
Biomaterials 23 (2002) 4523–4531
A bone substitute composed of polymethylmethacrylate
and a-tricalcium phosphate: results in terms of osteoblast
function and bone tissue formation
Milena Fini
a,
*, Gianluca Giavaresi
a
, Nicol
"o Nicoli Aldini
a
, Paola Torricelli
a
,
Rodolfo Botter
b
, Dario Beruto
b
, Roberto Giardino
a,c
a
Servizio di Chirurgia Sperimentale, Istituto di Ricerca Codivilla-Putti, Istituti Ortopedici Rizzoli via di Barbiano 1/10, 40136 Bologna, Italy
b
Dipartimento di Edilizia Urbanistica e Ingegneria dei Materiali, Universit
"a di Genova, Pzz le JF Kennedy-Pad D, 16129 Genova, Italy
c
Fisiopatologia Chirurgica, Facolt
"a di Medicina e Chirurgia, Universit"a di Bologna via di Barbiano 1/10, 40136 Bologna, Italy
Received 21 December 2001; accepted 16 May 2002
Abstract
The biological properties of a composite polymeric matrix (PMMA+a-TCP) made of polymethylmethacrylate (PMMA) and
alfa-tricalciumphosphate (a-TCP) was tested by means of in vitro and in vivo investigations. PMMA was used as a comparative
material. Osteoblast cultures (MG 63) demonstrated that PMMA+a-TCP significantly and positively affected osteoblast viability,
synthetic activity and interleukin-6 level as compared to PMMA. At 12 weeks, the PMMA+a-TCP implants in rabbit bone
successfully osteointegrated in trabecular and cortical tissue (affinity index: 57.14
78.84% and 68.3176.18%, respectively). The
newly formed bone after tetracycline labelling was histologically observed inside PMMA+a-TCP porosity. The microhardness test
at the bone–PMMA+a-TCP interface showed a significantly higher rate of newly formed bone mineralization compared with
PMMA (+83.5% and +58.5%, respectively), but differences still existed between newly formed and pre-existing normal bone. It is
herein hypothesized that the present positive results may be ascribed to the porous macroarchitecture of PMMA+a-TCP and the
presence of the bioactive ceramic material that could have a synergic effect and be responsible for the improvement of (a) the
material colonization by bone cells, (b) osteoblast activity, (c) osteoinduction and osteoconduction processes, (d) bone remodelling.
r
2002 Elsevier Science Ltd. All rights reserved.
Keywords: Bone substitutes; a-tricalciumphosphate; Polymethylmethacrylate; Biocompatibility; Osteoblasts; Osteointegration; Osteoinduction
1. Introduction
The need for bone substitutes is rapidly increasing in
the field of orthopaedic surgery, since advanced
procedures are now being performed in reconstructive
surgery after traumatic pathologies and iatrogenic bone
losses secondary to bone resections for tumours,
infections or pseudoarthroses. Moreover, the increasing
number of elderly patients or individuals with various
systemic pathologies and biological drawbacks related
to bone healing processes, often requires the use of bone
substitutes as an adjuvant therapy to be associated with
prosthetic implants in order to improve biological
fixation and osteointegration processes [1,2].
So far autologous bone has been considered as the
most effective bone substitute because of its osteocon-
ductive and osteoinductive properties [3]. However, its
limited availability and second site harvest morbidity
limit its application in favour of other biological bone
substitutes, such as banked bone and derivatives (i.e.
demineralised and morcelized bone), which unfortu-
nately have less osteoconductive capacity and poor
mechanical characteristics [3–5]. Additionally, synthetic
materials rarely show all the fundamental characteristics
of an optimal bone substitute: biocompatibility, os-
teoinductive or osteoconductive properties (bioactivity),
and biomechanical similarity to bone. Moreover, the
possibility that bone substitutes may release antibiotic
or chemotherapeutic agents seems to be greatly
*Corresponding author. Tel.: +39-51-6366557; fax: +39-51-
6366580.
E-mail address:
milena.fini@ior.it (M. Fini).
0142-9612/02/$ - see front matter r 2002 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 2 ) 0 0 1 9 6 - 5

appreciated by clinicians who search for improved anti-
infective or antitumoral therapies [6,7]. All of the above
features have rarely been found in one single material
and composite materials seem therefore to be the most
suitable for clinical applications.
Polymethylmethacrylate
(PMMA)
has
been
im-
planted in bone since 1960 [8] to improve implant
fixation. Although undesired side effects related to its in
vitro and in vivo application are well known [9–11], its
use is an effective way to improve primary implant
stability in patients with poor bone stock (revision
arthroplasty or osteoporosis), and prevents fractures
secondary to bone rarefaction [12–14].
Osteointegration of PMMA is known to be poor
[15,16] and its effect on enhancing bone attachment is
only mechanical. Consequently, many studies have
taken into consideration both mechanical and biological
properties, such as osteoconduction and osteoinduction,
and have obtained bioactivated PMMA by adding
bioactive materials to PMMA [17–21]. Moreover, the
addition of bioactive and resorbable materials to
PMMA may also improve its physical characteristics,
thus obtaining a porous composite with osteogenetic
activity [22].
Tricalcium phosphates (TCP) are frequently used as
bone substitutes. a-TCP, in particular, is known to be
biocompatible, osteoconductive, osteoinductive and
with a high biodegradation rate [23–25]. A porous
matrix of PMMA has already been developed in the past
where capillary cavities were obtained by adding a-TCP
powders as an aqueous dispersion to the matrix, as
described in previous papers [26,27]. The aim was to
create a porous and bioactive polymeric composite
whose resorbable ceramic should be slowly replaced
with natural bone. The total open porosity of the
material was found to be a function of the amount of
water added. The water, which is the pore-forming
agent, vaporises after the polymerisation process,
leaving behind empty spaces in the polymeric matrix
[26,27]. The initial characterisation of the material
demonstrated that it was comparable to the porous
bioceramics
currently
used
as
bone
substitute
compounds in terms of mechanical properties. The
obtained macrostructure could also promote osteoblast
and vascular colonization towards and inside the
material [27].
The aim of the present work was to evaluate the
biological characteristics of the biomaterial developed.
The
investigation
was
conducted
both
in
vitro
and in vivo using osteoblast-like cells and rabbits for
inserting bone implants in cortical and trabecular
bone. The viability test, biochemical and cytokine
dosages were performed on osteoblasts, and histomor-
phometry and microhardness were used to evaluate
PMMA+a-TCP osteointegration in comparison with
PMMA.
2. Materials and methods
2.1. Materials
A two-component bone cement currently used in
orthopaedic surgery [28] and previously decribed [26],
was used for the composite polymeric matrix through-
out this study. The prepolymeric component in bone
cement is constituted of PMMA spherical particles with
an appropriate size distribution [29] allowing a very low
use of monomer, about 25% of the total mass of the
bone cement.
A solid-state reaction between CaCO
3
and CaH-
PO
4
2H
2
O powders was carried out to produce a-TCP
at 1573 K [30,31]. Once the reaction was completed, a
rapid quenching treatment was performed to stabilize
the a-phase. The final solid product was sieved to
eliminate agglomerates greater than 250 mm. The phase
composition of the calcium phosphate powders used was
controlled by X-ray diffraction analysis using a Philips
PW 1050/81 powder diffractometer with CuKa radia-
tion (40 mA, 40 kV). All the peaks in the resulting
spectra belong to a-TCP phase. No traces of impurities
were recorded. The powder has particles of irregular
shape, somewhat elongated and interconnected with an
average width of about 10 mm.
PMMA and PMMA+a-TCP slabs for in vitro test
and nails for bone implant test were prepared as
described below.
Dense PMMA samples were prepared by adding the
methylmethacrylate
monomer
(MMA)
(33.3%
by
weight) to the PMMA prepolymerised powders. The
mixture was stirred until a smooth paste was formed.
The resulting dough was placed between two glass
slides and squeezed to form a 1 mm-thick slab. The
slab were marked following a 10 mm 10 mm square
array, with a blade of stainless steel before that
polymerisation was complete. After the curing time the
square chips of polymer were broken apart and stored in
a desiccator.
The porous PMMA+a-TCP composites were pre-
pared using the following method. Three grams of
calcium phosphate powders were mixed with distilled
water to obtain a dispersion with a concentration of
solid equal to 20% v/v, its weight was checked, and it
was then mixed with the previously prepared concen-
trated organic mixture (MMA and PMMA) [32] for 40 s.
The amount of PMMA and MMA to be mixed with the
cake was so calculated as to ensure the same amount of
dry calcium phosphate phase for all final composite
samples, i.e. 25.2% dry weight. The resulting organic/
inorganic dough was folded in on itself 6 times. This
method yielded a paste with a sufficient degree of
homogeneity. Finally, the paste was moulded into a
1 mm-thick slab and cut in a square shape as described
above and stored in desiccator.
M. Fini et al. / Biomaterials 23 (2002) 4523–4531
4524

A different moulding procedure was adopted to
produce PMMA+a-TCP composites and pure PMMA
samples (cylindrical nails) for bone implants. Glass tube
segments of about 12 mm in length and 2 mm in inner
diameter were filled with a polymerising composite
dough with the aid of a spatula. The paste was then
slightly pressed with two glass rods, after about 5 min
was pulled out from the moulds and stored in
desiccator. The nails were cut into a length of 6 mm.
The water, which was initially present in the
phosphate dispersion, was totally removed by treatment
in desiccator leaving a percolating pore network in its
place [26].
Both slabs and nails were sterilised with ethylene
oxide before their use.
2.2. In vitro tests
An MG-63 human osteoblast cell line was used. Cells
were maintained in DMEM supplemented with 10%
FCS, 100 IU/ml penicillin and 100 mg/ml streptomycin
solution, and placed in a cell incubator with 5% CO
2
at
371C until confluence. After the cells had formed the
monolayer, they were detached with 0.05% (w/v) trypsin
and 0.02% (w/v) EDTA, counted (cell Coulter) and
seeded onto 24-multiwell plates at the concentration of
1 10
4
cells/ml. Cells were cultured in contact with
PMMA+a-TCP (PMMA+a-TCP Group) and PMMA
(PMMA Group) specimens (10 mm 10 mm). Cells
cultured with no material were used as negative controls
(Polystyrene Group). After 72 h the following tests were
done: MTT (Sigma, UK), bone alkaline phosphatase (B-
ALP, Alkphase-B immunoassay, Metra Biosystems.
CA, USA), lactate dehydrogenase (LDH, Sigma kit,
UK), nitric oxide (NO, Sigma calorimetric assay, St
Louis, MO, USA), osteocalcin (OC, Novocalcin enzyme
immunoassay kit, Metra Biosystem, CA, USA), pro-
Collagen (PICP, Prolagen-C enzyme immunoassay kit,
Metra Biosystem, CA, USA), interleukin 6 (IL-6,
Human IL-6 Immunoassay Kit, Biosource Int, CA,
USA), Transforming Growth Factor-b1 (TGF-b1,
Quantikine human TGF-b1 Immunoassay, R&D Sys-
tems, MN, USA).
2.3. In vivo tests
The in vivo study was performed following European
and Italian Law on animal experimentation and
according to the Animal Welfare Assurance #A5424-
01 by the National Institute of Health (NIH-Rockville,
MD, USA). The experimental protocol was sent to the
Italian Ministry of Health.
2.3.1. Bone implants
Cylindrical nails, 2 mm in diameter and 6 mm in
length, were implanted in the femoral condylar trabe-
cular and diaphyseal cortical bone of 12 adult male New
Zealand rabbits (b.w. 3.250
70.350 kg). Both femurs
were used. The nails were made of PMMA+a-TCP and
PMMA (control material).
General anaesthesia was induced with an i.m. injec-
tion of 44 mg/kg ketamine (Ketavet 100, Farmaceutici
Gellini SpA, Aprilia Lt, Italy) and 3 mg/kg xylazine
(Rompun Bayer AG, Leverkusen, Germany), and
assisted ventilation (O
2
: 1 l/min; N
2
O: 0.4 l/min; iso-
fluorane: 2.5–3%). The distal femurs and middiaphyses
were exposed and 2 defects with a 1.9 mm diameter were
drilled at low speed and under continuous saline
irrigation in the trabecular (distal femurs) and cortical
(middiaphyses) bone of the right and left femurs. Using
press-fit techniques, PMMA cylinders were transversally
implanted in the left femurs of all rabbits, while
PMMA+a-TCP cylinders were positioned in the right
femurs, up to a total of 12 trabecular and 12 cortical
implants. Finally, the skin was sutured in 2 layers.
Antibiotic therapy (Cefazolin, 100 mg/kg) was adminis-
tered preoperatively, immediately after surgery and after
24 h. Analgesics (metamizole chloride, 50 mg/kg) were
prescribed in the immediate postoperative period. Seven
days prior to sacrifice, the animals received an i.m.
injection of oxytetracycline (30 mg/kg). Twelve weeks
after surgery, the animals were sacrificed by pharmaco-
logical euthanasia under general anaesthesia with
intravenous administration of Tanax (Hoechst, Frank-
furt am Main, Germany).
Femurs were removed, cleaned of soft tissues and
prepared for histomorphometry and microhardness test.
2.4. Histomorphometry
The distal parts of the middiaphyses and of the
femoral condylar trabecular bone were fixed in 4%
buffered paraformaldehyde for 48 h for undecalcified
bone processing. Each part contained an implant. The
samples were then dehydrated in graded series of
alcohols until the absolute was reached. Finally, they
were embedded in epoxy resin (Struers Co., Copenha-
gen, Denmark). Blocks were sectioned along a plane
perpendicular to the bone surface and a series of
sections of about 30 mm in thickness, spaced 200 mm
apart, were obtained with a Leica 1600 diamond saw
microtome (Leica SpA, Milan, Italy). They were stained
with Fast Green, and were processed for routine
histological and histomorphometric analyses by using
a transmission and polarized light Axioskop Microscope
(Carl Zeiss GmbH, Jena, Germany) and a computerized
image analysis system with Kontron KS 300 software
(Kontron Electronic GmbH, Eiching bei Munchen,
Germany). Bone histomorphometry measurements were
taken semi-automatically on three sections for each
samples. Affinity Index (AI: the length of bone directly
opposed to the implant without the presence of a fibrous
M. Fini et al. / Biomaterials 23 (2002) 4523–4531
4525
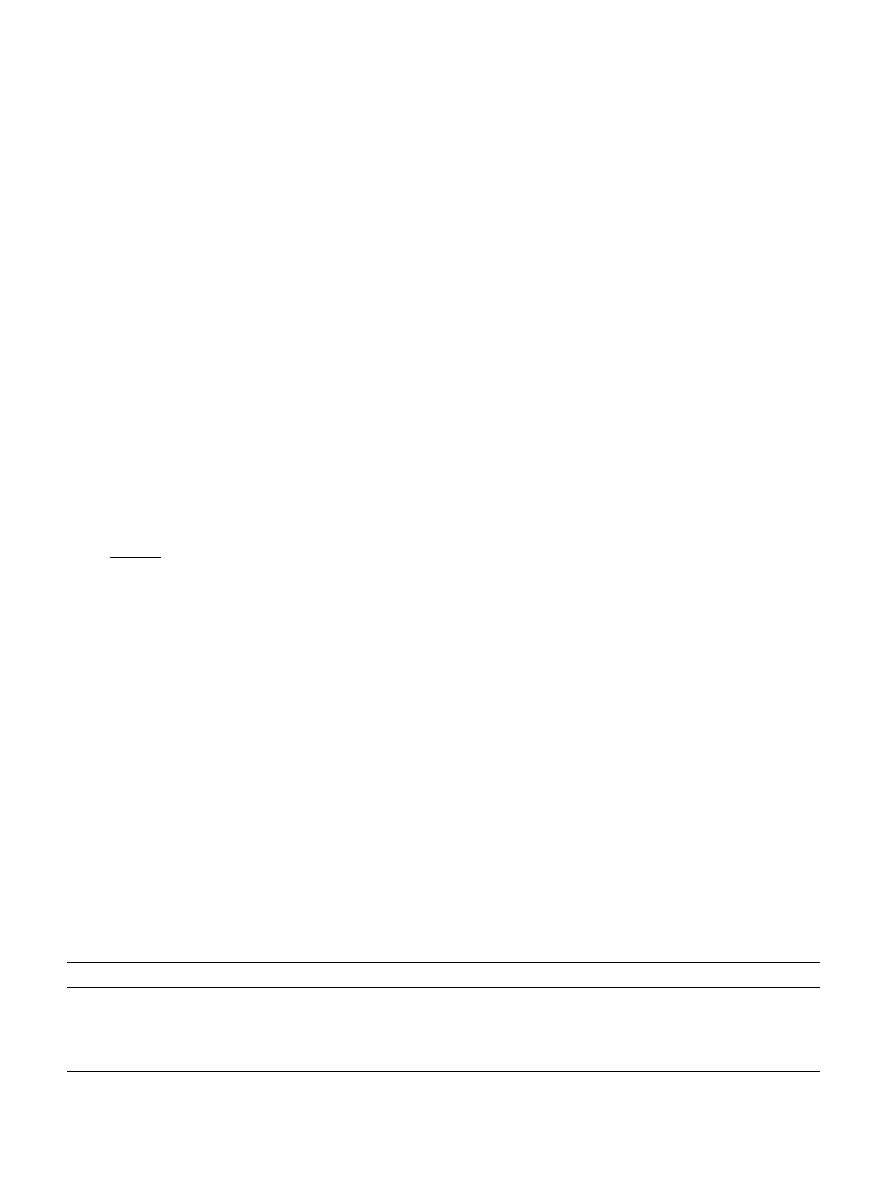
membrane/the total length of the bone–implant inter-
face multiplied by 100) and the newly formed bone
inside the implanted materials (the bone area/the total
implant area multipled by 100) were calculated at
12.5 . Each measurement was performed semiautoma-
tically by a blinded investigator.
2.5. Microhardness
After the histological analysis, the resin-embedded
blocks containing the residual part of the implanted
materials were ground and polished, and used to
measure the level of bone hardness by means of an
indentation test (Microhardness VMHT 30, Leica,
Wien, Austria). The microhardness measurements were
performed in the tangential direction to the interface
with a Vickers indenter (four-sided pyramid with square
base and an apex angle between opposite sides of
1361
715
0
) applied at a load of 0.05 kgf and dwell time of
5 s to the cortical and cancellous bone. The Vickers
hardness degree (HV) was calculated by dividing the
indentation force by the surface of the imprint (4
pyramid surfaces) observed at the microscope. The
resulting formula was
HV ¼
2F sin b
d
2
;
where F is the weight applied to the pyramid expressed
in kg, b is half of the pyramid angle, and d is the average
diagonal length of the imprint expressed in mm. The
average value for each sample was calculated on a mean
of 10 for each examined area (5 for each implant side) at
the following sites: within 200 mm from the interface and
at 1000 mm from it. A minimum distance of about 3d
was allowed between the imprints to avoid their mutual
influence.
2.6. Statistical analysis
Statistical analysis was performed using SPSS v.10.1
software (SPSS/PC Inc., Chicago, IL). Data are
reported as mean
7SD at a significance level of
p
o0:05: After having verified normal distribution and
homogeneity of variances, analysis of variance (ANO-
VA) and Scheff
!e’s post hoc multiple comparison tests
were done in order to highlight any significant in vitro
difference between groups. The student’s t-test was used
to compare in vivo data between the PMMA+a-TCP
and PMMA groups (histomorphometry and microhard-
ness).
3. Results
3.1. In vitro tests
One-way ANOVA showed significant differences
between groups for all biochemical parameters, except
for B-ALP, NO and TGF-b1 as reported in Table 1 and
Fig. 1. Significant decreases in MTT of about 26–29%
were found in MG63 cultures plated with PMMA when
compared
to
the
Polystyrene
(p
o0:001)
and
PMMA+a-TCP (p
o0:0005) groups. In the same way,
B-ALP, OC, PICP and TGF-b1 decreased in the
PMMA group as compared to other groups, while
LDH and IL-6 increased. The Scheff
!e’s test showed
significant decreases in OC and PICP of the PMMA
group compared to the Polystyrene (OC: 11%,
p
o0:0005; PICP: 27%, po0:005) and PMMA+a-
TCP (OC: 14%, p
o0:0005; PICP: 22%, po0:05)
groups. In the PMMA group, LDH (Table 1) increased
significantly of about 49% (p
o0:0005) when compared
to the other groups, while IL-6 (Fig. 1) increased of
about 35% (p
o0:001) and 16% (po0:01) compared to
the Polystyrene and PMMA+a-TCP groups, respec-
tively.
3.2. In vivo tests
All animals survived without any local or general
complications until the scheduled experimental time.
3.3. Histomorphometry
In PMMA+a-TCP implants the histological study
demonstrated that the surface of the material was
covered with newly formed bone, and direct bone
Table 1
In vitro study on bone cells after a 72-h culture without any material (polystyrene Group) and with PMMA and PMMA
6 -TCP. Mean7SD, n ¼ 5
triplicates
MG63
MTT (OD 550 nm)
B-ALP
LDH
NO (mmol)
OC (ng/ml)
PICP (ng/ml)
Polystyrene
0.919
70.039
8.47
71.35
13.47
70.74
5.74
70.30
21.46
70.70
15.83
70.27
PMMA
0.677
70.116
a,b
6.47
70.53
20.14
70.96
c
5.86
70.36
19.00
70.68
c
11.50
72.47
d,c
PMMA
6 -TCP
0.960
70.057
8.01
71.87
13.61
70.67
5.95
70.13
22.02
70.25
14.82
71.26
ANOVAF, p
19.46,
o0.0005
2.85, ns
113.05,
o0.0005
0.73, ns
38.06,
o0.0005
9.88,
o0.005
Scheff
!e’s post hoc multiple comparison test:
a
, p
o0.001 vs Polystyrene;
b
, p
o0.005 vs PMMA
6 -TCP;
c
, p
o0.005 vs others;
d
, p
o0.005 vs
Polystyrene;
e
, p
o0.05 vs PMMA
6 -TCP.
M. Fini et al. / Biomaterials 23 (2002) 4523–4531
4526
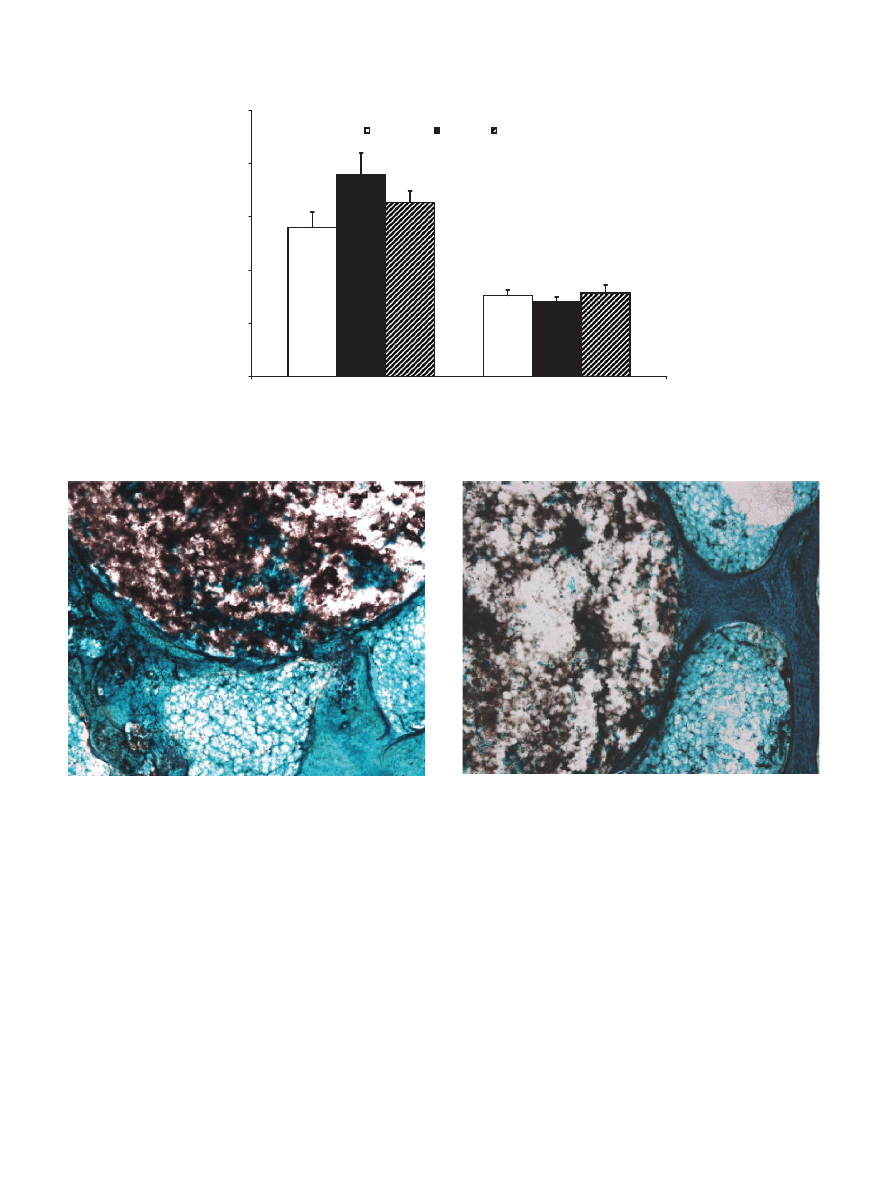
bonding was observed both in cancellous and in cortical
bone. In PMMA implants an intervening fibrous tissue
layer of 40
75 mm in thickness was observed in various
areas at the interface, both in cancellous and in cortical
bone. Some pictures of the PMMA+a-TCP implants in
cancellous bone are shown in Figs. 2 and 3, while the
histological appearance in cortical bone is shown in
Figs. 4 and 5. Fig. 6 depicts the presence of newly
formed bone inside the porosity of PMMA+a-TCP
implanted in trabecular bone.
AI results for both cortical and trabecular bone
revealed significantly (p
o0:0005) higher percentages for
PMMA+a-TCP Versus PMMA (Table 2). The newly
formed bone regrown inside the porosity of PMMA+a-
TCP averaged 18.33
72.85% and 8.3374.88% in
trabecular and cortical bone, respectively.
3.4. Microhardness
Table 3 reports the microhardness measurements
taken at the bone–biomaterial interface and in the pre-
existing bone. The microhardness values of the cortical
bone adjacent to the implant surface and at 1000 mm
from the implant were significantly higher than those of
the trabecular bone for both materials. Additionally,
there were significant differences between data measured
at 200 and 1000 mm for both materials and both tissues.
Bone at the interface was significantly less hard than the
Fig. 2. PMMA+a-TCP implanted in cancellous bone. Direct apposi-
tion of bone to the material surface. (Undecalcified section, Fast
Green, 4 .)
Fig. 3. PMMA+a-TCP implanted in cancellous bone. A bone
trabecula is rounding the material surface. (Undecalcified section,
Fast Green, 4 .)
0
50
100
150
200
250
pg/ml
Polystyrene
PMMA
PMMA+aTCP
IL-6 (pg/ml)
TGF-
β
1 (*10 pg/ml)
a
b
Fig. 1. In vitro study on bone cells after a 72 h culture without any material (Polystyrene) and with PMMA and PMMA+a-TCP. Data on IL-6 and
TGF-b1. Mean
7SD, n ¼ 5 triplicates. Scheff!e’s post hoc multiple comparison test: PMMA versus Polystyrene (
a
, p
o0:001); PMMA versus
PMMA+a-TCP (
b
, p
o0:01).
M. Fini et al. / Biomaterials 23 (2002) 4523–4531
4527

pre-existing healthy bone (PMMA: cortical bone: –55%,
p
o0:0005;
trabecular
bone:
–53%,
p
o0:001;
PMMA+a-TCP: cortical bone: 26%, p
o0:001; tra-
becular bone: 12%, p
o0:05). Both trabecular and
cortical bone revealed significant decreases (p
o0:0005)
at 200 mm both for the PMMA and PMMA+a-TCP
groups (cortical bone: 37%; trabecular bone: 46%).
4. Discussion
Among the various characteristics required for a bone
substitute to be used in reconstructive orthopaedic
surgery, the capability to improve cell colonization
inside and on the surface of a material is now recognised
to be of basic importance [3,33]. The synthetic activity of
the cells around and inside the materials should in fact
allow implant incorporation in tissue with time.
Fig. 4. PMMA+a-TCP implanted in cortical bone. Direct apposition
of the cortical bone to material surface. (Undecalcified section, Fast
Green, 1.25 .)
Fig. 5. PMMA+a-TCP in cortical bone. A particular of the bone–
material interface at the endosteal perimeter. (Undecalcified section,
Fast Green 4 .)
Fig. 6. PMMA+a-TCP implanted in cancellous bone. Newly formed
bone regrown inside the pores of the implanted PMMA+a-TCP (a).
Under fluorescence the newly formed bone incorporated the fluor-
escent dye (b) (Undecalcified section, Fast Green and fluorescent stain,
20 ): M=Material; B=Bone.
Table 2
Affinity Index (%) of PMMA
6 -TCP in cortical and trabecular bone.
Mean
7SD, n ¼ 5
Material
Cortical bone
Trabecular bone
PMMA
6 -TCP
68.31
76.18
a
57.14
78.84
b
PMMA
13.16
710.04
9.63
77.17
Student’s t test between:
a
, p
o0.0005 vs PMMA implants in cortical
bone:
b
, p
o0.0005 vs PMMA implants in trabecular bone.
M. Fini et al. / Biomaterials 23 (2002) 4523–4531
4528

A porous polymeric composite material containing an
osteoinductive ceramic in the cavities was developed and
described in previous studies [26,27], as part of a larger
project investigating bone substitutes [34]. The addition
of a-TCP as an aqueous dispersion to a PMMA matrix
was shown to produce a class of composites that due to
their macrostructure and mechanical properties may be
suitable for application as bone substitutes in orthopae-
dics. After adding the inorganic component as an
aqueous dispersion, the PMMA formed a cellular
polymeric matrix with open cells of about 100 mm. The
mechanical properties of the new composite were similar
to those of the porous hydroxyapatite currently used as
a bone substitute [27].
However, when investigating a biomaterial for ortho-
paedic purposes, biological investigations by means of in
vitro and in vivo tests are mandatory in order to obtain
a true overall picture of biocompatibility, including
osteogenetic and osteointegration properties. The com-
monest tests on bone implants are histologic, histomor-
phometric and biomechanical investigations which may
follow in vitro tests [25]. They provide a complete
characterization of the osteogenetic and osteointegra-
tion properties of the material showing potential as bone
substitute for orthopaedic surgery [35].
In the present study, PMMA+a-TCP did not
negatively affect cell vitality and synthetic activity and
its positive effect on osteoblast function could be
demonstrated by OC and PICP increased levels.
Regarding PMMA in vitro behaviour, the present
findings concerning its inhibitory effect on cell vitality
and collagen synthesis were consistent with those
obtained by other authors, with the exception of the
OC production that was similarly inhibited by PMMA
in the present study, whereas it was stimulated by
PMMA in other studies [11]. The current discrepancy
between OC behaviours could be ascribed to the fact
that most in vitro studies on PMMA have been
conducted on polymerised PMMA in powder form.
However, a higher OC production in osteoblast cultures
is usually considered as a positive stimulation factor for
bone formation [36,37]. The production of IL-6 by cells
is recognized to increase with bone resorption around
implants, since the level of this cytokine is higher in
patients with loosened prostheses [38]. The expression of
IL-6 by the osteoblasts lining the surface of newly
formed bone around the implant is assumed to maintain
the inflammatory response within the interface and to
have an inhibitory effect on bone remodelling [39].
Therefore,
the
good
osteointegration
rate
of
PMMA+a-TCP versus PMMA observed in vivo could
also be partially explained by its action which decreases
IL-6 production by cells at the interface.
The material, in fact, successfully osteointegrated
both in trabecular and in cortical bone, while the
capability of PMMA to achieve a direct contact with
bone was negligible, as reported by other authors [15,1].
AI values were higher in cortical bone as compared to
trabecular bone. These findings are consistent with those
obtained by other authors who have implanted ceramic
materials into rabbits [40]. The implant site in fact
greatly affects both osteointegration and the rate of
biodegradation in those ceramic materials with higher
osteogenesis in cortical than in trabecular sites and
higher material degradation in trabecular than in
cortical sites [41]. The lower rate of TCP degradation
in cortical bone could be partially responsible for the
lower amount of bone regrown inside the porosity of the
material in this implant site.
The microhardness test confirmed the histomorpho-
metric data and demonstrated that the rate of miner-
alization and maturation was higher for the bone
around PMMA+a-TCP than for the bone in contact
with PMMA. As reported by Huja et al. [41], this non-
destructive technique enables the comparative study of
bone hardness variations at different distances from the
interface. This information should be considered in the
evaluation of bone adaptation around an implant.
Additionally, a good correlation coefficient was ob-
served between microhardness, elastic modulus, yield
stress, volume fraction of mineral and calcium content,
together with a close relationship between mineraliza-
tion and hardness [41]. Microhardness results demon-
strated that PMMA+a-TCP significantly improved
cortical and trabecular bone status at the bone-material
interface as compared to PMMA. However, the
Table 3
Microhardness measurements (HV) at the bone-PMMA and bone-PMMA
6 -TCP interface (200 mm from the material surface) and at 1000 mm from
it, both in cortical and in trabecular bone (Media
7SD, n ¼ 5)
Material
200 mm
1000 mm
Cortical bone
Trabecular bone
Cortical bone
Trabecular bone
PMMA
42.97
76.53
a
32.19
76.03
b
95.28
75.39
c
68.28
76.73
d
PMMA
6 -TCP
68.10
72.71
59.09
73.16
91.57
74.98
c
67.07
75.65
f
Student’s t test:
a
, p
o0.0005 vs 200 mm PMMA
6 -TCP in cortical bone;
b
, p
o0.0005 vs 200 mm PMMA
6 -TCP in trabecular bone;
c
, p
o0.0005 vs
200 mm PMMA in cortical bone;
d
, p
o0.001 vs 200 mm PMMA in trabecular bone;
e
, p
o0.001 vs 200 mm PMMA
6 -TCP in cortical bone;
f
, p
o0.05
vs 200 mm PMMA
6 -TCP in trabecular bone.
M. Fini et al. / Biomaterials 23 (2002) 4523–4531
4529

difference still existing between newly formed and pre-
existing normal tissue confirms that bone mineralization
and maturation around implants are slow processes
which take a long time. The present and other authors
have also experienced that bone at the interface does not
reach physiologic hardness values at 6 or 12 weeks even
after implanting osteoconductive materials, such as
titanium and hydroxyapatite [42–44].
The positive results obtained are probably due to the
porous
macroarchitecture
of
the
bone
substitute
brought about by adding a-TCP as an aqueous
dispersion. Its porosity and the presence of a bioactive
ceramic material could have a synergic effect and be
responsible for the improvement of (a) the material
colonization by bone cells, (b) osteoblast activity, (c)
osteoinduction and osteoconduction processes (d) bone
remodelling.
Acknowledgements
This work was partially supported by Progetto
Finalizzato ‘‘Materiali Speciali per Tecnologie Avanzate
II’’ and Rizzoli Orthopaedic Institute (ricerca corrente).
The authors are indebted to P. Di Denia, C. Dal Fiume,
N. Corrado, P. Nini and F. Rambaldi for their technical
assistance.
References
[1] Ignatius AA, Augat P, Ohnmacht M, Pokinsky JP, Kock HJ,
Claes LE. A new bioresorbable polymer for screw augmentation
in the osteosynthesis of osteoporotic cancellous bone: a biome-
chanical evaluation. J Biomed Mater Res (Appl Biomater)
2001;58:254–60.
[2] Griffon DJ, Dunlop DG, Howie CR, Pratt JN, Gilchrist TJ,
Smith N. An ovine model to evaluate the biological properties of
impacted morselized bone graft substitutes. J Biomed Mater Res
2001;56:444–51.
[3] Murphey MD, Sartoris DJ, Bramble JM. Radiograph assessment
of bone grafts. In: Habal MB, Hari Reddi A, editors. Bone grafts
and bone substitutes. Philadelphia: W.B. Saunders, 1992. p. 9–36.
[4] Malinin T. Acquisition and banking of bone allografts. In: Habal
MB, Hari Reddi A, editors. Bone grafts and bone substitutes.
Philadelphia: W.B. Saunders, 1992. p. 206–25.
[5] Goldberg VM. Selection of bone grafts for revision total hip
arthroplasty. Clin Orthop Rel Res 2000;381:68–76.
[6] Arcos D, Ragel CV, Vallet-Regi. Bioactivity in glass/PMMA
composites used as drug delivery system. Biomaterials 2001;
22(79):701–708.
[7] Gautier H, Daculsi G, Merle C. Association of vancomycin and
calcium phosphate by dynamic compaction: in vitro charateriza-
tion and microbiological activity. Biomaterials 2001;22:2481–7.
[8] Charnley J. Anchorage of the femoral head prosthesis to the shaft
of the femur. J Bone Joint Surg Br 1960;42:28–30.
[9] Ohsawa K, Neo M, Matsuoka H, Akijama H, Ito H, Nakamura
T. Tissue responses around polymethylmethacrylate particles
implanted into bone: analysis of expression of bone matrix
protein mRNA by in situ hybridization. J Biomed Mater Res
2001;54:501–8.
[10] Herman JH, Sowder WG, Anderson D, Applel AM, Hopson CN.
PMMA-induced release of bone-resorbing factors. J Bone Joint
Surg Am 1989;71:1530–41.
[11] Zambonin G, Colucci S, Cantatore F, Grano M. Response of
human osteoblasts to polymethylmethacrylate in vitro. Calcif
Tissue Int 1998;62:362–5.
[12] Heini PF, Walchli B, Berlemann U. Percutaneous transpedicular
vertebroplasty with PMMA: operative technique and early
results. A prospective study for the treatment of osteoporotic
compression fractures. Eur Spine J 2000;9:445–50.
[13] Ranawat CS, Peters LE, Ulmas ME. Fixation of the acetabular
component. The case for cement. Clin Orthop 1997;344:207–15.
[14] Giddings VL, Kurtz SM, Jewett CW, Foulds JR, Edidin AA. A
small punch test technique for characterizing the elastic modulus
and fracture behaviour of PMMA bone cement used in total joint
replacement. Biomaterials 2001;22:1875–81.
[15] Heikkila JT, Aho AJ, Kangasniemi I, Yli-Urpo A. Polymethyl-
methacrylate composites: disturbed bone formation at the surface
of bioactive glass and hydroxyapatite. Biomaterials 1996;17:
1755–60.
[16] Mousa WF, Kobayashi M, Shinzato S, Kamimura M, Neo M,
Yoshihara S, Nakamura T. Biological and mechanical properties
of PMMA-based bioactive bone cements. Biomaterials 2000;21:
2137–46.
[17] Vallo CI, Montemartini PE, Fanovich MA, Porto Lopez JM,
Cuadrato T. Polymethylmethacrylate-based bone cement mod-
ified with hydroxyapatite. J Biomed Mater Res 1999;48:150–8.
[18] Okada Y, Kawanabe K, Fujita H, Nishio K, Nakamura T.
Repair of segmental bone defects using bioactive bone cement:
comparison with PMMA bone cement. J Biomed Mater Res 1999;
47:353–9.
[19] Fujita H, Kazuhiro I, Hirozaku I, Oka M, Kitamura Y,
Nakamura T. Evaluation of bioactive bone cement in canine
total hip arthroplasty. J Biomed Mater Res 2000;49:273–88.
[20] Shinzato S, Kobayashi M, Mousa WF, Kamimura M, Neo M,
Kitamura Y, Kokubo T, Nakamura T. Bioactive polymethyl-
methacrylate-based bone cement: comparison of glass beads,
apatite- and wollastonite-containing glass–ceramic, and hydro-
xyapatite fillers on mechanical and biological properties. J
Biomed Mater Res 2000;51:258–72.
[21] Shinzato S, Nakamura T, Kokubo T, Kitamura Y. A new
bioactive bone cement: effect of glass bead filler content on
mechanical and biological properties. J Biomed Mater Res 2001;
54:491–500.
[22] Mayr-Wohlfart U, Fiedler J, Gunther KP, Puhl W, Kessler S.
Proliferation and differentiation rates of a human osteoblast-like
cell line (SaOS-2) in contact with different bone substitutes
materials. J Biomed Mater Res 2001;57:132–9.
[23] Herten HA, Wiltfang J, Grohmann U, Hoenig JF. Intraindividual
comparative animal study of a- and b-tricalcium phosphate
degradation in conjunction with simultaneous insertion of dental
implants. J Craniofac Surg 2001;12:59–68.
[24] Yuan H, Yang Z, de Bruijn J, de Groot K, Zhang X. Material-
dependent bone induction by calcium phosphate ceramics: a 2.5-
year study in dogs. Biomaterials 2001;22:2617–23.
[25] Knabe C, Driessens FCM, Plannel JA, Gildenhar R, Berger G,
Reif D, Fitzner R, Radlanski RJ, Gross U. Evaluation of
calcium phosphates and experimental calcium phosphate bone
cements using osteogenic cultures. J Biomed Mater Res 2000;52:
498–508.
[26] Beruto DT, Mezzasalma SA, Capurro M, Botter R, Cirillo P. Use
of a-tricalcium phosphate (TCP) as powders and as an aqueous
dispersion to modify processing, microstructure, and mechanical
properties of polymethylmethacrylate (PMMA) bone cements and
to produce bone-substitute compounds. J Biomed Mater Res
2000;49:498–505.
M. Fini et al. / Biomaterials 23 (2002) 4523–4531
4530

[27] Beruto D, Botter R, Fini M. The effects of water in inorganic
microsponges of calcium phosphates on the porosity and
permeability of composites made with polymethylmethacrylate.
Biomaterials, in press.
[28] Freeman MA, Tennant R. The scientific basis of cement versus
cementless fixation. Clin Orthop 1992;276:19–25.
[29] De Bastiani B, Faccioli G, Magnan B, Soffiatti R. Two phase
cement mixture, particularly suitable for orthopaedics. Patent no.
US5004501, 1991.
[30] Elliott JC. General chemistry of the calcium orthophosphates. In:
Elliott JC, editor. Structure and chemistry of the apatites and
other calcium orthophosphates. Amsterdam: Elsevier Science,
1994. p. 1–61.
[31] Bigi A, Boanini E, Botter R, Panzavolta S, Rubini K. a-tricalcium
phosphate hydrolysis to octacalcium phosphate: effect of sodium
polyacrylate. Biomaterials, in press.
[32] Nzihou A, Attias L, Sharrok P, Ricard A. A rheological, thermal
and mechanical study of bone cement-from a suspension to a solid
biomaterial. Powder Technol 1998;99:60–9.
[33] Dalton BA, McFarland CD, Gengenbach TR, Griesser HJ, Steele
JG. Polymer surface chemistry and bone cell migration. J
Biomater Sci Polym Ed 1998;9:781–99.
[34] Project MSTA II-Biomaterials P.F. CNR Italy.
[35] Lopes MA, Santos JD, Monteriro FJ, Ohtsuki C, Osaka A,
Kaneko S, Inoue H. Push-out testing and histological evaluation
of glass reinforced hydroxyapatite composites implanted in the
tibia of rabbits. J Biomed Mater Res 2001;54:463–9.
[36] Yamaguchi M, Sugimoto E, Hachya S. Stimulatory effect of
menaquinone-7 (vitamin K2) on osteoblastic bone formation in
vitro. Mol Cell Biochem 2001;223:131–7.
[37] Mayr-Wohlfart U, Fiedler J, Gunther KP, Puhl W, Kessler S.
Proliferation and differentiation rates of a human osteoblast-like
cell line (SaOS-2) in contact with different bone substitute
materials. J Biomed Mater Res 2001;57:132–9.
[38] Goodman SB, Huie P, Song Y, Schurman D, Maloney W,
Woolson S, Sibley R. Cellular profile and cytokine production at
prosthetic interfaces. Study of tissues retrieved from revised
hip and knee replacements. J Bone Joint Surg Br 1998;80:
531–9.
[39] Al Saffar N, Revell PA, Khwaja HA, Bonfield W. Assessment of
the role of cytokines in bone resorption in patients with total joint
replacement. J Mater Sci Mater Med 1995;6:762–7.
[40] Lu XJ, Gallur A, Flautre B, Anselme K, Descamps M, Thierry B,
Hardouin P. Comparative study of tissue reactions to calcium
phosphate ceramics among cancellous, cortical, and medullar
bone in rabbits. J Biomed Mater Res 1998;42:357–67.
[41] Huja SS, Katona TR, Moore BK, Roberts WE. Microhardness
and anisotropy of the vital osseous interface and endosseous
implants supporting bone. J Orthop Res 1998;16:54–60.
[42] Fini M, Giavaresi G, Rimondini L, Giardino R. Titanium
alloy osteointegration in cancellous and cortical bone of
ovariectomized
animals:
histomorphometric
and
bone
hardness measurements. Int J Oral Maxillofac Implants 2002;17:
28–37.
[43] Fini M, Cadossi R, Can
"e V, Cavani F, Giavaresi G, Krajewski A,
Martini L, Nicoli Aldini N, Ravaglioli A, Rimondini L, Torricelli
P, Giardino R. The effect of pulsed electromagnetic fields on the
osteointegration of hydroxyapatite implants in cancellous bone: a
morphologic and microstructural in vivo study. J Orthop Res, in
press.
[44] Stea S, Visentin M, Savarino L, Ciapetti G, Donati ME, Moroni
A, Caja V, Pizzoferrato A. Microhardness of bone at the interface
with ceramic-coated metal implants. J Biomed Mater Res 1995;29:
695–9.
M. Fini et al. / Biomaterials 23 (2002) 4523–4531
4531
Document Outline
Wyszukiwarka
Podobne podstrony:
Resorbability of bone substitute biomaterials by human osteo
bone graft substitutes
Palestrina O Bone Jesu (Satb)
Maternal Bone Lead Contribution to Blood Lead during and after Pregnancy
Metastatic bone disease,Wykł B Drozdzowska
Acetylenic Polymers, Substituted
T 6 Narzędzia KJ fish bone
Cancer induced bone dis Wyk B Drozdzowska
le bone, psychologia tłumu streszczenie
Bone formers id 91722 Nieznany (2)
ATX power switch substitute
Stek „bavette” i T bone stek
Metastatic bone disease,Wyk B Drozdzowska 2
Cancer induced bone dis Wykł B Drozdzowska
firstword bone
patomorfologia - nowotwory kosci, Cancer-induced bone disease; osteolytic tumour-induced bone diseas
więcej podobnych podstron