
C H A P T E R
3
Acute Mesenteric Ischemia
YARON STERNBACH
•
BRUCE A. PERLER
Acute mesenteric ischemia (AMI) may be defined as an
abrupt reduction in blood flow to the intestinal circula-
tion of sufficient magnitude to compromise the metabolic
requirements and potentially threaten the viability of the
affected organs. It is a disease that has been recognized
with increasing frequency since the original description
of a successful superior mesenteric artery (SMA) throm-
boembolectomy nearly 50 years ago. Chronic occlusive
disease of the mesenteric circulation is not uncommon.
In one unselected autopsy series, varying degrees of ath-
erosclerotic occlusive disease were identified in the mes-
enteric circulation in 29% of patients, although the preva-
lence in those 80 years of age or older was 67%.
1
The diagnosis of acute AMI may be present in up to 1
in every 1000 patients admitted to acute care hospitals
2
and will likely increase in the future as our society ages
because AMI typically affects individuals of advanced age,
often in the seventh and eighth decades. These individu-
als frequently also suffer from systemic atherosclerosis,
including coronary artery and peripheral arterial occlu-
sive disease, cardiac arrhythmias, congestive heart failure
or valvular disease, and other significant comorbidity.
Despite the growing awareness of mesenteric ischemia
among health care providers and significant advances in
the diagnosis and treatment of vascular disease in general,
the morbidity and mortality associated with AMI remain
high.
In addition to the relatively older patient population
with significant comorbidity in which AMI occurs, such
dismal outcomes continue to result, at least in part, from
delay in recognition and accurate diagnosis of the condi-
tion. Clearly, optimizing patient outcome is intimately
dependent on the clinician maintaining a high index of
suspicion, on early and aggressive evaluation of patients
suspected of experiencing mesenteric ischemia, and on
expeditious therapeutic intervention. Consequently, al-
though this illness has typically been managed by the
vascular or general surgeon, it should be of interest to
emergency room physicians, internists, and all primary
care providers.
A number of pathophysiologic mechanisms may be
responsible for acute mesenteric insufficiency. Acute
thrombosis of pre-existent atherosclerotic disease in the
mesenteric arterial circulation or, more commonly, sud-
den embolic arterial occlusion may occur. In addition,
AMI may be precipitated by compromise of mesenteric
arterial blood flow without anatomic occlusion in the
setting of low cardiac output or in association with vaso-
spastic disorders and the vasculitides. Less often, mesen-
teric venous thrombosis (MVT) may be identified as the
cause of compromised perfusion to the intestine.
17
This chapter discusses the differences in clinical pre-
sentation and pathogenesis between the various visceral
ischemic syndromes, provides an overview of appropriate
diagnostic modalities, and reviews contemporary therapy
and results.
ANATOMY
Intestinal blood supply occurs predominantly through
three major branches of the abdominal aorta: the celiac
axis, the SMA, and the inferior mesenteric artery (IMA).
Anastomotic connections between branches of these
three major trunks play an important role in maintaining
adequate visceral perfusion in patients with significant
mesenteric arterial occlusive disease. All three vessels
originate anteriorly from the aorta, with the celiac axis
emerging at a perpendicular angle just under the median
arcuate ligament near the level of the diaphragm. The
celiac axis is the largest of these arteries, and it trifurcates
about 1 to 2 cm beyond its origin into the splenic, the
left gastric, and the common hepatic arteries. It is this
latter branch that may provide significant collateral flow
to the intestine through its first branch, the gastroduode-
nal artery, as well as the anterior and posterior pancreat-
icoduodenal arcades.
The SMA forms a more acute angle at its origin, which
is generally 1 to 3 cm distal to the celiac axis. It courses
almost parallel to the aorta proximally before curving
toward the right lower quadrant and giving off branches
that supply blood to the pancreaticoduodenal arcade, the
entire small bowel, as well as the right and transverse
portions of the colon. Small arterial branches from the
SMA and middle colic artery form an anastomotic net-
work with vessels arising from the IMA. This occurs in
the mesentery near the splenic flexure of the colon
through the marginal artery of Drummond and the arc of
Riolan and may provide vital collateral flow in patients
with significant occlusive disease in the mesenteric arte-
rial circulation. Also serving as a potential source of
significant collateral blood flow is the meandering mesen-
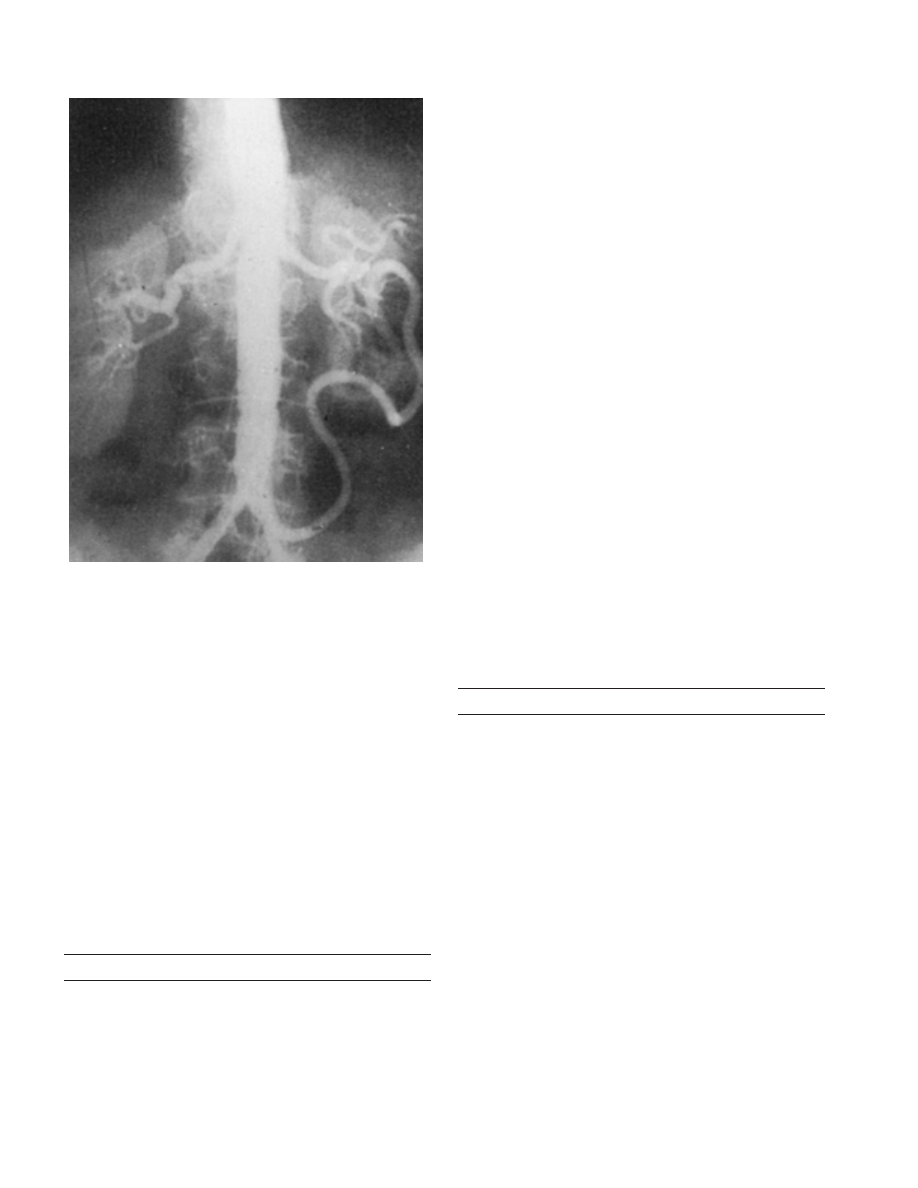
teric artery (Fig. 3–1). It appears as a tortuous, hypertro-
phic vessel within the mesentery and may be distinct
from the arc of Riolan. Its presence generally signifies
proximal hemodynamically significant mesenteric arterial
occlusive disease.
3
The IMA is smaller in caliber, originating from the
infrarenal aorta, 5 to 8 cm distal to the SMA, and a
variable distance from the aortic bifurcation. It provides
perfusion to the distal transverse colon, left colon, and
18
Volume V
• Mesenteric Circulation
Figure 3–1. Aortogram demonstrating a large meandering mesenteric
artery. (From Kornblith, P. L., Boley, S. J., and Whitehouse, B. S.: Anat-
omy of the splanchnic circulation. Surg. Clin. North Am., 72:28, 1992,
with permission.)
rectum. Another important anastomotic network exists
between the IMA and branches of the internal iliac arter-
ies, which contribute to the rectal blood supply, and may
provide collateral flow to the colon when the IMA is
severely stenotic or occluded.
Other, less common collateral pathways exist as well.
A direct connection between the celiac artery and the
SMA may be identified on occasion. This represents an
embryonic remnant and is known as the arch of Buhler.
In the hindgut, blood flow may be derived from the
aorta through lumbar arteries as well as from circumflex
branches of the external iliac arteries. Angiographic as-
sessment of the significance of these mesenteric collateral
pathways often requires obtaining delayed views with
each contrast injection and careful documentation of the
order in which the dye opacifies each vessel.
PATHOPHYSIOLOGY
Intestinal blood flow accounts for 10 to 20% of the resting
cardiac output but may, on occasion, exceed 30%. It is
widely regulated by a variety of mechanisms, including
the autonomic nervous system, a broad array of secreted
neurohormonal factors such as gastrin, glucagon, and
secretin, as well as other vasoactive peptides such as
bradykinin, serotonin, histamine, and the prostaglandins.
Of the blood reaching the intestinal wall, most is directed
toward the mucosa, the layer with the greatest metabolic
demand and highest rate of cell turnover.
A sudden reduction of the blood supply to the viscera
initiates the changes associated with organ ischemia in
general and specifically compromises the mucosal barrier
function. A common histopathologic sequence is ob-
served, regardless of anatomic distribution. The earliest
ultrastructural changes are noted in the mucosal layer,
with alterations observed as soon as 10 minutes after
injury in the canine model.
4
Histologic changes follow
with an inflammatory cell infiltrate. Bowel wall edema
ensues as a result of loss of capillary integrity. Absence
of this natural barrier permits bacterial translocation, pro-
motion of endotoxemia, as well as exudation of fluid
into the bowel lumen. Schoeffel and associates
5
have
implicated these changes, rather than various therapeutic
measures and the inflammatory response, in determining
the clinical course of patients with mesenteric ischemia.
The injured mucosa sloughs, leaving ulcerations of the
bowel wall. Although the bowel may still be viable when
the mucosa is threatened, prolonged interruption of
blood flow ultimately leads to necrosis of the muscularis
and serosa, a point at which the compromised segment
is no longer salvageable.
Whereas the interruption of mesenteric blood flow
initiates tissue injury and systemic illness, its restoration
may also be associated with further deleterious effects
catalyzed by oxygen free radicals and other toxins. Clini-
cally, an already compromised and perhaps frail patient
may experience myocardial depression, a progressive in-
flammatory response with a generalized increase in capil-
lary permeability, resulting in edema and organ dysfunc-
tion.
ETIOLOGY
Arterial Embolism
Embolization to the SMA is the most frequent cause of
AMI, accounting for about half of all cases.
6
Typically, the
emboli originate in the heart in an akinetic or aneurysmal
portion of the left ventricle after myocardial infarction,
in the left atrium in patients with atrial fibrillation, or,
less frequently, in valve cusps harboring vegetations in
patients with bacterial endocarditis (Table 3–1). Rarely,
an unrecognized intracardiac shunt may allow the right-
to-left passage of lower extremity venous thrombi, a so-
called paradoxical embolus. The proximal aorta also may
be a source of atheroemboli which may dislodge sponta-
neously into the bloodstream. Catheter manipulation dur-
ing an endovascular procedure may also be a precipitat-
ing factor. In some cases, however, the source of the
embolic occlusion is never identified.
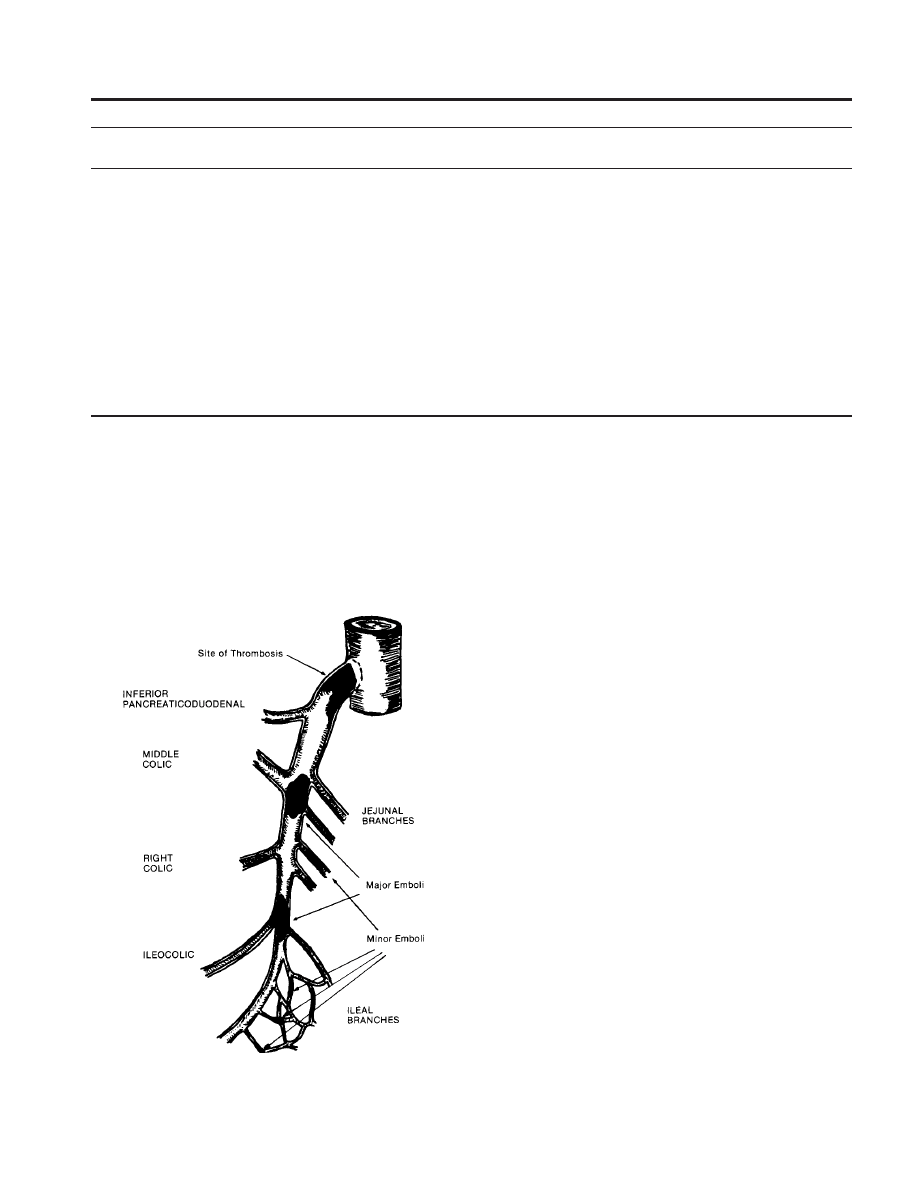
Emboli usually become lodged at major branch points
within the SMA where the distal vessel tends to taper
somewhat (Fig. 3–2). This is typically just beyond the
origin of the middle colic artery, although emboli at more
distal branch points have been identified (see Fig. 3–2).
Proximal SMA perfusion may be maintained, ensuring
Chapter 3
• Acute Mesenteric Ischemia
19
Table 3–1. Clinical Profile of Acute Mesenteric Ischemia
Incidence
(%)
Age
Prior Symptoms
Risk Factors
Mortality
Thrombosis
50
Elderly
Intestinal angina (
)
Systemic atherosclerosis
Very high
Embolism
25
Elderly
No
Recent myocardial infarction
High
Congestive heart failure
Arrhythmias
Rheumatic fever
Nonocclusive
20
Elderly
No
Cardiogenic shock
Highest
mesenteric
Cardiopulmonary bypass
ischemia
Vasopressor agents
Sepsis
Burns
Pancreatitis
Mesenteric
5
Younger
Asymptomatic
Hypercoagulability
Lowest
venous
thrombosis
Portal hypertension
thrombosis
Indolent (
)
Inflammation
Prior surgery
Trauma
viability of the jejunum and resulting in a clear demarca-
tion of the affected intestinal segment at the time of
laparotomy. A minority of emboli (15%) occlude the SMA
at its origin, with ischemic regions extending proximally
to the ligament of Treitz. Smaller emboli may occlude
distal arterioles, resulting in a patchy bowel appearance
with segmental ischemia. In up to 20% of patients suffer-
Figure 3–2. Schematic representation of common sites of superior
mesenteric artery emboli and thrombosis. (From Kaleya, R. N., Sammar-
tano, R. J., and Boley, S. J.: Aggressive approach to acute mesenteric
ischemia. Surg. Clin. North Am., 72:161, 1992, with permission.)
ing mesenteric arterial embolism, more than one arterial
bed may be affected.
7
In response to acute occlusion,
vasoconstriction may ensue, further compromising arte-
rial perfusion and exacerbating the ischemic injury.
Mesenteric Artery Thrombosis
Atherosclerotic occlusive lesions tend to occur at the
origins, or very proximal segments, of the mesenteric
arteries. Not infrequently, a proximal stenosis identified
angiographically may in fact reflect an extension of aortic
plaque into the vessel’s origin. Typically, a stenosis prog-
resses slowly over a period of years, and the patient
remains symptom free if adequate collateral flow exists.
Although the mesenteric arterial circulation is a common
location for atherosclerotic occlusive disease among older
people, in view of the extensive potential collateral net-
work within the mesenteric circulation, patients with
symptoms of chronic mesenteric ischemia are encoun-
tered infrequently. Therefore, it is not uncommon for
AMI to develop secondary to acute arterial thrombosis in
patients with no prior symptoms suggestive of mesen-
teric insufficiency.
Thrombosis of the residual lumen of a diseased mesen-
teric artery often occurs during a period of relative hypo-
tension, or reduced flow, and may be responsible for up
to 25% of cases of AMI.
6
Dehydration is not an uncom-
mon contributing factor among elderly patients. In some
cases, there may be hemorrhage into the wall of an
atherosclerotic plaque, leading to complete occlusion of
the vessel lumen. In contrast to most embolic occlusions,
SMA and celiac thromboses are usually proximal, ostial
occlusions and generally result in ischemia of more exten-
sive segments of bowel (see Fig. 3–2). Although collateral
blood supply may be adequate to obviate symptoms in
the setting of chronic occlusive lesions, in the context of
an acute proximal mesenteric arterial thrombosis, these
collateral networks may not be sufficient to compensate
adequately to sustain bowel viability.

20
Volume V
• Mesenteric Circulation
Although chronic arteriosclerotic arterial occlusive dis-
ease is the most common etiology associated with acute
mesenteric arterial thrombosis, other entities should be
kept in mind. AMI may result from arterial dissection,
and most often, this results from extension of an aortic
dissection producing the malperfusion syndrome. Less
commonly, there may be an isolated dissection of the
mesenteric vessel either spontaneously or as a complica-
tion of a catheter-based intervention. Fibromuscular dys-
plasia and Takayasu’s arteritis may also be associated with
an acute mesenteric arterial occlusion. Conversely, arte-
rial thrombosis may occur in vessels with no, or minimal,
pre-existing disease secondary to an underlying hyperco-
agulable state.
Nonocclusive Mesenteric Ischemia
Compromise of intestinal blood flow may occur in the
absence of an anatomic arterial occlusion or venous
thrombosis as a result of severe mesenteric vasoconstric-
tion. Nonocclusive mesenteric ischemia (NOMI) may ac-
count for as many as 20% of cases of AMI.
8
Although
others have suggested that this entity is seen less fre-
quently,
9,10
this condition may be underdiagnosed be-
cause of the severity of concurrent or associated illnesses
in this patient population.
This pathophysiologic mechanism occurs as a manifes-
tation of a shock state due to sepsis, hemorrhage, or
cardiac decompensation. In essence, the normal mesen-
teric autoregulatory mechanisms are overwhelmed by
neurohumeral agents such as angiotensin II and vasopres-
sin in the setting of profound physiologic stress.
8
This
pathophysiologic process can persist well after normal
flow is restored and the underlying cause is corrected.
NOMI affects the small intestine, the colon, or a combina-
tion of the two. It likely represents the disease state
analogous to ischemic colitis, in which compromise of
the blood flow to the left colon (and particularly the
splenic flexure) is typical. As in occlusive mesenteric
syndromes, ischemia followed by reperfusion may exacer-
bate tissue injury mediated by oxygen free radicals. Ex-
perimental studies indicate that the repetitive hemody-
namic changes in NOMI may eventuate in an injury more
severe than the single insult induced in occlusive mesen-
teric disease.
11
These observations are consistent with a
reported mortality rate in excess of 90% in the most
severe cases.
8
Early diagnosis of this condition and therapeutic inter-
vention before diffuse mesenteric vasospasm induces this
cascade of pathophysiologic events is crucial in minimiz-
ing the mortality associated with NOMI.
12
The clinical
diagnosis, however, is often confounded by the severity
of underlying illnesses that predispose to NOMI. Clearly,
a high index of suspicion is crucial. Elderly patients are
at risk for NOMI, particularly those with low cardiac
output. In addition, any disease with relative dehydration
or hypoperfusion may be associated with NOMI. Com-
mon examples include septicemia, hemorrhage, shock,
severe diarrhea, and any process associated with signifi-
cant third-space fluid sequestration, such as pancreatitis
and burns. In addition to the fluid resuscitation delivered
to these critically ill patients, administration of alpha-
adrenergic agonists, such as phenylephrine, norepineph-
rine, and epinephrine, in the intensive care setting may
potentiate this vasospastic process (see Table 3–1).
Other pharmacologic agents have also been associated
with NOMI. The ergot alkaloids, frequently prescribed in
the treatment of migraine headaches, have been impli-
cated in peripheral and mesenteric arterial vasospasm.
Diuretics may induce a state of relative dehydration while
stimulating antidiuretic hormone release as well as the
renin-angiotensin system. This cascade may contribute
to the persistent vasoconstriction associated with this
syndrome.
13
Digitalis preparations, generally used to po-
tentiate cardiac contractility in this patient population,
have been associated with NOMI due to arterial and
venous smooth muscle contraction.
14–16
Recent reports
have documented the role of cocaine, consumed both
intranasally and intravenously, in inducing mesenteric is-
chemia.
17–19
Affected patients have generally been much
younger than the typical population at risk. Occlusive
changes in the mesenteric circulation may develop with
prolonged use.
17
Other agents that can potentially induce
this clinical state by decreasing mesenteric blood flow
and promoting vascular smooth muscle contraction in-
clude somatostatin, beta-blockers, norepinephrine, and
dopamine in high doses.
20,21
Mesenteric Venous Thrombosis
The development of thrombus in the portal and superior
mesenteric venous system may induce intestinal ische-
mia, which threatens viability of the affected bowel. A
similar pathologic process in the inferior mesenteric vein
is rarely of clinical significance because of more extensive
collateral venous drainage. Initially described in 1895,
this condition is the least frequent etiology of AMI, ac-
counting for just more than 6% of all cases presenting to
the Mayo Clinic between 1972 and 1993.
22
The disease
has been classified according to duration of symptoms,
with acute MVT encompassing those patients suffering
symptoms for less than 4 weeks. Patients with more
prolonged symptoms or those who remain symptom free
are referred to as suffering from a chronic form of the
disorder.
22
Alternatively, one may classify MVT in terms of
its etiology. Primary MVT is diagnosed when no precipi-
tating factor is identified. Most patients, however, in
whom there is an apparent precipitating factor, suffer
from secondary MVT.
These conditions may be related to a variety of hyper-
coagulability states, traumatic injury, obstruction of ve-
nous flow, and intra-abdominal infection. In contradistinc-
tion to the other etiologies of AMI, patients with MVT
are typically younger, often between 30 and 60 years of
age, and the condition predominates in women (see Table
3–1). In the Mayo clinic series,
22
the most common condi-
tions, encountered in at least one third of patients who
were ultimately diagnosed with MVT, were previous ab-
dominal surgery, a hypercoagulable state, previous MVT,
and use of tobacco. Among the hypercoagulable states,
polycythemia vera was the most common.
The primary pathophysiologic process associated with

Chapter 3
• Acute Mesenteric Ischemia
21
MVT is a rise in portal and superior mesenteric venous
pressures. In the intestine, increased hydrostatic pressure
leads to luminal fluid sequestration as well as bowel wall
edema. The ensuing relative hypovolemia and hemo-
concentration may contribute to vasoconstriction. Ulti-
mately, infarction of the affected intestinal segments may
develop. Eventual focal hemorrhage and necrosis lead to
loss of the gut barrier function, which ultimately allows
for bacterial translocation and possible endotoxemia. The
arterial response to MVT may persist well after the ve-
nous obstruction has been corrected.
DIAGNOSIS
Clinical Presentation
Expedient diagnosis of the patient with AMI is the key to
maximizing patient survival and remains a difficult clini-
cal challenge. The clinician must maintain a high index
of suspicion and appreciate the similarities and subtle
differences in the clinical patterns of presentation associ-
ated with the various etiologies of AMI and the clinical
profiles of the patient at risk for these respective etiolo-
gies (see Table 3–1).
AMI secondary to acute arterial thrombosis or embo-
lism typically occurs in patients in the seventh or eighth
decades of life. Coronary and peripheral arterial occlusive
disease, or cardiac dysrhythmias, are ubiquitous in this
patient population. Unlike most clinical manifestations of
arteriosclerotic occlusive disease, however, AMI second-
ary to arterial thrombosis or embolism does not predomi-
nate in men. The most important symptom of acute
mesenteric arterial occlusion, either due to thrombosis
or embolism, is severe abdominal pain, which is out of
character to the physical findings early in the course
of the illness. This apparent inconsistency between the
patient’s presenting complaints and the paucity of physi-
cal findings, which has clearly been a major factor respon-
sible for delay in establishing the correct diagnosis and
poor survival over the years, is ironically the sine qua
non of AMI.
When physical findings suggestive of an acute intra-
abdominal catastrophe are present, bowel infarction has
in most cases already occurred, and the chance of sur-
vival in this elderly patient population with significant
associated comorbidity is dramatically reduced. The pain
may be colicky in character initially, but it then becomes
more sustained as bowel viability is compromised. It may
be diffuse or localized to any quadrant of the abdomen,
generally with anterior projection. Vomiting, and less
commonly diarrhea, may be seen. Occult blood in the
stool and, later, frankly bloody diarrhea are not uncom-
mon. Feculent breath may herald intestinal necrosis. In
most cases, the onset of abdominal pain is acute, with
rapid progression over a few hours. This is most typical of
the patient who has experienced an embolic occlusion.
In some cases, especially among patients who have
experienced thrombosis of a previously diseased mesen-
teric vessel and in whom some collateral reserve is there-
fore present, the onset of the illness may be more insidi-
ous, with a prodrome of anorexia, malaise, and other
vague symptoms, which evolve to frank distress over a
period of a few days. In evaluating the past medical
history, evidence of a recent illness, weight loss, changes
in eating habits, or postprandial discomfort leading to
food aversion may be helpful in differentiating throm-
botic from embolic etiologies, although as noted pre-
viously, many patients who experience acute mesenteric
arterial thrombosis have no symptoms until the acute
event.
The diagnosis of NOMI is especially difficult because
many of these patients are already hospitalized for treat-
ment of other critical illnesses, and initial clinical manifes-
tations of AMI may be masked. In many cases, these
patients are obtunded so that a history is not obtainable,
and the physical examination is not revealing. Among
conscious patients, the history is similar to that described
by patients who have experienced acute mesenteric arte-
rial thrombosis. Frequently, the surgeon is asked to evalu-
ate the patient in the intensive care unit for possible
NOMI. This is a rare, but potentially life-threatening, com-
plication among patients who have undergone cardiac
surgical procedures. The incidence of mesenteric ische-
mia among this patient population has ranged from 0.06
to 0.36% in several recent series.
23–25
Risk factors identi-
fied include emergent procedures, prolonged pump time,
use of intra-aortic balloon pumps, advanced age, and
failed coronary angioplasty. The presentation tends to
occur days after the initial procedure, and the mean time
to abdominal exploration has ranged from 4 to 9 days
after cardiac surgery. In part, this delay may be due to
the use of ventilatory support and sedation, resulting in
a less accurate physical examination.
Although the presentation of patients with MVT may
be similar to that experienced by patients with acute
mesenteric arterial insufficiency, more often, symptoms
develop more insidiously, generally over a period of 7 to
14 days. The abdominal pain experienced by patients in
the Mayo Clinic experience was present for more than
48 hours in 75% of the cases, with only 9% describing
the onset within 24 hours of presentation.
26
Abdominal
pain may be poorly localized and is often associated
with abdominal distention, ascites, anorexia, and in some
cases, nausea, vomiting, and diarrhea.
As noted previously, when seen early in the course
of AMI, the physical examination may be deceptively
unimpressive. The patient’s appearance may range from
mild discomfort with intermittent exacerbation of colicky
pain to obvious extremis after bowel infarction has devel-
oped. Dry mucous membranes with decreased skin tur-
gor and flat neck veins may indicate a dehydrated state.
In addition, cool extremities with faint or absent pulses
and mottling of the skin may also be indicative of hypo-
perfusion and are a frequent finding among patients with
NOMI. Early, the abdomen may be soft, and bowel sounds
may be normal. As the ischemic process progresses, how-
ever, there may be guarding, hypoactive or absent bowel
sounds with distention, and later progressive guarding
and peritonitis as full-thickness intestinal necrosis
evolves. Although patients with MVT may have a more
protracted and insidious onset of symptoms, once bowel
viability is compromised, the physical findings are gener-
22
Volume V
• Mesenteric Circulation
ally similar to what is seen in the other intestinal ischemic
syndromes.
Laboratory Testing
A complete blood count with differential, electrolyte
panel, coagulation studies, liver function tests, and an
amylase level should be drawn in any patient suspected
of experiencing an acute abdominal process, including
AMI. The findings of leukocytosis, acidosis, and elevated
amylase level, however, are consistent with more ad-
vanced intestinal ischemia and, most likely, nonviable
bowel. Therefore, absence of one or several of these
abnormalities should not dissuade the clinician from sus-
pecting the diagnosis of mesenteric ischemia. Although
efforts have been made to establish elevated levels of a
number of enzymes, including amylase, alkaline phospha-
tase, lactic dehydrogenase, creatine phosphokinase, and
mucosal diamine oxidase, as early markers for mesenteric
ischemia, all have proved nonspecific and therefore unre-
liable.
27
It is generally not possible to differentiate the
etiology of NOMI from acute arterial thrombosis or embo-
lism on the basis of the laboratory profile, although hemo-
concentration, consistent with a generalized dehydration
state, is ubiquitous among those experiencing NOMI.
15
Likewise, no specific laboratory test is helpful in identi-
fying the patient with acute MVT, although documenta-
tion of a hypercoagulability state may be suggestive.
Radiologic Evaluation
Plain abdominal radiographs should be obtained early in
the evaluation of patients with suspected AMI, primarily
to rule out other causes of abdominal pain, such as a
perforated viscus, small or large bowel obstruction, or
gallstones. In about one fourth of patients with confirmed
AMI, no abnormality may be detected at all.
28
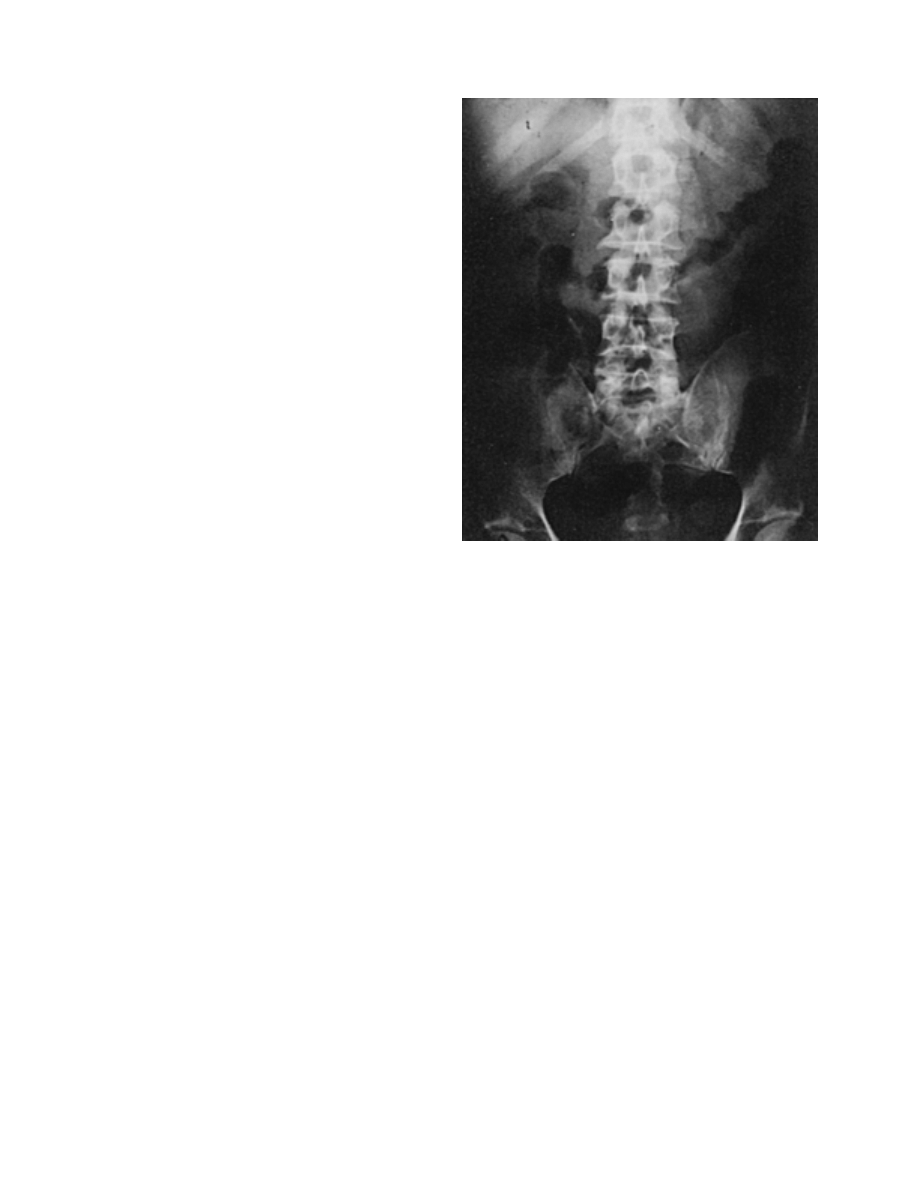
Occasion-
ally, one may see a fixed small bowel loop, ‘‘thumb-
printing,’’ or bowel wall thickening, which although con-
sistent with the diagnosis, are nonspecific findings (Fig.
3–3). Pneumatosis intestinalis is seen only rarely (5%)
29
and has also been associated with numerous other benign
conditions, including chronic obstructive pulmonary dis-
ease, inflammatory bowel disease, and mechanical ventila-
tion. In the patient with mesenteric ischemia, however,
this finding is indicative of bowel infarction. Likewise,
air in the portal venous circulation, biliary tree, or free
intraperitoneal cavity are also late findings consistent
with bowel necrosis. Abdominal radiographs may reveal
a paucity of bowel gas and adynamic ileus, the most
frequent finding in MVT.
23
Barium studies are contraindi-
cated in the patient with suspected AMI because the
increased intraluminal pressure generated may precipitate
bowel perforation and because residual barium within
the bowel may obscure crucial angiographic findings.
Although Duplex examination of the mesenteric circu-
lation is playing an increasingly valuable role in the evalu-
ation of chronic mesenteric arterial occlusive disease, the
technical difficulty of imaging the mesenteric vessels in
patients with distended bowel loops has significantly lim-
Figure 3–3. Plain abdominal film in patient with acute mesenteric
ischemia. Note ‘‘thumbprinting’’ in the transverse colon. (From Wolfe,
E. L., Sprayregen, S., and Bakal, C. W.: Radiology in intestinal ischemia.
Surg. Clin. North Am., 72:108, 1992, with permission.)
ited the role of this modality in assessing patients with
suspected AMI. Furthermore, even if flow is seen in the
proximal SMA or celiac arteries, an embolic etiology is
not necessarily excluded. On the other hand, the pres-
ence of normal flow in the portal and mesenteric venous
system may help to exclude the diagnosis of portal ve-
nous thrombosis, whereas absent flow and the presence
of ascites are highly suggestive of MVT.
30
Transgastric
ultrasonography has been suggested as a means of visual-
izing the visceral aorta,
31
although its utility in diagnosing
AMI is unknown.
Abdominal computed tomography (CT) has assumed
an important role in contemporary practice in the evalua-
tion of the patient with acute abdominal pain. It can
detect luminal contrast distribution, and therefore masses
as well as ascites, and inflammatory changes of the bowel
wall and the surrounding fat plains. Among patients with
AMI secondary to arterial thrombosis or embolism, how-
ever, the CT scan may be normal or nondiagnostic. Al-
though CT may identify calcified plaque in the orifice of
the mesenteric arteries and rapid bolus contrast adminis-
tration may demonstrate vessel occlusion, a chronic oc-
clusive process cannot be differentiated from acute vessel
thrombosis. In one series of 39 patients, the finding of at
least one of a number of signs, including arterial or
venous thrombosis, intramural gas, portal venous gas,
lack of bowel enhancement, or liver and spleen infarcts
on dynamic scanning, resulted in a sensitivity of 64%
and specificity of 92%.
32
Other investigators have shown
Chapter 3
• Acute Mesenteric Ischemia
23
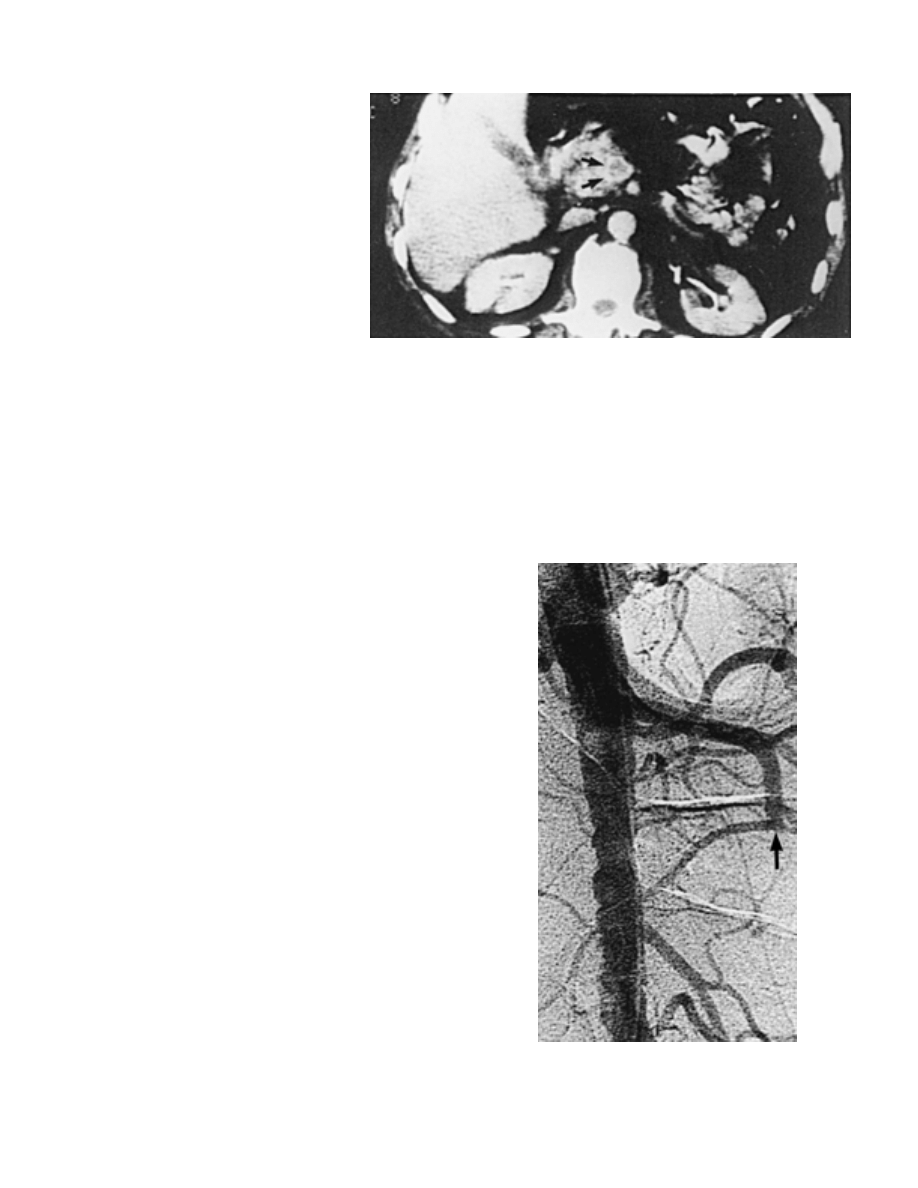
Figure 3–4. Abdominal computed tomography
scan in patient with mesenteric venous thrombo-
sis. Note the dilated superior mesenteric vein
with central thrombus. Arrows indicate the rim
of increased density surrounding the thrombus.
(From Boley, S. J., Kaleya, R. N., and Brandt, L. J.:
Mesenteric venous thrombosis. Surg. Clin. North
Am., 72:194, 1992, with permission.)
generally poor correlation between plain radiographs and
CT scans in this clinical scenario,
28
and each modality was
thought to be diagnostic in only a minority of cases.
On the other hand, CT has become the diagnostic
modality of choice in acute MVT, with sensitivity ex-
ceeding 90%.
33–35
The superior mesenteric or portal vein
appears enlarged, with a central area of low attenuation,
suggestive of thrombus. In the contrast phase, a rim may
enhance at the vein wall, yielding a bull’s eye appearance
(Fig. 3–4). Bowel wall thickening and the presence of
ascites are also suggestive of the diagnosis of MVT. Al-
though the use of magnetic resonance imaging is much
more limited, this modality does appear to offer a high
degree of accuracy among patients with MVT.
36
Arteriography
Arteriography is unequivocally the gold-standard diagnos-
tic methodology in the evaluation of the patient with
acute abdominal pain with presumed AMI. One of the
most difficult judgments that the clinician caring for the
patient with suspected AMI must make is whether the
information that may be derived from mesenteric angiog-
raphy justifies the time required to perform the study and
the resultant delay in carrying out surgical intervention,
if required. Among patients in whom the diagnosis has
not been firmly established and who are stable hemody-
namically
37
and presumably seen early in the course of
the pathologic process, angiography should be per-
formed. It can establish the diagnosis; localize the site of
occlusion and therefore differentiate among thrombotic,
embolic, and nonocclusive etiologies; and may allow early
nonoperative therapeutic intervention in selected cases.
Among patients who will require operative intervention,
the diagnostic information afforded by angiography
allows the surgeon to select the appropriate operative
approach.
Biplanar views of the aorta and its branches must be
obtained. The lateral projection is of particular impor-
tance in assessing the proximal celiac axis and SMA.
Acute thrombotic occlusion usually occurs at the origin
of the SMA or celiac axis so that opacification of a short
segment of these vessels is highly suggestive of this diag-
nosis. In such cases, collateral flow should be seen, and
prolonged angiographic runs with delayed images may
demonstrate retrograde filling of these vessels through
these collateral networks. Typically, diffuse atheromatous
disease is seen in the abdominal aorta, including the
suprarenal and supraceliac segments. In contrast, an em-
bolic occlusion is visualized as an inverted meniscus sign
several centimeters distal to the origin of the SMA, typi-
cally at the origin of the middle colic artery (Fig. 3–5).
Therefore, the proximal jejunal branches of the SMA
are opacified. If the embolus is acute and therefore not
Figure 3–5. Lateral aortogram demonstrating an embolus (arrow) in
the superior mesenteric artery, several centimeters beyond its origin.
(From Eldrup-Jorgensen, J., Hawkins, R. E., and Bredenberg, C. E.:
Abdominal vascular catastrophies. Surg. Clin. North Am., 77:1313, 1997,
with permission.)

24
Volume V
• Mesenteric Circulation
completely occlusive, there may be some flow around it.
On the other hand, if the embolus is chronic, there may
be significant secondary thrombus deposition so that the
meniscus sign is obscured and differentiation from a pri-
mary thrombotic occlusion is more problematic.
The SMA and other mesenteric vessels, as well as the
abdominal aorta, appear relatively undiseased. In view of
this, and in contradistinction to thrombotic occlusions,
there is poor collateral vessel development and opacifica-
tion. Less commonly, the embolus lodges in the distal
SMA or a branch vessel. Rarely, an embolic occlusion may
lodge in the proximal vessel, mimicking a thrombotic
occlusion. Multiple emboli may be identified in other
visceral vessels. Differentiation of an embolic versus
thrombotic occlusion is of more than academic interest
because the surgical approach is different for these two
etiologies.
In fact, an even more important benefit of angiography
in patients with AMI is in differentiating occlusive disease
from NOMI because the management of patients with
NOMI is very different from the management of patients
with anatomic arterial occlusions. In NOMI, the mesen-
teric vessels may be patent, with or without evidence of
chronic disease. Typically, narrowing at the origin of the
major SMA branches, or intermittent areas of narrowing
and dilation (‘‘string of sausages’’ or ‘‘string of lakes’’
sign), is seen (Fig. 3–6). The runoff vessels may appear
tapered or small in caliber throughout their length, a
finding consistent with diffuse arterial vasoconstriction
or spasm, and the mesenteric arcades may not be visual-
Figure 3–6. Superior mesenteric arteriogram in patient with nonocclu-
sive mesenteric ischemia. Note the spasm at the origins of several major
branches as well as the intermittent areas of spasm and dilatation
(‘‘string of sausages’’ or ‘‘string of lakes’’ sign). (From Boley, S. J., Brandt,
L. J., and Veith, F. J.: Ischemic disorders of the intestines. Curr. Probl.
Surg., 15(4):30, 1978, with permission.)
ized at all. Contrast injected into the SMA through a
selectively placed catheter may reflux back into the aorta
because of elevated vascular resistance.
Because these angiographic findings are nonspecific
and can often be demonstrated in hypotensive or hypovo-
lemic patients or in patients taking high-dose vasopressor
pharmacologic agents, it is important to restore normovo-
lemia and reduce vasopressor support as much as possi-
ble before performing the angiographic study. Direct infu-
sion of a bolus of papaverine (60 mg) into the SMA is a
useful diagnostic maneuver. Reversal of vasoconstriction
demonstrated angiographically confirms the diagnosis,
and a catheter can be left in place for a continuous
therapeutic infusion. Tolazoline and nitroglycerin have
also been used in this setting (see later).
On the other hand, angiography may not be as helpful
in confirming the diagnosis of MVT, especially if there is
segmental venous thrombosis. Perhaps most importantly,
arteriography can exclude acute arterial thrombosis, em-
bolism, and NOMI. Signs consistent with MVT include
spasm in the SMA branches, possible reflux of contrast
back into the aorta because of vasoconstriction, a pro-
longed arterial phase, and more intense capillary opacifi-
cation with selective SMA injection. There may be a more
intense nonvisualization of the portal and mesenteric
veins on venous-phase images, particularly if venous col-
laterals are present in the vicinity. Venous-phase filming
during arteriography may demonstrate thrombus within
the mesenteric venous circulation, although the definitive
filling defect in the superior mesenteric vein is rarely
encountered.
38
Likewise, smaller venous channels are not
visualized.
MEDICAL TREATMENT
Treatment of mesenteric ischemia must begin during the
initial evaluation. Substantial protein-rich fluid losses oc-
cur into the gut in patients experiencing AMI and con-
tinue after successful revascularization. As a result, al-
though splanchnic vasoconstriction is the primary
etiologic mechanism in NOMI as well as an important
component of the pathophysiologic process in MVT, it
also plays a role in promoting the visceral ischemic pro-
cess among patients with mesenteric arterial thrombosis
and embolism. Therefore, aggressive fluid resuscitation is
vital and can be guided by placement of a urinary drain-
age catheter as well as a central venous or Swan-Ganz
catheter in the patient with significant cardiac disease.
An arterial line is indicated for systemic blood pressure
monitoring if significant hemodynamic instability is
noted.
In patients with more advanced mesenteric ischemia
and hemodynamic compromise, a fundamental goal of
volume resuscitation is to allow weaning and, if possible,
removal of pharmacologic vasopressor support because
many of these agents can further contribute to the mesen-
teric ischemic process (see Table 3–1). Norepinephrine
and phenylephrine (Neo-Synephrine) are particularly del-
eterious in this regard. Conversely, dopamine is a more
appropriate inotropic agent among patients with AMI

Chapter 3
• Acute Mesenteric Ischemia
25
because, in low doses, it may act as a mesenteric vasodila-
tor, and in higher doses, it produces less severe mesen-
teric vasoconstriction than the latter agents. Digitalis is a
well-recognized vasoconstrictor of SMA smooth muscle
and should be discontinued if possible. A nasogastric
tube should be placed to decompress the fluid-filled and
distended intestinal tract and thus promote intestinal per-
fusion, reduce the risk for bowel perforation, and mini-
mize the chance of aspiration.
39
In view of the potential
for bacterial translocation through the compromised in-
testinal barrier and the documented high incidence of
positive blood cultures among patients with severe mes-
enteric ischemia, early institution of broad-spectrum anti-
biotics, including anaerobic coverage, is mandatory.
The role of formal anticoagulation is dependent on the
etiology of the AMI. Systemic anticoagulation with hepa-
rin is indicated acutely among patients with MVT to
minimize thrombus progression, and most of these pa-
tients require long-term anticoagulation with warfarin,
especially if an underlying hypercoagulability disorder is
uncovered. Among those who present with an acute
arterial thrombosis or embolism, the role of anticoagula-
tion is more problematic. Although early heparin adminis-
tration may prevent thrombus extension, this potential
benefit must be weighed against the risk for significant
gastrointestinal bleeding in patients with established
bowel ischemia. In most cases, because urgent operative
exploration is indicated, anticoagulation should be with-
held preoperatively. Indeed, many of these patients are
already experiencing a hypocoagulable state as a systemic
manifestation of the mesenteric ischemic process. Postop-
eratively, therapeutic anticoagulation is indicated in pa-
tients who have experienced an embolic occlusion to
minimize the risk for recurrent embolization to the mes-
enteric circulation or other arterial beds. Anticoagulation
is not necessary, however, in most patients after revascu-
larization for acute mesenteric arterial thrombosis.
INTERVENTIONAL RADIOLOGY
In contradistinction to the other etiologies of AMI, the
primary treatment of NOMI is pharmacologic. Specifi-
cally, selective catheter-directed administration of a num-
ber of vasodilating agents, including papaverine, tolazo-
line, glucagon, nitroglycerin, nitroprusside, prostaglandin
E, phenoxybenzamine, and isoproterenol, have been used
in this clinical setting. Most experience to date, however,
has been reported with papaverine.
37
As noted previously,
a selective SMA test injection of papaverine, 60 mg,
should be administered, followed by a repeat contrast
injection. If this demonstrates reversal of vasoconstric-
tion, the catheter is left in place, and a continuous infu-
sion at 30 to 60 mg/hour is delivered. Potential side
effects of papaverine include cardiac arrhythmia as well
as hypotension and reflex tachycardia, which may be
particularly deleterious in patients with significant under-
lying coronary artery disease. Because about 90% of the
drug is metabolized during its initial pass through the
liver, however, these systemic side effects are much less
commonly seen when compared with the intravenous
administration of the drug. Tolazoline may also be admin-
istered in this clinical setting, with a 25-mg bolus fol-
lowed by a continuous infusion at 10 to 25 mg/hour.
Transcatheter infusion of vasodilators is generally ac-
companied by the administration of heparin to prevent
possible propagation of thrombus formed during the low-
flow state or its formation at the catheter site. The infu-
sion may be maintained initially for a period of 8 to 24
hours before angiographic re-evaluation. The follow-up
angiogram should be repeated only after flushing the
drug out of the infusion line for 30 minutes with saline.
Based on the angiographic findings and the patient’s clini-
cal course, the infusion may be stopped or continued for
an additional 24 hours. Infusions continued for as long
as 4 and 5 days have been reported.
It is axiomatic that this nonoperative therapy places
the onus on the physician managing the patient to moni-
tor closely the patient’s abdominal examination and labo-
ratory parameters to make certain that the patient is
improving. Failure to improve or any evidence of a deteri-
orating clinical state, such as the development of perito-
neal signs suggestive of progressive intestinal ischemia,
mandates immediate surgical exploration. Even in this
case, however, the infusion catheter may be left in place
to allow postoperative drug infusion to maximize perfu-
sion of marginally viable bowel after resection of frankly
gangrenous segments. Selective intra-arterial vasodilator
therapy has also been used as an adjunct in the manage-
ment of patients with acute mesenteric arterial occlusion
due to embolism or thrombosis because, as noted earlier,
mesenteric vasoconstriction plays a role in exacerbating
the ischemic insult in many of these patients.
37
Other interventional radiologic techniques used in re-
cent years in the management of AMI include catheter-
directed thrombolysis, percutaneous transluminal angio-
plasty (PTA), and fenestration of aortic dissection.
Jamieson and associates
40
first reported the successful
lysis of an SMA embolus with streptokinase 20 years ago.
In another report, a thromboembolic occlusion of the
SMA was successfully lysed with streptokinase.
41
More
recently, a patient at the Johns Hopkins Hospital under-
went successful thrombolysis of an SMA occlusion with
urokinase, followed by laparoscopy to assess bowel viabil-
ity.
42
Likewise, several workers have reported the treat-
ment of MVT with catheter-directed thrombolysis, using
either the transjugular venous
43
or selective arterial ap-
proach.
44–46
Experience with thrombolytic therapy for AMI can
best be characterized as anecdotal at this time. In addition
to the time required to achieve complete clot lysis, one
must be concerned about the risk for intestinal hemor-
rhage as a complication of the thrombolytic infusion.
Nevertheless, in elderly patients who have severe medical
comorbidity and in whom the clinical presentation sug-
gests that the ischemic process is early, this endovascular
approach may avoid a long and potentially morbid surgi-
cal operation, especially if bowel viability can be con-
firmed through laparoscopy.
42,47
Furthermore, in patients
who present with AMI secondary to arterial thrombosis,
successful lysis may allow treatment of the underlying
chronic occlusive disease with PTA.
48
Although there is scant reported experience with PTA

26
Volume V
• Mesenteric Circulation
in the setting of AMI, Matsumoto and colleagues
49
re-
cently documented an initial technical success in 102
(86%) of 126 patients who underwent PTA of chronic
visceral arterial lesions, with a major complication rate
of only 6%. Similarly, in patients who develop AMI as a
secondary complication of an aortic dissection, endovas-
cular fenestration of the false lumen may restore visceral
arterial perfusion.
50
SURGERY
Operative intervention remains the mainstay of manage-
ment of almost all patients who present with AMI. The
surgeon’s goal is to confirm the diagnosis of mesenteric
ischemia and assess bowel viability, determine the respon-
sible etiology, perform revascularization where possible,
and resect nonviable bowel. Although the underlying
etiology significantly influences the surgical approach and
operative strategy, and although preoperative studies,
such as angiography, can be helpful in answering this
question, laparotomy should not be unduly delayed in
the compromised patient because, in most cases, surgical
exploration allows accurate identification of the responsi-
ble pathologic process. It is axiomatic that operative
delay is the most important determinant of an adverse
outcome.
The operating room should be equipped with a
Wood’s lamp, fluorescein, and a continuous-wave Dop-
pler ultrasound unit. The patient is placed in the supine
position on the operating table and a wide field extending
from the nipples to the knees should be prepped and
draped into the operative field to allow harvesting of
the greater saphenous veins, if necessary. The abdomen
should be entered through a long midline incision and
the bowel carefully examined from the stomach to the
rectosigmoid. A definitive determination of intestinal via-
bility should not be made until after revascularization has
been performed.
Palpation of pulsations or detection of Doppler signals
in the periphery of the mesentery may reflect collateral
flow; therefore, this finding does not rule out an SMA
occlusion. The SMA should be carefully isolated at the
base of the mesentery as it exits from under the pancreas
and exposed for several centimeters distally. The pres-
ence of a strong pulsation in the proximal vessel, which
weakens or is not palpable more distally, is highly sugges-
tive of an embolus, whereas an absent pulsation in the
proximal SMA is most consistent with arterial thrombosis.
The continuous-wave Doppler should be used to detect
flow if no pulses are palpable. The presence of a pulse
or attenuated Doppler signal at the SMA origin, but with
an absent signal in the mesentery, implies embolic occlu-
sion of the vessel. Similarly, the celiac axis and its main
branches should be examined, and the trunk can be
approached either through the gastrohepatic ligament or
by medial visceral rotation.
Inspection of the bowel can also elucidate the underly-
ing etiology. An acute SMA thrombosis typically compro-
mises viability of the right colon and entire small intes-
tine. In contrast, because an embolic occlusion tends to
lodge more distally in the vessel, the proximal jejunum
may be spared, and there may be more patchy involve-
ment in patients with multiple distal embolic occlusions.
Marked edema of the intestine and mesentery, cyanotic
discoloration of the bowel wall, and palpable mesenteric
arterial pulsations are most suggestive of MVT. In this
setting, the superior mesenteric vein (SMV) should be
isolated inferior to the pancreas to confirm the diagnosis
and allow venous thrombectomy, if necessary. Finally, if
preoperative arteriography was not performed, and if
peripheral arterial pulsations with distal attenuation are
noted throughout the mesentery, in the absence of appar-
ent venous thrombosis, the most likely diagnosis is NOMI.
In this setting, the surgeon must minimize arterial manip-
ulation to avoid further exacerbating mesenteric vasocon-
striction, and in the absence of frankly necrotic bowel,
the abdomen should be closed and the patient urgently
taken to the angiography department for vasodilator ther-
apy.
Revascularization
Embolus
The SMA should be controlled just distal to the origin of
the middle colic artery and proximal to the jejunal
branches and an arteriotomy performed. If the diagnosis
of embolus is certain, we prefer a transverse arteriotomy,
although if there is any doubt, a longitudinal incision
should be made so that it can serve as the site for the
distal anastomosis of a bypass graft. Not infrequently, the
embolus can be localized at and directly extracted from
this site. A No. 3 thromboembolectomy catheter is passed
proximally and should easily advance into the aorta, with
brisk inflow confirmed. This can be followed by passage
of a No. 4 thromboembolectomy catheter. Next, the No.
3 catheter should be gently advanced distally to retrieve
the embolus and associated thrombotic material. This
should be followed by passage of a No. 2 thromboembo-
lectomy catheter until no further clot can be extracted.
In some cases, it may be possible to ‘‘milk’’ clot manu-
ally out of the distal vasculature. Dilute heparin solution
should be infused distally. It may not be possible to
extract smaller, more peripheral thromboemboli com-
pletely, and in this case, there may be a role for a brief
thrombolytic infusion into the distal vessel, although
there is only anecdotal experience with this strategy.
Likewise, it is not unreasonable to infuse a vasodilator,
such as papaverine, into the distal vessel before closing
the arteriotomy. If a transverse arteriotomy was used,
it may be possible to perform a primary closure with
interrupted 6-0 or 7-0 monofilament synthetic sutures,
although in an especially small vessel or when a longitudi-
nal arteriotomy was used, a patch angioplasty should be
performed.
Thrombosis
When a retrograde embolectomy performed from the
distal vessel cannot achieve adequate inflow, suggesting
that the diagnosis is thrombosis rather than embolus,

Chapter 3
• Acute Mesenteric Ischemia
27
or when the preoperative evaluation or intraoperative
findings suggest SMA thrombosis, revascularization be-
comes more complex. Because acute mesenteric arterial
thrombosis typically occurs in the proximal vessel, throm-
boendarterectomy procedures have been attempted in
this setting. However, ‘‘blind’’ thromboendarterectomy,
performed from an arteriotomy distal to the origin of
the involved vessel and without aortic control, has been
associated with poor results and is not recommended.
Therefore, suprarenal aortic and proximal mesenteric ar-
terial exposure, achieved either anteriorly through the
lesser sac or retroperitoneally through medial visceral
rotation, is favored to perform this procedure.
In patients with significant intraperitoneal soilage and
no autogenous vein available to perform a bypass graft,
thromboendarterectomy may be a reasonable option to
achieve revascularization in order to avoid the use of a
prosthetic conduit. In most cases, however, we favor
performance of a bypass graft as the most expeditious
means of achieving successful revascularization in the
patient with an acute mesenteric arterial thrombosis.
There are a number of options to consider in performing
an emergency bypass operation in patients with AMI,
including the conduit used, the choice of inflow, and the
extent of revascularization. Although experience with
elective revascularization for chronic mesenteric ischemia
attests to the durability (and possible superiority) of pros-
thetic conduits, in most patients with AMI, because
bowel viability may be marginal at best or if intestinal
resection will likely be required, autogenous saphenous
vein should be used as the bypass conduit. If not avail-
able, or if bowel viability is certain, a synthetic conduit
may be selected, including either Dacron or polytetraflu-
oroethylene, and there is some evidence that the latter
material may be more resistant to bacterial adherence.
In patients with acute SMA thrombosis, the distal anas-
tomosis can usually be performed at the level of the
middle colic artery or just proximal or distal to this. If
the patient has experienced a celiac axis occlusion, a
distal anastomosis may be performed to the vessel just
beyond its origin from the aorta or to any of its major
branch vessels. It is important to pass a thromboembolec-
tomy catheter distally at the time of performing the distal
anastomosis to make certain that there has not been
significant distal clot propagation.
The choice of inflow is somewhat controversial and
is largely influenced by surgeon preference. Either the
infrarenal aorta or supraceliac aorta is selected in most
cases, although in some patients, the right iliac artery
may provide inflow for an SMA bypass. The major advan-
tage of retrograde bypass from the infrarenal aorta is the
relatively easy and rapid exposure of the aorta at this
level. We perform an end-to-side anastomosis to the ante-
rior aortic wall using 3-0 monofilament synthetic suture
and prefer an end-to-side anastomosis to the SMA using
6-0 monofilament synthetic suture when performing a
retrograde bypass. In many patients, however, significant
atherosclerotic disease in the aorta, including aneurysmal
dilation in some cases, may compromise this approach.
Partial occlusion with a side-biting clamp is often not
advisable, or possible, so that the aorta must be com-
pletely clamped proximal and distal to the site of the
proximal anastomosis. Furthermore, this retrograde by-
pass to the SMA, which assumes the configuration of an
inverted U, may potentially kink, especially when vein is
used as the conduit, if the graft length is not precise.
On the other hand, although exposure of the suprace-
liac aorta may be more time-consuming and technically
challenging, it obviates these limitations. In most patients,
the supraceliac aorta is relatively less diseased, and in
many cases, only a partial side-biting aortic clamp is
required, which limits the increase in afterload and associ-
ated cardiac stress during performance of the proximal
anastomosis. The supraceliac aorta may be exposed either
through the lesser sac after division of diaphragmatic
fibers or through medial visceral rotation, as noted pre-
viously.
An aortotomy is made about 10 to 15 mm in length.
The proximal anastomosis is then performed with 3-0
monofilament synthetic suture. When the aorta is ex-
posed through the lesser sac, the graft is tunneled in
antegrade fashion posterior to the pancreas to the SMA.
In this scenario, we prefer to ligate the SMA proximally
and perform the distal anastomosis in end-to-end fashion
using 6-0 monofilament synthetic suture. In view of the
limited exposure afforded through the lesser sac and the
potential difficulty in tunneling the graft posterior to the
pancreas, however, we prefer approaching the suprace-
liac aorta through the retroperitoneum. In addition to the
superior exposure of the aorta afforded by this approach,
it allows exposure of the proximal portions of both the
SMA and celiac axis so that a relatively short antegrade
bypass graft may be placed in end-to-end fashion to the
SMA, the celiac axis (or one of its branches), or both.
Although there is growing evidence among patients un-
dergoing elective revascularization for chronic mesen-
teric ischemia that long-term results are superior when
revascularization is performed to multiple vessels, in all
but the most unusual cases, bypass of a single mesenteric
artery should be undertaken in critically ill patients with
AMI.
Mesenteric Venous Thrombosis
When MVT is confirmed at exploration, although the
primary treatment for this condition is anticoagulation, if
there appears to be clot in the SMV, thrombectomy
should be attempted. The SMV is controlled inferior to
the pancreas, and a transverse venotomy is performed. A
thromboembolectomy catheter is used to extract clot
from the portal vein, and the more peripheral veins
should be ‘‘milked’’ to extract as much thrombus as possi-
ble. When the thrombotic process involves only the more
distal small venous channels, bowel resection may be the
only option. It is common in MVT for the thrombotic
process to extend well beyond what appears to be the
compromised bowel; therefore, a wide margin of resec-
tion, and a low threshold for a second-look procedure, is
imperative.
Resection
After revascularization, the intestines should be returned
to the abdominal cavity, the anesthesia team should make
28
Volume V
• Mesenteric Circulation
every effort to maximize the patient’s hemodynamic
state, and a period of 30 to 45 minutes of observation
should be allowed before making a definitive assessment
of intestinal viability and the necessity for, and in most
cases extent of, bowel resection. The challenge is to
resect all nonviable bowel while leaving the patient with
sufficient intestinal length to avoid development of the
short bowel syndrome. Clinical signs, such as absence of
peristalsis, bowel wall edema, discoloration of the bowel
and mesentery, mucosal hemorrhage, and absence of
bleeding from cut bowel edges, are imprecise markers
and may lead to resection of intestinal segments that
have potential for recovery. Therefore, a number of more
objective modalities have been used to support this clini-
cal judgment.
A continuous-wave Doppler ultrasound probe can be
used to assess arterial pulsations in the bowel wall. Al-
though rapidly and easily performed, one significant
drawback of this technique is the potential to miss rela-
tively limited patchy segments of bowel wall with inade-
quate perfusion. Nevertheless, in areas of the bowel
where clinical signs are ambiguous, a normal Doppler
signal provides some objective evidence of bowel viabil-
ity. We have found examination of the bowel fluorescence
pattern after administration of fluorescein to be a useful
adjunct in assessing bowel viability. Sodium fluorescein
(1 g) is administered intravenously over 30 to 60 seconds,
and the bowel is then examined with a hand-held long-
wave ultraviolet Wood’s light with the operating room
lights dimmed.
In a prospective study performed at the Johns Hopkins
Hospital, the fluorescein test was found to be 100% accu-
rate, whereas clinical judgment was 89% accurate and
Doppler examination 84% accurate, in predicting bowel
viability.
51
Other investigators, however, have not con-
firmed this degree of accuracy using fluoroscein.
52
This
may relate to the fact that interpretation of the fluores-
cein test, both in terms of the pattern and intensity of
the fluorescence, is somewhat subjective, which is one
drawback of this technique. In addition, the agent may
remain in the circulation for up to 48 hours; hence, this
is a one-time examination and should be performed after
every effort to achieve maximum revascularization has
been performed. A number of other modalities for as-
sessing bowel viability in this setting have been investi-
gated, including pulse oximetry,
53
infrared photoplethys-
mography,
54
and bowel surface oximetry,
55
although there
is minimal clinical experience with these techniques.
All bowel judged to be nonviable must be resected.
Primary anastomoses, especially in the small intestine,
can be performed if brisk bleeding from the edges of
the bowel wall is observed and the patient is stable.
Alternatively, long segments of marginal bowel left in situ
may be stapled or oversewn, with continuity established
during a second-look procedure.
After resection of the colon, creation of a stoma is
generally indicated. An important consideration is the
patient’s hemodynamic status. If cardiac output is com-
promised or there is an ongoing requirement for vaso-
pressors and inotropic support, delay in intestinal recon-
struction may be safer, avoiding the risk for an
anastomotic leak or dehiscence. When the cause is ve-
nous thrombosis, wide intestinal resection should be per-
formed because, as noted previously, venous drainage of
apparently viable bowel may be involved in the patho-
logic process, and there is the potential for extension of
the thrombotic process during the postoperative period.
On the other hand, when the diagnosis is an acute arterial
occlusion or NOMI, and especially when extended seg-
ments of bowel appear marginal, it is best to be more
conservative in performing intestinal resection and plan
to reassess the marginal segments during a second-look
procedure 18 to 24 hours later.
The decision to re-explore the patient should be made
at the time of the original operation, and the surgeon
should not deviate from this plan after leaving the op-
erating room because it has been well demonstrated that
the patient’s early postoperative course, including the
physical examination and laboratory parameters, can be
misleading with respect to the viability of the remaining
intestinal tract.
POSTOPERATIVE CARE
Patients who undergo revascularization for AMI are at
risk for a number of serious and, in many cases, poten-
tially life-threatening complications (Table 3–2). Indeed,
multisystem failure is commonly seen in this patient pop-
ulation, and growing evidence suggests that reperfusion
injury is a major etiologic component of this pathologic
process.
56–58
The primary focus during the early postoper-
ative period must be vigorous cardiopulmonary resuscita-
tion, including aggressive blood and electrolyte-rich fluid
replacement, correction of arrhythmias, and pharmaco-
logic inotropic support, if necessary. This is especially
paramount in patients with NOMI. Metabolic abnormali-
ties, including acidosis, should be corrected in a timely
fashion. Sepsis is common in these patients, and paren-
Table 3–2. Postoperative Complications of Revascularization
Cardiac
Myocardial infarction
Congestive heart failure
Arrhythmias
Pulmonary
Respiratory insufficiency
Pneumonia
Adult respiratory distress syndrome
Renal Failure
Liver Dysfunction
Coagulopathy
Recurrent or Progressive Mesenteric Ischemia
Recurrent embolization
Graft thrombosis
Gastrointestinal Problems
Anastomotic breakdown or fistula
Bleeding
Short bowel syndrome
Chapter 3
• Acute Mesenteric Ischemia
29
teral broad-spectrum antibiotics, with anaerobic cover-
age, should be administered for at least 5 days. Prolonged
gastrointestinal decompression reduces bowel distention,
which can compromise healing of intestinal anastomoses.
Likewise, early institution of parenteral nutrition is indi-
cated in most cases, with careful monitoring of liver
function tests. The issue of formal anticoagulation is more
problematic because the potential benefit of anticoagula-
tion must be weighed against the risk for gastrointestinal
hemorrhage.
Among patients with MVT, even after successful ve-
nous thrombectomy, anticoagulation is the mainstay of
therapy; thus, heparin anticoagulation should be started
at the time the diagnosis is made and continued postoper-
atively. The appropriate duration of long-term warfarin
anticoagulation depends on the underlying cause. Like-
wise, patients who have experienced an embolus to the
mesenteric arterial circulation are at significant risk for
recurrent embolization to the mesenteric, cerebral, and
peripheral circulations; therefore, in most cases, heparin
should be administered. On the other hand, among pa-
tients with NOMI, and even among those who have
experienced an acute arterial thrombosis and undergone
successful revascularization, anticoagulation is generally
not necessary. In the most critically ill patients with AMI
after mesenteric revascularization, a hypocoagulable state
may exist secondary to liver dysfunction, and replen-
ishment of coagulation factors may be necessary to treat
gastrointestinal bleeding.
RESULTS: ACUTE
Although some improvement in outcome has been real-
ized in recent years, AMI continues to extract consider-
able mortality. Even recent series have documented an
overall mortality rate in excess of 60%.
7,59,60
In addition to
the associated comorbidity of the patients so afflicted
and the delay in establishing the correct diagnosis, overall
mortality is significantly influenced by the responsible
etiology. Survival is highest among patients with MVT,
poorest among those who have suffered NOMI, and inter-
mediate when the underlying cause is acute arterial
thrombosis or embolism (see Table 3–1).
Acute Arterial Thrombosis and Embolism
Acute mesenteric arterial occlusion, the most common
cause of AMI, has traditionally been associated with an
acute mortality rate of 70% to 90%.
60,61
Survival appears
superior in the setting of an embolic, as opposed to a
thrombotic, arterial occlusion. In one study, the mortality
rate was 96% among patients with an SMA thrombosis
and 77% among those with an SMA embolus.
62
In a more
recent series of more than 200 patients, the mortality
rate was 92% among 86 patients with an acute mesenteric
arterial thrombosis, compared with 70% among patients
who experienced a mesenteric embolus.
63
In another
recent review, the mortality rate was reported to be less
than 50% among patients who experienced a mesenteric
arterial embolism but 80% among those who suffered a
thrombotic occlusion.
64
It is well recognized that mortality increases with the
extent of bowel ischemia or infarction. In one report, for
example, excessive mortality was associated with leuko-
cytosis, peritonitis, and resection of more than 1.5 m
of intestine.
65
Therefore, the relatively poorer outcome
associated with mesenteric arterial thrombosis in large
measure reflects the greater extent of ischemic injury
secondary to proximal arterial occlusion, whereas emboli
tend to lodge in the more distal vessel, resulting in more
limited bowel infarction. In addition, patients experienc-
ing acute mesenteric arterial thrombosis tend to be older
and have greater comorbidity than those experiencing an
embolic occlusion.
As noted previously, in the absence of frank signs of
peritonitis, emergency mesenteric angiography can be
helpful in identifying the responsible etiology among pa-
tients presenting with AMI. More importantly, there is
evidence that intra-arterial vasodilator therapy may be
associated with better survival. In one series that in-
cluded 47 patients with SMA emboli, this strategy was
associated with a mortality rate of 45%, compared with
80% for cases treated with more conventional means in
this institution.
8
In a more recent report, 61% of patients
who presented with SMA emboli and 67% of those with
SMA thromboses survived after treatment with intra-arte-
rial vasodilator therapy.
7
Nonocclusive Mesenteric Ischemia
Historically, NOMI has been associated with the highest
rates of mortality, ranging from 70% to 90% in some
series.
14,66,67
A reduction in mortality associated with this
condition has occurred in recent years for two reasons.
First, there appears to have been a decline in the inci-
dence of NOMI as a result of greater awareness of this
condition among intensive care unit physicians and the
more liberal administration of intravenous vasodilating
agents in critically ill patients at risk for this mesenteric
vasospastic pathophysiologic process. More importantly,
catheter-directed intra-arterial vasodilator administration
has emerged as the primary treatment of this condition
and has improved outcome among patients so afflicted.
In a recent report, for example, intra-arterial papaverine
administration was associated with an overall mortality
rate of 50% to 55%, a remarkable improvement when
compared with previous series.
68
Mesenteric Venous Thrombosis
MVT, the least common cause of AMI, is also associated
with the lowest risk for mortality, for several reasons
(see Table 3–1). First, a relatively younger and generally
healthier patient population presents with this condition.
Second, recognition of predisposing conditions, the more
indolent course, and the wide availability and accuracy
of CT in establishing the diagnosis allow a significant
fraction of these patients to be treated medically before
bowel infarction occurs (see Fig. 3–4). In one report, for

30
Volume V
• Mesenteric Circulation
example, 11 (69%) of 16 patients with MVT were man-
aged without laparotomy, and the overall mortality rate
was only 12%.
34
In addition, intestinal infarction tends to
involve shorter segments of bowel than mesenteric arte-
rial occlusion. Since 1989, several series have reported a
mortality ranging from 13% to 38%.
24,34,69–71
Mortality ap-
pears to be higher among patients with secondary MVT.
65
Recurrent episodes may be seen in 20% to 25% of pa-
tients, although institution of formal anticoagulation may
reduce the incidence of recurrent MVT by 50%.
23
Further-
more, in a review of nearly 400 cases worldwide, the
combination of surgery and anticoagulation was associ-
ated with superior long-term survival.
72
RESULTS: LONG-TERM
Much less attention has been paid to the long-term out-
come of patients who survive treatment of AMI. This
limited experience, however, has identified a relatively
favorable prognosis. For example, in one report that in-
cluded 20 patients, the 2-year survival rate was 70%.
73
In
another recent series of 31 patients, the survival rates
were 70% and 50% at 2-year and 5-year follow-up, respec-
tively.
74
Mortality appears highest during the first year
after recovery from the acute episode of mesenteric is-
chemia. Predictably, the most common causes of long-
term mortality are cardiovascular. Recurrent bowel ische-
mia has been observed infrequently, however, in part
related to aggressive long-term anticoagulation therapy in
at least one series.
74
Furthermore, a reasonable quality of
life was achieved among most survivors in one report,
with only 38% reporting weight loss and 19% noting
reduced appetite.
74
In one study of 23 patients who
underwent bowel resection for AMI, symptoms of the
short bowel syndrome had developed in only 4 (20%),
and none required parenteral nutrition, with follow-up
extending to 5 years.
74
SUMMARY
Although there has clearly been some improvement in
outcome among patients who present with AMI in con-
temporary practice, this remains a particularly lethal con-
dition. Although the incidence of NOMI may be declining
as a result of refinements in intensive care management
of critically ill patients in recent years, in view of the
aging of our population, one may anticipate stability or
even an increase in the prevalence of mesenteric arterial
occlusive disease in the future, and MVT remains an
important diagnostic consideration, especially in younger
patients with severe abdominal pain. The key to salvage
of the patient with AMI remains recognition of the diag-
nosis and identification of the responsible pathologic eti-
ology expeditiously to allow early intervention. Although
surgical exploration continues to play a central role in the
management of most of these patients, early diagnostic
angiography and administration of catheter-directed intra-
arterial pharmacologic vasodilating agents will continue
to play an adjunctive (and, in some cases, primary) thera-
peutic role. Investigative efforts are focusing on methods
of earlier diagnosis of ischemic bowel preoperatively,
more accurate assessment of bowel viability at laparot-
omy, and the means to promote survival of ischemic but
viable bowel through inhibition of reperfusion injury and
other confounding pathophysiologic insults.
7
References
1. Jarvinen, O., Lqaurikka, J., Sisto, T., et al.: Atherosclerosis of the
visceral arteries. Vasa. 24:1, 9–14, 1995.
2. Kauraluoma, M.I., Karkola, P., Heikinnen, E., et al.: Mesenteric
infarction. Am. J. Surg. 133:188–193, 1977.
3. Rosenblum, J.D., Boyle, C.M., and Schwartz, L.B.: The mesenteric
circulation: Anatomy and physiology. Surg. Clin. North Am.
77
(2):289–306, 1997.
4. Brown, R.A., Chiv, C., Scott, H.J., et al.: Ultrastructural changes in
the canine mucosal cell after mesenteric arterial occlusion. Arch.
Surg. 101:290–297, 1970.
5. Schoeffel, U., Baumgartner, U., Imdahl, A., et al.: The influence of
ischemic bowel wall damage on translocation, inflammatory re-
sponse and clinical course. Am. J. Surg. 174(1):39–44, 1997.
6. Stoney, R.J., and Cunninham, C.G.: Acute mesenteric ischemia.
Surgery 114:489–490, 1993.
7. Kaleya, R.N., Summartano, R.J., and Boley, S.J.: Aggressive approach
to acute mesenteric ischemia. Surg. Clin. North Am. 72(1):157–
182, 1992.
8. Boley, S.J., Brandt, L.J., and Veith, F.J.: Ischemic disorders of the
intestine. Curr. Probl. Surg. 15:1–85, 1978.
9. Howard, T.J., Plaskon, L.A., Wiebke, E.A., et al.: Nonocclusive mes-
enteric ischemia remains a diagnostic dilemma. Am. J Surg.
171
(4):405–408, 1996.
10. McNeill, J.R., Stark, R.D., and Greenway, C.V.: Intestinal vasocon-
striction after hemorrhage: Roles of vasopressin and angiotensin.
Am. J. Physiol. 219:1342–1347, 1970.
11. Clark, E.T., and Gewerz, B.L.: Intermittent ischemia potentiates
intestinal reperfusion injury. J. Vasc. Surg. 13:606–61, 1991.
12. Bryant, D.S., Pellicane, J.V., and Davies R.S.: Nonocclusive intestinal
ischemia: Improved outcome with early diagnosis and therapy. Am.
Surg. 63(4):334–337, 1997.
13. Bailey, R.W., Bulkley, G.B., and Hamilton, S.R.: Protection of the
small intestine from non-occlusive mesenteric ischemia. Am. J. Surg.
153:
108–116, 1987.
14. Kim, E.H., and Gewertz, B.L.: Chronic digitalis administration alters
mesenteric vascular reactivity. J. Vasc. Surg. 5:382–389, 1987.
15. Britt, L.G., and Cheek, R.C.: Non-occlusive mesenteric vascular
disease: Clinical and experimental observations. Ann. Surg.
169:
704–711, 1969.
16. Ferrer, M., and Bradley, S.: The effects of digoxin in the splanchnic
circulation in ventricular failure. Circulation 32:524–537, 1965.
17. Hoang, M.P., Lee, E.L., and Anand, A.: Histologic spectrum of arte-
rial and arteriolar lesions in acute and chronic cocaine-induced
mesenteric ischemia: Report of three cases and literature review.
Am. J. Surg. Pathol. 22(11)1404–1410, 1998.
18. Herrine, S.K., Park, P.K., and Wechsler, R.J.: Acute mesenteric ische-
mia following intranasal cocaine use. Dig. Dis. Sci. 43(3):586–589,
1998.
19. Wattoo, M.A., and Osundeko, O.: Cocaine-induced intestinal ische-
mia. West. J. Med. 170(1):47–49, 1999.
20. Harris, M.T., and Lewis, B.S.: Systemic diseases affecting the mesen-
teric circulation. Surg. Clin. North Am. 72:245–261, 1992.
21. Pettei, M.J., Levy, J., and Abramson, S.: Non-occlusive mesenteric
ischemia associated with propranolol overdose: Implications regard-
ing splanchnic circulation. J. Pediatr. Gastroenterol. Nutr. 10:544–
547, 1990.
22. Rhee, R.Y., Gloviczki, P., Medonca, C.T., et al.: Mesenteric venous
thrombosis: Still a lethal disease in the 1990s. J. Vasc. Surg. 20:688–
697, 1994.
23. Gennaro, M., Ascer, E., Matano, R., et al.: Acute mesenteric ischemia
after cardiopulmonary bypass. Am. J. Surg. 166:231–236, 1993.
24. Allen, K.B., Salam, A.A., and Lumsden, A.B.: Acute mesenteric
ischemia after cardiopulmonary bypass. J. Vasc. Surg. 16:391–396,
1992.

Chapter 3
• Acute Mesenteric Ischemia
31
25. Klempnauer, J., Grothues, F., Bektas, T.H., et al.: Acute mesenteric
ischemia following cardiac surgery. J. Cardiovasc. Surg. 38:639–
643, 1997.
26. Rhee, R.Y., and Gloviczki, P.: Mesenteric venous thrombosis. Surg.
Clin. North Am. 77(2):327–338, 1997.
27. Thompson, J.S., Bragg, L.E., and West, W.W.: Serum enzyme levels
during intestinal ischemia. Ann. Surg. 211:369–373, 1990.
28. Smerud, M.J., Johnson, C.D., and Stephens, D.H.: Diagnosis of
bowel infarction: A comparison of plain films and CT scans in 23
cases. Am. J. Roentgenol. 154:99–103, 1990.
29. Tomchick, F.S., Wittenberg, J., and Ottinger, L.W.: The roentgenolo-
gic spectrum of bowel infarction. Radiology 96:249–260, 1970.
30. Miller, V.E., and Beland, L.L.: Pulsed Doppler duplex sonography
and CT of portal vein thrombosis. Am. J. Roentgenol. 145:73–76,
1985.
31. Keen, R.R., Yao, J.S., Astleford, P. et al.: Feasibility of transgastric
ultrasonography of the abdominal aorta. J. Vasc. Surg. 24(5):834–
842, 1996.
32. Taourel, P.G., Deneuville, M., Pradel, J.A.: Acute mesenteric ische-
mia: diagnosis with contrast enhanced CT. Radiology 199(3):632–
636, 1996.
33. Rahmouni, A., Mathieu, D., Golli, M., et al.: Value of CT and
sonography in the management of acute spleno-renal and superior
mesenteric venous thrombosis. Gastrointest. Radiol. 17(2):135–
140, 1992.
34. Harward, T.R.S., Green, D., Bergan, J.J., et al: Mesenteric venous
thrombosis. J. Vasc. Surg. 9:328–333, 1989.
35. Vogelzang, R.L., Gore, R.M., and Anschnetz, S.L.: Thrombosis of the
splanchnic veins: CT diagnoses. Am. J. Roentgenol. 150:93–96,
1988.
36. Gehl, H.B., Bohndorf, K., Klose, K.C., et al.: Two-dimensional MR
angiography in the evaluation of abdominal veins with gradient
refocused sequences. J. Comput. Assist. Tomogr. 14(4):319–324,
1990.
37. Boley, S.J., Feinstein, F.R., Sammartano, R., et al.: New concepts
in the management of superior mesenteric artery embolus. Surg.
Gynecol. Obstet. 153:561–566, 1981.
38. Bakal, C.W., Sprayregen, S., and Wolf, E.L.: Radiology in intestinal
ischemia: Angiographic diagnosis and management. Surg. Clin.
North Am. 72(1):125–141, 1992.
39. McKinsey, J.F., and Gewertz, B.L.: Acute mesenteric ischemia. Surg.
Clin. North Am. 77(2):307–318, 1997.
40. Jamieson, A.C., Thomas, R.J.F., and Cade, J.F.: Lysis of a superior
mesenteric artery embolus following local infusion of streptokinase
and heparin. Aust. N. Z. J. Surg. 49:355–356, 1979.
41. Flickinger, E.J., Johsrude, I.S., Ogburn, N.L., et al.: Local streptoki-
nase infusion for superior mesenteric artery thromboembolism.
Am. J. Roentgenol. 140:771–773, 1983.
42. Regan, F., Karlstad, R.R., and Magnuson, T.H.: Minimally invasive
management of acute superior mesenteric artery occlusion: Com-
bined urokinase and laparoscopic therapy. Am. J. Gastroenterol.
91
(5):1019–1021, 1996.
43. Rivitz, S.M., Geller, S.C., Hahn, C., and Waltman, A.C.: Treatment
of acute mesenteric venous thrombosis with transjugular intrames-
enteric urokinase infusion. J. Vasc. Interv. Radiol. 6(2):219–223,
1995.
44. Rijs, J., Depreitere, B., Beckers, A., et al.: Mesenteric venous throm-
bosis: diagnostic and therapeutic approach. Acta. Chir. Belg.
97
(5):247–249, 1997.
45. Train, J.S., Ross, H., Weiss, J.D., et al.: Mesenteric venous thrombo-
sis: Successful treatment by intraarterial lytic therpy. J. Vasc. Interv.
Radiol. 9(3):461–464, 1998.
46. Poplausky, M.R., Kaufman, J.A., Geller, S.C., et al.: Mesenteric ve-
nous thrombosis treated with urokinase via the superior mesenteric
artery. Gastroenterology 110(5):1633–1635, 1996.
47. Walsh, R.M., Popovich, M.J., and Hoadley, J.: Bedside diagnostic
laparoscopy and peritoneal lavage in the intensive care unit. Surg.
Endosc. 12(12):1405–1409, 1998.
48. VanDeinse, W.H., Zawacki, J.K., and Phillips, D.: Treatment of acute
mesenteric ischemia by percutaneous transluminal angioplasty. Gas-
troenterology 91:475–478, 1986.
49. Matsumoto, A.H., Angle, J.F., and Tegtmeyer, C.J.: Mesenteric angio-
plasty and stenting for chronic mesenteric ischemia. In Perler, B.A.,
and Becker, G.J. (eds.): Vascular Intervention: A Clinical Approach.
New York, Thieme, 1998.
50. Williams, D.M., Brothers, T.E., and Messina, L.E.: Relief of mesen-
teric ischemia in type III aortic dissection with percutaneous fenes-
tration of the aortic septum. Radiology 174:450–452, 1990.
51. Bulkley, G.B., Zuidema, G.D., Hamilton, S.R., et al.: Intraoperative
determination of small intestinal viability following ischemic injury.
Ann. Surg. 193:628–637, 1981.
52. Ballard, J.L., Stone, W.M., and Hallett, J.W.: A critical analysis of
adjuvant techniques used to assess bowel viability in acute mesen-
teric ischemia. Ann. Surg. 59(5):309–311, 1993.
53. Denobile, J., Guzzetta, P., and Patterson, K.: Pulse oximetry as a
means of assessing bowel viability. J. Surg. Res. 48:21–23, 1990.
54. Pearce, W.H., Joness, D.N., Warren, G.H., et al.: The use of infrared
photoplethysmography in identifying early intestinal ischemia.
Arch. Surg. 193:628–637, 1987.
55. Ferrara, J., Dyess, D., Lasecki, M., et al.: Surface oximetry: A new
method to evaluate intestinal perfusion. Am. Surg. 54:10–14, 1988.
56. Harward, T.R.S., Brooks, D.L., Flynn, T.C., et al.: Multiple organ
dysfunction after mesenteric artery revascularization. J. Vasc. Surg.
18:
459–469, 1993.
57. Granger, D.N., Rutili, G., McCord, J.M., et al.: Superoxide radicals
in feline intestinal ischemia. Gastroenterology 81:22–29, 1981.
58. Parks, D.A., Bulkley, G.B., Granger, D.N., et al.: Ischemic injury to
the cat small intestine: Role of superoxide radicals. Gastroenterol-
ogy 82:9–15, 1982.
59. Heys, S.D., Brittenden, J., and Crofts, T.J.: Acute mesenteric ische-
mia: The continuing difficulty in early diagnosis. Postgrad. Med. J.
69:
48–51, 1993.
60. Klempnauer, J., Grothues, F., Bektas, H., and Pichlmayr, R.: Long-
term results after surgery for acute mesenteric ischemia. Surgery
121:
239–243, 1997.
61. Hunter, G.C., and Guernsey, J.M.: Mesenteric ischemia. Med. Clin.
North Am. 72:1091–1115, 1988.
62. Ottinger, L.W.: The surgical management of acute occlusion of the
superior mesenteric artery. Ann. Surg. 188:721–731, 1978.
63. Taylor, L.M., and Monetta, G.L.: Basic data underlying decision
making in vascular surgery: Intestinal ischemia. Ann. Vasc. Surg.
5:
403–406, 1991.
64. Ricotta, J.J., and d’Audiffret A.: Acute mesenteric occlusion. In
Cameron, J.L. (ed.): Current Surgical Therapy, 6th ed. St. Louis, C.V.
Mosby, 1998.
65. Clavien, P.A., Muller, C., and Harder, F.: Treatment of mesenteric
infarction. Br. J. Surg. 74:500–503, 1987.
66. Berger, R.L., and Byrne, J.J.: Intestinal gangrene associated with
heart disease. Surg. Gynecol. Obstet. 113:522–529, 1961.
67. Wilson, G.S.M., and Block, J.: Mesenteric vascular occlusion. Arch.
Surg. 73:330–345, 1956.
68. Bassiouny, H.S.: Nonocclusive mesenteric ischemia. Surg. Clin.
North Am. 77:319–326, 1997.
69. Boley, S.J., Kaleya, R.N., and Brandt, L.J.: Mesenteric venous throm-
bosis. Surg. Clin. North Am. 72:183–201, 1992.
70. Levy, P.J., Krausz, M.M., and Manny, J.: The role of second-look
procedure in improving survival time for patients with mesenteric
venous thrombosis. Surg. Gynecol. Obstet. 170:287–291, 1990.
71. Grieshop, R.J., Dalsing, M.C., Ckirit, D.F., et al.: Acute mesenteric
venous thrombosis: Revisited in a time of diagnostic clarity. Am.
Surg. 57:573–578, 1991.
72. Abdu, R., Zakhour, B.J., and Dallis, D.J.: Mesenteric venous thrombo-
sis: 1971 to 1984. Surgery 101:383–388, 1987.
73. Bottger, T., Schafer, W., and Jungiger, T.: Eine prospektive studie zur
evaluiering des postoperativen risikos sowie der langzeitprognose
des mesenterialinfarkts. Med. Klin. 86:198–203, 1991.
74. Klempnauer, J., Grothues, F., Bektas, H., et al.: Long-term results
after surgery for acute mesenteric ischemia. Surgery 121:239–243,
1997.
Wyszukiwarka
Podobne podstrony:
Acute mesenteric ischemia Text 02
Acute extremities ischemia Text
Visceral Ischemia Text 01
text 03 06 13
text 17 03 13(1)
text 17 03 13
2001 03 Using the Text Editor Joe
03 Sejsmika04 plytkieid 4624 ppt
03 Odświeżanie pamięci DRAMid 4244 ppt
podrecznik 2 18 03 05
od Elwiry, prawo gospodarcze 03
Probl inter i kard 06'03
TT Sem III 14 03
03 skąd Państwo ma pieniądze podatki zus nfzid 4477 ppt
03 PODSTAWY GENETYKI
więcej podobnych podstron