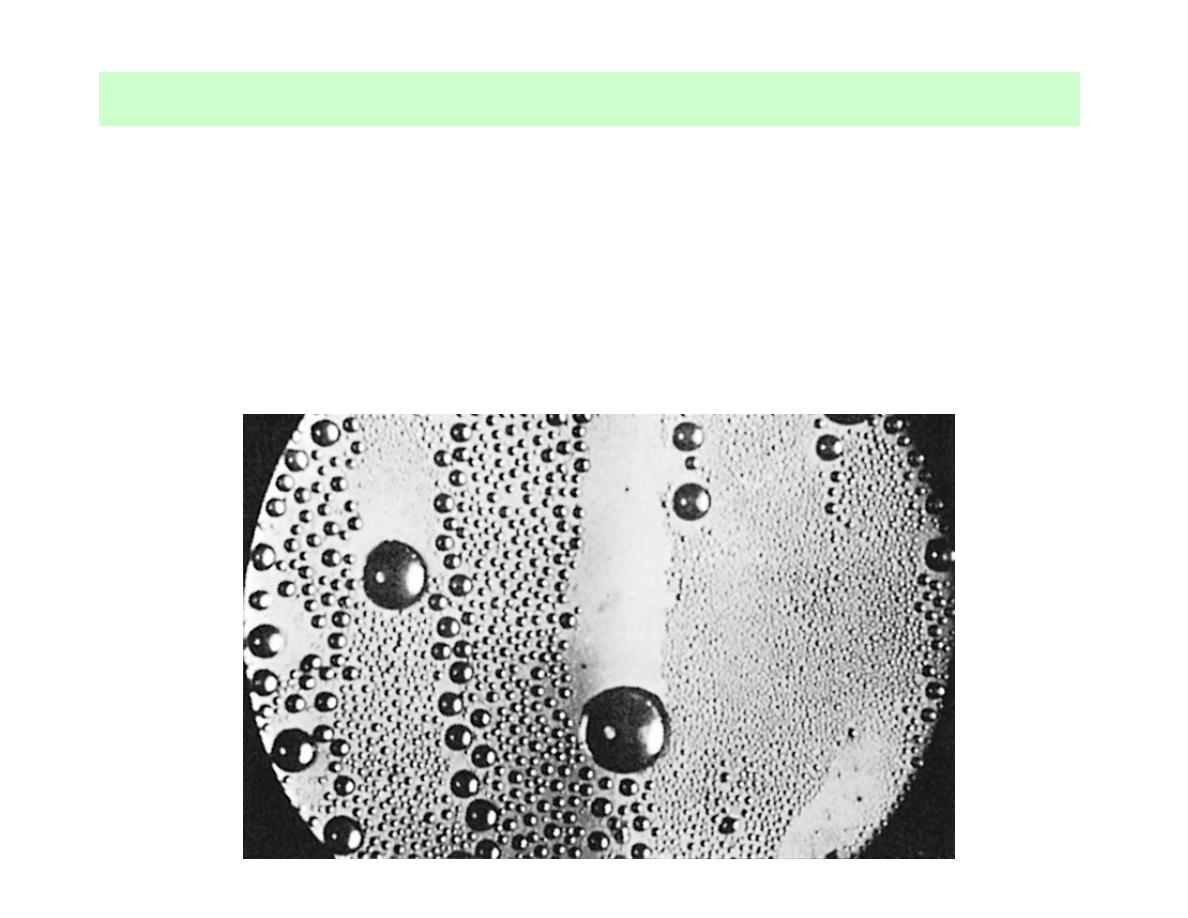
Dropwise condensation of steam on a vertical surface
One of the most effective mechanisms of heat transfer →
→
→
→
extremely large heat transfer coefficients (more than 10 times
larger than in case of film condensation) →
→
→
→ preferred mode of
condensation (efficient condensers) →
→
→
→ adding a promoting
chemical into the vapour, treating the surface with a promoter
chemical or coating the surface with a polymer (teflon) or a
noble metal (gold, platinum, silver)
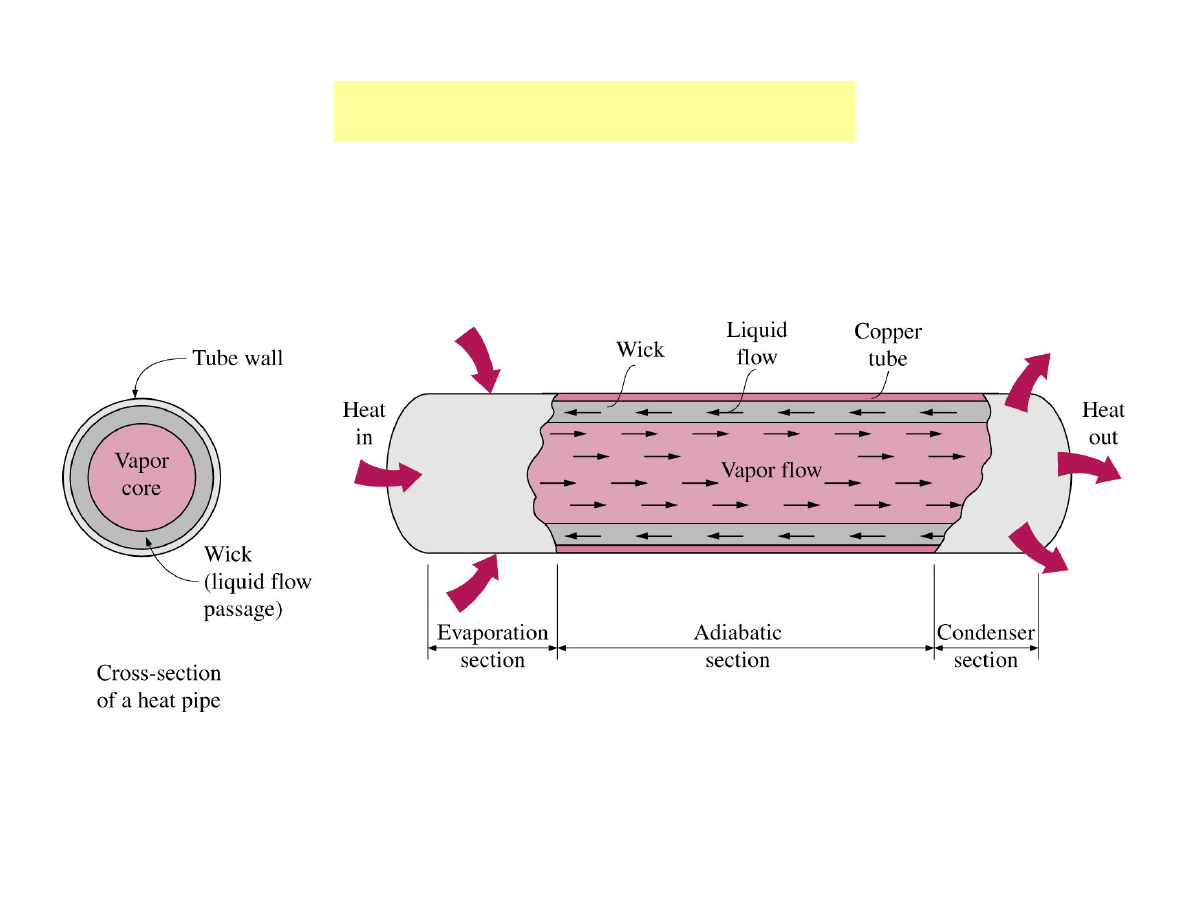
Heat exchangers
Wyszukiwarka
Podobne podstrony:
13 ZMIANY WSTECZNE (2)id 14517 ppt
13 zakrzepowo zatorowa
Zatrucia 13
pz wyklad 13
13 ALUid 14602 ppt
pz wyklad 13
ZARZ SRODOWISKIEM wyklad 13
Biotechnologia zamkniete użycie (2012 13)
Prezentacja 13 Dojrzewanie 2
SEM odcinek szyjny kregoslupa gr 13 pdg 1
w 13 III rok VI sem
Wykład 13 UKS
fundusze 7 13
13 ZACHOWANIA ZDROWOTNE gr wtorek 17;00
auksologia 13 02 2010
więcej podobnych podstron