
14.1 Introduction: technofunctional uses of egg constituents
Hen egg was categorised by Baldwin in 1986 as a polyfunctional ingredient, as it
can simultaneously realise several technological functions in the same
formulated foodstuff. Its emulsifying, foaming, gelling, thickening, colouring
and aromatic properties make it still today a universal basic ingredient for the
domestic kitchen and the food processing industry. Whereas egg yolk is well
recognised for its emulsifying properties, egg white (or albumen) is a reference
in terms of foaming and both parts are used as gelling ingredient in many foods.
Yolk takes part in the formation and the stabilisation of emulsions. In spite of
the intensive use of yolk in formulated foodstuffs, and since the invention of
mayonnaise three centuries ago, the role of its major constituents is not clear
because of its complex structure. Yolk is a mixture of proteins and lipids
forming natural assemblies at various scales. These natural assemblies contri-
bute to the nano- and the microstructure of yolk. Thus, an understanding of the
emulsifying properties of yolk lies in the comprehension of these various levels
of structure.
14
Egg proteins
M. Anton, INRA Nantes Unite 1268 BiopolymeÁres Interactions
Assemblages, France and F. Nau and V. Lechevalier, UMR INRA
Science et Technologie du Lait et de l'Oeuf, France
Abstract: This chapter deals with the chemical composition and structural
characteristics of egg yolk and white in relation to three important functional
properties: emulsifying, foaming and gelling properties.
Key words: egg, yolk, white, emulsions, foams, gels, structure, assemblies,
interfaces.

The exceptional foaming properties of the albumen are also the base of
traditional recipes among which meringues act certainly as reference. Indeed, the
extreme simplicity of their formula (albumen and sugar, possibly added with
flavours) allows albumen to express in an optimal way its foaming properties.
However, the technological parameters influence the final quality of foam
obtained, and three types of meringues (traditional meringue, Swiss meringue
and Italian meringue) can be distinguished, depending on whether whipping is
achieved in the presence or absence of sugar, and at ambient or warm
temperature. But there is also a great number of other products in which
previously foamed albumen is added, which are either fat-free formulas (angel
food cake) or lipid-containing formulas (spoon biscuits, `sponge' cake, blown).
In such products, the complexity of the phenomena is extreme, foaming and
emulsification taking place simultaneously, which makes the control of the
physico-chemical and technological parameters of these operations very delicate.
Concerning the gelling properties of albumen and yolk, they are related to the
heat-gelation capacity of egg proteins. Then, these properties imply a cooking
step during the food processing. The heat gelation of egg proteins completely
conforms to the model of heat gelation of globular proteins. The corresponding
mechanisms have been extensively studied, on egg proteins as well as on other
ones, and the key technological parameters have now been identified. However,
the addition of other ingredients in mixture with egg (polysaccharides, for
example) complicates the understanding of the egg gelation behaviour, and
developments with more complex models are still needed.
14.2 Physico-chemistry and structure of egg constituents
14.2.1 Egg yolk
Chemical composition
Yolk correspond to 36% of whole hen egg weight. Its dry matter is about 50±
52% according to the age of the laying hen and the duration of preservation
(Kiosseoglou, 1989; Thapon and Bourgeois, 1994; Li-Chan et al., 1995). The
compositions of fresh and dry yolks are presented in Table 14.1: the main
components are lipids (about 65% of the dry matter) and the lipid to protein ratio
is about 2:1. Yolk lipids are exclusively associated with lipoprotein assemblies.
They are made up of 62% triglycerides, 33% phospholipids, and less than 5%
cholesterol. Carotenoids represent less than 1% of yolk lipids, and give it its
colour. Proteins are present as free proteins or apoproteins (included in
lipoprotein assemblies). The interactions between lipids and proteins result in
the formation of lipoproteins (low and high density), which represent the main
constituents of yolk.
Macrostructure and main constituents
Yolk is a complex system with different structuration levels consisting in
aggregates (granules) in suspension in a clear yellow fluid (plasma) that contains
360 Handbook of hydrocolloids
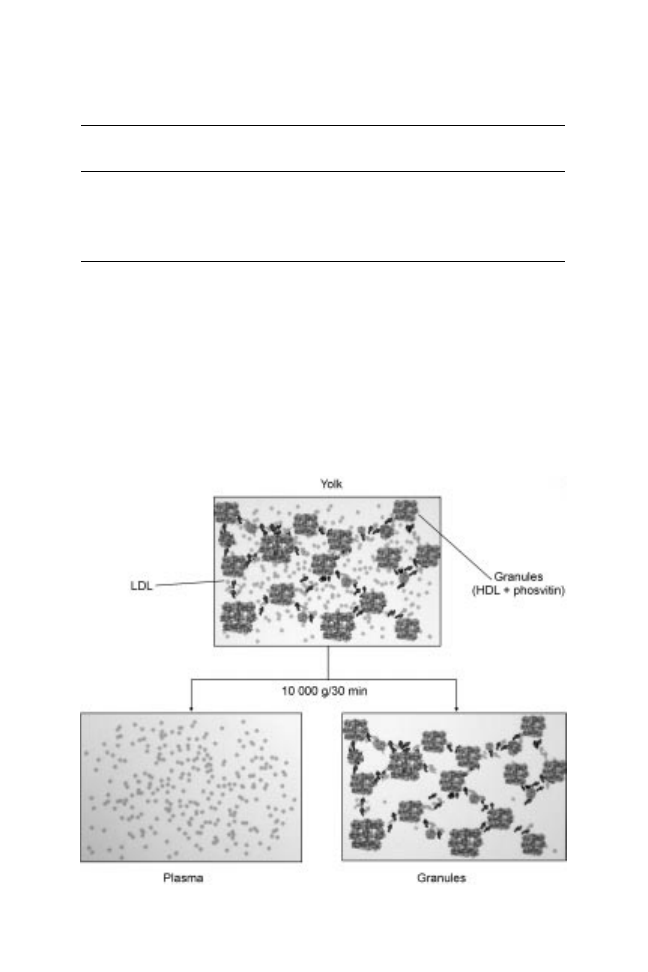
lipoproteins and proteins. Granules consist in circular complexes ranging in
diameter from 0.3 m to 2 m (Chang et al., 1977). Consequently, yolk can be
easily separated into two fractions after a dilution (two times) with 0.3 M NaCl
and a centrifugation at 10,000 g (30 min) according to the method of McBee and
Cotterill (1979): a dark orange supernatant called plasma and a pale pellet called
granules (Fig. 14.1).
Granules represent 22% of yolk dry matter, accounting for about 50% of yolk
proteins and 7% of yolk lipids. The dry matter content of granules is about 44%,
with about 64% proteins, 31% lipids and 5% ash (Dyer-Hurdon and Nnanna,
Table 14.1 Composition of hen egg yolk
Fresh yolk
Dry yolk
(%)
(%)
Water
51.1
Ð
Lipids
3.6
62.5
Proteins
16.0
33.0
Carbohydrates
0.6
1.2
Minerals
1.7
3.5
Source: Powrie and Nakai (1986)
Fig. 14.1 Fractionation of plasma and granules from hen egg yolk.
Egg proteins 361

1993; Anton and Gandemer, 1997). They are mainly constituted by high density
lipoproteins (HDL) (70%) and phosvitin (16%) linked by phosphocalcic bridges
between the phosphate groups of their phosphoseryl residues (Burley and Cook,
1961; Saari et al., 1964). Low density lipoproteins (LDL) (12%) are included in
the granular structure (Table 14.2).
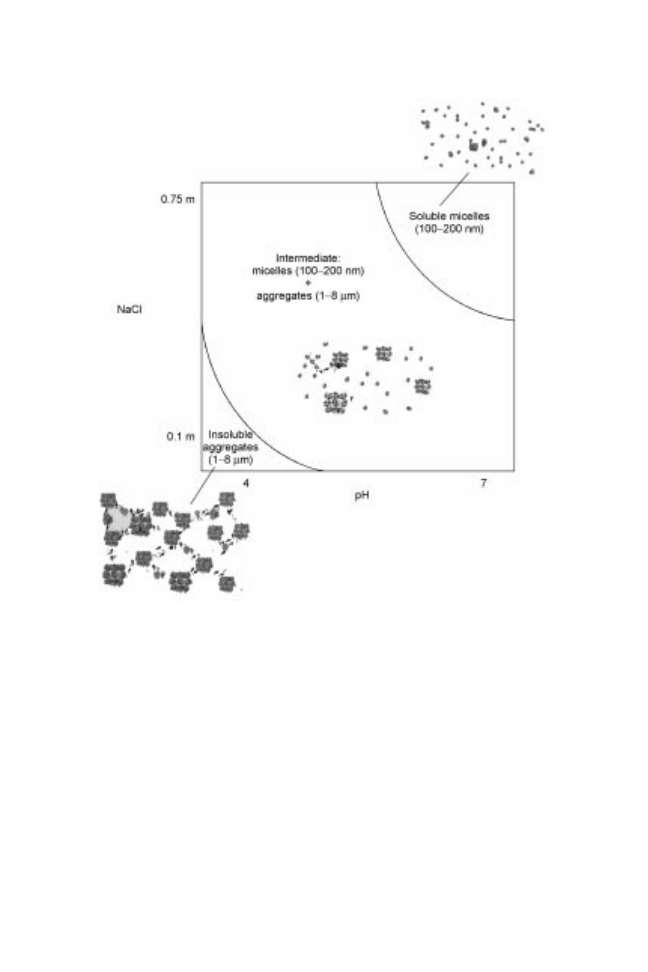
At low ionic strength, granules mainly form insoluble HDL-phosvitin
complexes linked by phosphocalcic bridges as HDL and phosvitin contain a high
proportion of phosphoserin amino acids able to bind calcium (Causeret et al.,
1991). The numerous phosphocalcic bridges make the granule structure very
compact, poorly hydrated, weakly accessible to enzymes, and lead to an efficient
protection against thermal denaturation and heat gelation.
At an ionic strength over 0.3 M NaCl, the phosphocalcic bridges are disrupted
because monovalent sodium replaces divalent calcium. In such conditions, the
solubility of granules reaches 80% because phosvitin is a soluble protein and
HDL behave like soluble proteins (Cook and Martin, 1969; Anton and
Gandemer, 1997). Complete disruption of granules occurs when ionic strength
reaches 1.71 M NaCl. Acidification or alkalinisation similarly cause the disrup-
tion of granules and the solubilisation of these constituents by increasing the
number of the positive (NH
3
+
) or negative (COO
-
) charges inducing electrostatic
repulsions between granule constituents. Recently, we have established
(Sirvente, 2007) a phase diagram drawing the different states of granules as a
function of pH and ionic strength (Fig. 14.2).
Plasma comprises 78% of yolk dry matter and is composed of 85% LDL and
15% livetins (Burley and Cook, 1961; Table 14.2). It forms the aqueous phase
where yolk particles are in suspension. It accounts for about 90% of yolk lipids
(including nearly all the carotenoids), and 50% of yolk proteins. Plasma contains
about 73% lipids, 25% proteins and 2% ash. Lipids of plasma are distributed
thus: 70% triglycerides, 25% phospholipids and 5% cholesterol.
Table 14.2 Repartition of hen egg yolk constituents
Yolk
Yolk
Yolk
Lipids
Proteins
D.M.
lipids
proteins
(%)
(%)
(%)
(%)
(%)
Yolk
100
100
100
64
32
Plasma
78
93
53
73
25
LDL
66
61
22
88
10
Livetins
10
Ð
30
Ð
96
Others
2
Ð
1
Ð
90
Granules
22
7
47
31
64
HDL
16
6
35
24
75
Phosvitin
4
Ð
11
Ð
95
LDLg
2
1
1
88
10
Source: Powrie and Nakai (1986)
362 Handbook of hydrocolloids
LDL are spherical particles (17±60 nm in diameter with a mean of about
35 nm) with a lipid core in a liquid state (triglycerides and cholesterol esters)
surrounded by a monofilm of phospholipid and protein (Cook and Martin, 1969;
Evans et al., 1973). LDL are soluble in aqueous solution (whatever the pH and
ionic conditions) due to their low density (0.982). Phospholipids take an
essential part in the stability of the LDL structure because association forces are
essentially hydrophobic (Burley, 1975). Some cholesterol is included in the
phospholipid film, increasing its rigidity. LDL are composed of 11±17% protein
and 83±89% lipid, out of which 74% is neutral lipid and 26% phospholipid
(Martin et al., 1964).
14.2.2 Egg white
Egg white represents about 60% of the total egg weight. It consists of an
aqueous protein solution, containing few minerals and carbohydrates (Table
Fig. 14.2 Physical state of granules as function of pH and ionic strength.
Egg proteins 363

14.3). During egg storage, different physico-chemical modifications happen,
among them the CO
2
departure that induces a pH increase, from 7.5 at the laying
moment to 9.5 after a few days. This pH modification should be the cause of the
egg white liquefaction, because of the dissociation of a protein complex
(ovomucin-lysozyme complex) (Kato et al., 1975). Another evolution observed
concerns the ovalbumin modification toward S-ovalbumin, which is a more
heat-stable form (Smith and Back, 1965), resulting from isomerisation of three
serine residues (Yamasaki et al., 2003).
Proteins
Proteins represent more than 90% of the dry matter of egg white, but until very
recently, only the major ones have been identified. However, the recent and
powerful techniques for separation and analysis enabled the identification of
many minor proteins (Table 14.4) (GueÂrin-Dubiard et al., 2006; Mann, 2007).
The egg white proteins are predominantly globular proteins, and acidic or
neutral, except lysozyme and avidin which are highly alkaline proteins. All are
glycosylated, except cystatin and the major form of lysozyme. Some of them are
very heat-sensitive and/or sensitive to surface denaturation, explaining their
noteworthy functional properties.
The major egg white protein (more than 50% of the total proteins) is
ovalbumin, a 45 kDa globular and phosphorylated protein. Half of its amino
acids are hydrophobic, and one-third are electrically charged, essentially
negatively at physiologic pH. Ovalbumin possess six buried Cys residues, two
being involved in a disulfide bridge (Cys
73
-Cys
120
). Ovalbumin is then the only
egg white protein with free thiol groups, capable of inducing some
rearrangements with variations of storage conditions, pH and surface
denaturation.
Ovotransferrin (13% of total proteins) molecular weight is around 78 kDa.
This protein consists of two lobes, each containing a specific binding site for
iron (or copper, zinc, aluminium) (Kurakawa et al., 1995). It is the most heat-
sensitive egg white protein, but the complexation of iron or aluminium
significantly increases its heat stability (Lin et al., 1994).
OvomucoõÈde is a highly glycosylated protein (up to 25% carbohydrates, w/w)
of 28 kDa. At pH 7, its denaturation temperature is around 77 ëC, but this protein
Table 14.3 Composition of hen egg white
% of hen egg white
Water
88.0
Lipids
Ð
Proteins
10.6
Carbohydrates
0.8
Minerals
0.6
Source: Thapon and Bourgeois (1994)
364 Handbook of hydrocolloids

is much more heat resistant at acidic pH (Lineweaver and Murray, 1947).
Ovomucin is also a highly glycosylated protein, with a very high molecular
weight (10
4
kDa). Electrostatic interactions can be observed between ovomucin
and some of the other egg white proteins. In the freshly laid eggs (pH 7.5), the
carboxylic groups of the ovomucin sialic acids especially interacts with the -
NH
3+
of lysozyme lysine residues to form a lysozyme-ovomucin complex that
may be responsible for the gel-like structure of egg white (Kato et al., 1975).
Lysozyme is a small (14 kDa) globular, and strongly basic protein. Its structure
is very rigid, stabilised by four disulfide bridges.
Glucidic and mineral fractions
The glucidic fraction of egg white consists of free glucose (0.5% w/w) and carbo-
hydrates linked to proteins (0.5% w/w). The mineral fraction is predominantly
composed of Na
+
, K
+
and Cl
ÿ
, as free minerals, whereas P and S are essentially
constitutive elements of proteins. Egg white also contains CO
2
, in equilibrium
with bicarbonate, which plays a major role for pH control (Thapon, 1994).
14.3 Egg yolk emulsions
14.3.1 Basic principles
Emulsifying activity is related to the capacity of surface active molecules to
cover the oil±water interface created by mechanical homogenisation, thus
Table 14.4 Composition and some physico-chemical and functional properties of egg
white proteins
Protein
%
M
w
(kDa)
pI
Major biological properties
Ovalbumin
54
45
5
Immunogenic phosphoproteine
Ovalbumin Y
5
44
5.2
nd
Ovalbumin X
0.5
56
6.5
nd
Ovotransferrin
13
76
6.7
Iron binding, bacteriostatic activity
OvomucoõÈd
11
28
4.8
Trypsin inhibitor
Ovomucin
1.5±3.5
230±8300
4.5±5
Highly glycosylated, viral
hemaglutination inhibition
Lysozyme
3.5
14.4
10.7
Lysis of Gram bacteria wall
Ovoinhibitor
0.1±1.5
49
5.1
Serine protease inhibitor
Ovoglycoprotein
0.5±1
24.4
3.9
nd
Flavoprotein
0.8
32
4
Riboflavin (vitamin B2) binding
Ovostatin
0.5
760±900
4.6
Serine protease inhibitor
Cystatin
0.05
12.7
5.1
Cysteine protease inhibitor
Avidin
0.05
68.3
10
Biotine binding
Ex-FABP
nd
18
5.5
Lipocaline family
Cal gamma
nd
20.8
6
Lipocaline family
TENP
nd
47.4
5.6
BPI (bactericidal permeability-
increasing protein) family
Hep 21
nd
18
6.4
uPar/Ly6/Snake neurotoxin family
Sources: Li-Chan and Nakai (1989), Stevens (1991), GueÂrin et al. (2006).
Egg proteins 365

reducing the interfacial tension. Consequently, the more active the emulsifying
agent, the more the interfacial tension is lowered. Emulsion stability indicates
the capacity to avoid flocculation, creaming, and/or coalescence of oil droplets.
Creaming and flocculation are reversible phenomena which can be avoided by a
simple agitation of the emulsion. Coalescence is the irreversible fusion of oil
droplets due to the rupture of the interfacial film created by emulsifying agents.
This phenomenon leads to a complete destruction of the emulsion. This relates
the importance of the structure and the viscoelasticity of the interfacial film.
14.3.2 Role of egg yolk constituents
In researching the principal contributor to yolk emulsifying properties,
numerous authors have separated yolk into its main fractions: plasma and
granules. Large similarities have been observed between emulsifying properties
of yolk and plasma, whereas emulsions made with granules behaved very
differently (Dyer-Hurdon and Nnanna, 1993; Anton and Gandemer, 1997; Le
Denmat et al., 2000). Specifically, emulsions made with granules are more
coarse (more important oil droplet size) than emulsions made with yolk and
plasma, and notably at acidic pH where granules are not soluble (Le Denmat et
al., 2000) (Fig. 14.3).
Concerning the parameters of emulsion stability (creaming), we showed (Le
Denmat et al., 2000) that emulsions made with yolk and plasma had the same
creaming rate, in function of the medium conditions, whereas emulsions made
with granules behaved very differently (Fig. 14.3). Consequently, these studies
demonstrated that yolk emulsifying power was situated in plasma.
Among plasma constituents, some authors demonstrated that LDL are better
emulsifiers than bovine serum albumin (BSA) (Mizutani and Nakamura, 1984)
and casein (Shenton, 1979). Even though some authors suggested that, in certain
conditions, HDL were more efficient than LDL to form and stabilise O/W
emulsions (Hatta et al., 1997; Mine, 1998), a large number of studies confirm
the prevalent role of LDL in yolk emulsions. These findings have been
confirmed recently (Aluko et al., 1998; Mine and Keeratiurai, 2000; Anton et
al., 2003; Martinet et al., 2003). In particular, it has been established that LDL
made emulsions finer than HDL, along different conditions of pH and ionic
strength (Martinet et al., 2003). The next question is how to explain the
exceptional efficiency of LDL at the interfaces.
14.3.3 Importance of assemblies
Given that any destructurating treatment affects the emulsifying properties of
LDL, it appears that the integrity of the structure of LDL seems essential to
ensure their interfacial properties (Tsutsui, 1988). Direct adsorption of
apoproteins and phospholipids from LDL is not easy because of the non-
solubility of these species in water or in aqueous buffer. So the interactions
between apoproteins and lipids to assemble the LDL particles are essential to
366 Handbook of hydrocolloids
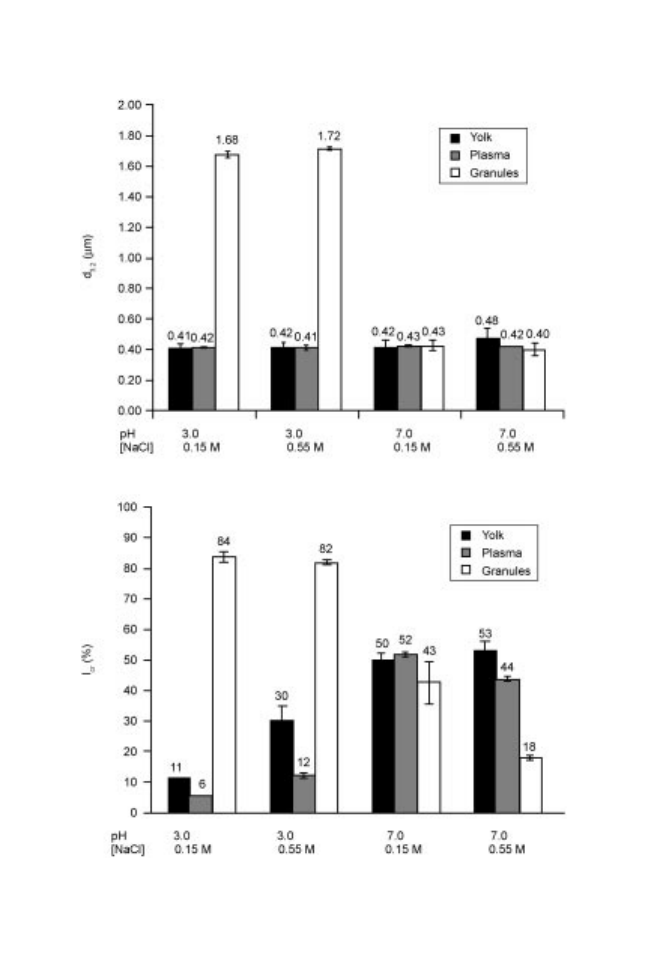
Fig. 14.3 Mean droplet diameter (d3.2) and creaming index (Icr) in oil/water emulsions
(30 : 70) prepared with yolk, plasma and granules, protein concentration: 25 mg/ml,
homogenisation pressure: 200 bars, n 3.
Egg proteins 367
transport the surfactants in a soluble form in the neighbourhood of the interface
and then to release them at the interface.
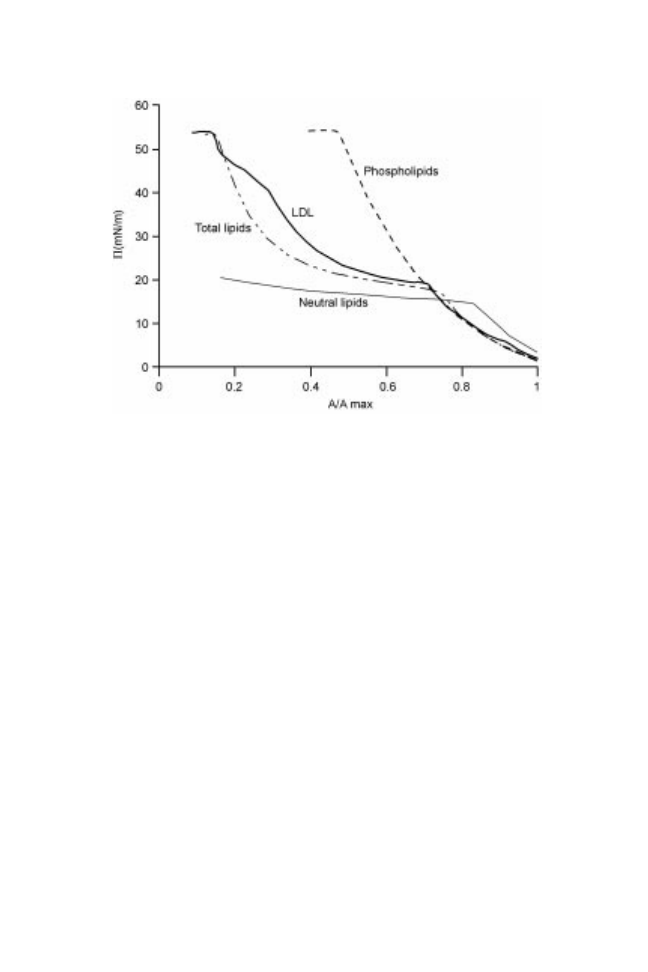
Using Langmuir film balance (air±water interface), three phase transitions
have been detected in compression isotherms and these three transitions (19, 41
and 54 mN/m) have been attributed, respectively, to neutral lipids, apoproteins
and phospholipids by comparison with films of neutral lipids, phospholipids and
total lipids extracted from LDL (Fig. 14.4) (Martinet et al., 2003). The transition
observed at 19 mN/m corresponds to the collapse of neutral lipids, and the
transition at 54 mN/m corresponds to phospholipid collapse. These different
transitions show that LDL actually break down when they come into contact
with the interface to release neutral lipids, phospholipids and apoproteins from
the lipoprotein core and to allow their spreading. In a recent study made with
atomic force microscopy (AFM) after a Langmuir±Blodgett transfer of the
layers from the air±water interface to a silica plate, it has been shown that the
second transition (previously attributed to apoproteins alone) is not due to
apoproteins alone, but to apoprotein±lipids complexes (Dauphas et al., 2006).
So, it has been deduced that LDL serve as vectors of surfactant constituents
(apoproteins and phospholipids) that could not be soluble in water, until the
interface. At this step the conservation of the LDL structure is essential. Once
LDL are near the interface, the structure is then broken up to release surfactant
constituents at the interface (Fig. 14.5).
Furthermore, comparing interfacial behaviour of LDL and liposomes (double
phospholipid layer not containing proteins), it has been shown that the
apoproteins situated on the LDL surface start the LDL disruption mechanism by
Fig. 14.4 /A isotherms of the different lipid constituents extracted from LDL and
spread at the air±water interface; neutral lipids = 85 g, phospholipids = 198 g, total
lipids = 287 g, compression rate = 100 cm
2
/min.
368 Handbook of hydrocolloids
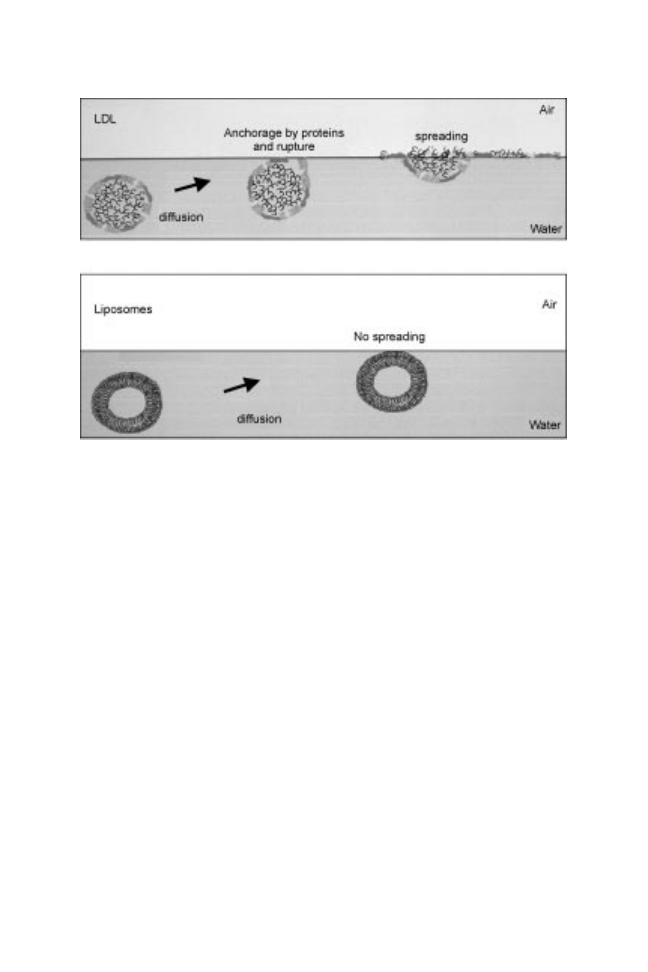
their initial anchorage. This anchorage provokes an unfolding of the protein
leading to the destabilisation of the external layer of the LDL. Then this
phenomenon could be followed by a deformation of the particle due to the
creation of a neutral lipid lens conducive to the spreading of the LDL
constituents. In the case of liposomes, without external proteins, the structure
remains steady at the interface and then this structure is not able to adsorb
efficiently and to decrease interfacial tension (Fig. 14.5).
14.4 Egg white foams
14.4.1 Formation and stabilisation mechanisms
Foam formation is a highly energetic and dynamic process, in which interfacial
area is created. The ability of a protein solution, such as egg white, to foam
depends on protein structure and conformation, depending themselves on
extrinsic factors such as pH, ionic strength, etc. The formation mechanism of
globular protein foams can be divided into three phases happening near gas
bubbles: protein diffusion towards the air±solution interface, conformation
changes of adsorbed proteins, and irreversible rearrangement of the protein film
(McRitchie, 1991).
Foams are short-lived states and there is any correlation between foam
stability and protein adsorption kinetic (Dickinson, 1996). Foam stability,
indeed, depends on protein association at the air±solution interface to form a
Fig. 14.5 Hypothetical mechanism of LDL adsorption at an oil±water interface as
compared with liposome behaviour.
Egg proteins 369

continuous intermolecular network. Foam stability is affected by the protein film
cohesion, drainage and Ostwald disproportionation.
14.4.2 Interfacial properties of egg white proteins
Interfacial properties of egg white proteins are responsible for egg white's
excellent foaming properties. Table 14.5 gathers some data on the kinetics of
diffusion towards the air±solution interface of three major egg white proteins.
Ovalbumin interfacial behaviour is well known, since a large set of data is
available about its tensioactivity, adsorption kinetics, interfacial shear and
dilatational rheology (de Feijter et al., 1978; de Feijter and Benjamins, 1987;
Benjamins and van Voorst Vader, 1992; Benjamins and Lucassen-Reynders,
1998; Damodaran et al., 1998; Lucassen-Reynders and Benjamins, 1999;
Pezennec et al.; 2000; Razumovsky and Damodaran, 2001; Croguennec et al.,
2007) but also on its structure at the air±water interface (Renault et al., 2002;
Lechevalier et al., 2003, 2005; Kudryashova et al., 2003). It is now known that
ovalbumin forms a single layer at the air±water interface, whatever its
concentration in the bulk (Renault et al., 2002). As for ovalbumin, lysozyme
interfacial behaviour has been extensively studied (de Feijter and Benjamins,
1987; Damodaran et al., 1998; Razumovsky and Damodaran, 2001; Kim et al.,
2002; Postel et al., 2003; Chang et al., 2005; Roberts et al., 2005; Perriman and
White, 2006) as well as its structure at the air±water interface (Lechevalier et al.,
2003, 2005). However, its interfacial behaviour differs as lysozyme forms films
that are much thicker than a protein monolayer whereas the surface pressure is
definitely smaller than the ovalbumin one (Le Floch-FoueÂre et al., 2009). These
different behaviours observed on planed air±water interface result in different
foaming properties. Ovalbumin foaming properties are much better than those of
lysozyme, since in native state at pH 7.0, the foaming capacity of lysozyme is
very weak (Townsend and Nakai, 1983), probably because of its little surface
hydrophobicity and its rigidity due to its four disulfide bonds.
Egg white proteins thus show different behaviour at 2D and 3D air±water
interfaces. When they are in mixture, their behaviour is again different. Indeed,
Damodaran et al. (1998) showed that the adsorption kinetics of egg white
proteins are different depending on whether they are in single protein systems or
in mixture. They suggested the formation of electrostatic complexes between
positively charged lysozyme and other negatively charged egg white proteins.
Moreover, the mixture ovalbumin-lysozyme forms films that are much thicker
than those of both proteins in single protein systems, suggesting a synergy in
interfacial adsorption between the two proteins (Le Floch-FoueÂre et al., 2007).
14.4.3 Egg white foams
Egg white is the reference for foaming properties: compared with other protein
ingredient of vegetable or animal origin, it still offers the best foaming
properties (Vani and Zayas, 1995; Matringe et al., 1999; Pernell et al., 2002;
370 Handbook of hydrocolloids

Table 14.5 Parameters of the kinetic of diffusion towards the air±solution interface of three major egg white proteins
Parameters
Ovalbumin
Ovotransferrine
Lysozyme
Reference
Apparent diffusion
0.5 (C=10
ÿ4
% prot.)
0.2 (C=10
ÿ4
% prot.)
De Feijter and Benjamins
coefficient
(in solution: 0.7)
(in solution: 1)
(1987)
(10
ÿ10
m
2
s
ÿ1
)
0.15 (C=1.510
ÿ4
% prot.)
Xu and Damodaran (1993)
0.5 to 1 (C=0.1% prot.)
Pezennec et al. (2000)
Surface concentration
1.6 (C=10
ÿ4
% prot.)
2.4 (C=10
ÿ4
% prot.)
De Feijter and Benjamins
(mg m
ÿ2
)
(1987)
1.5 (C=5.410
ÿ4
% prot.)
0.8 (C=1.210
ÿ4
% prot.)
0.5 (C=0.3510
ÿ4
% prot.)
Damodaran et al. (1998)
2.1 (native protein) to 2.9
Pezennec et al. (2000)
(heat-treated protein)
Croguennec et al. (2007)
(C=0.01% prot.)
1.4 (pH 4) to 3 (pH 11)
Perriman and White (2006)
(C=0.1% prot.)
Surface pressure
1 (C=10
ÿ4
% prot.)
3.5 (C=10
ÿ4
% prot.)
De Feijter and Benjamins
(mN m
ÿ1
)
(1987)
14 (C=5.410
ÿ4
% prot.)
2.5 (C=1.210
ÿ4
% prot.)
2.5 (C=0.3510
ÿ4
% prot.)
Damodaran et al. (1998)
24 (C=0.01% prot.)
Pezennec et al. (2000)
8 (pH 5.6) to 14 (pH 11)
Roberts et al. (2005)
(C=0.012% prot.)
9 (C=510
ÿ4
% prot.) to
Chang et al. (2005)
24.5 (C=0.1% prot.)
Lag phase
YES if C<0.01%
YES if C<0.01%
De Feijter and Benjamins
Not enough molecules at
Not enough molecules at
(1987)
the interface to create
the interface to create
an increase of
an increase of
NO
YES
YES
Damodaran et al. (1998)

Foegeding et al., 2006; Davis and Foegeding, 2007). Egg white can be
considered as a solution of efficient surfactants. Its proteins are amphiphilic and
show a relatively high surface hydrophobicity, thus diffusing quite rapidly
towards the air±water interface where they adsorb efficiently. Their molecular
flexibility ensures conformational rearrangement at the interface, resulting in a
great decrease in surface tension. Their ability to form a continuous inter-
molecular network, especially when a certain denaturation level is previously
obtained, enable them to form a viscoelastic interfacial film responsible for foam
stability. However, egg white proteins do not present at the same level these
different characteristics, and thus do not participate in the same way to egg white
foaming properties (Table 14.6). A lot of studies have been performed on the
different egg white protein foaming properties (Nakamura, 1963; Johnson and
Zabik, 1981a; Mine, 1995). However, it is now well known that the complexity
and the synergy of the phenomena mean that it is impractical to distinguish the
role of the different egg white properties (Lechevalier et al., 2005). It is thus
quite difficult to predict foaming properties of any mixture of egg white proteins
since phenomena of competition for the interface and possible exchange
between proteins at the air±water interface may occur. Nevertheless, foaming
properties of isolated egg white proteins are always lower than those of egg
white, which tend to confirm the existence and the role of the interactions
between proteins. It is thus generally admitted that the natural coexistence in egg
white of alkaline protein (lysozyme) and acid ones (most of the other) enables
electrostatic interactions, thus explaining the good foaming properties of egg
white (Poole et al., 1984; Damodaran et al., 1998).
Egg white foams are an integral component of many foods such as meringue,
nougat and angel food cake. A recent study showed that the properties of foams
do not predict performance in angel food cake (Foegeding et al., 2006).
14.4.4 Key parameters
Many physico-chemical parameters are susceptible to influence foam formation
and stability. In the specific case of egg white proteins, surface hydrophobicity
(that conditions the efficiency of protein adsorption at the air±water interface),
Table 14.6 Interfacial characteristics of the main egg white proteins
Protein
Surface tension (mN m
ÿ1
)
Foamability (cm
3
g
ÿ1
min
ÿ1
)
Globulins
45.4
4.71
Ovalbumin
51.8
0.59
Ovotransferrin
42.4
0.34
Lysozyme
42.0
0.12
OvomucoõÈd
39.0
0
Ovomucin
nd
0
Mixture in the egg white ratio
46.7
3.08
Source: Mine (1995)
372 Handbook of hydrocolloids

the number of disulfide bonds (that conditions protein flexibility/rigidity) and
the number of free sulfhydryl groups (that conditions protein reactivity) are
decisive in the structural modifications that occur at the air±water interface.
Moreover, the number and the nature of inter- and intramolecular interactions
determine the rheological properties of the interfacial film and so foam stability.
These interactions are favoured by a certain degree of denaturation, however,
too much denaturation weakens the interfacial film and the foam collapses
(Kinsella, 1976; Trziszka, 1993; Kato et al., 1994; Van der Plancken et al.,
2007). Another special feature of egg white foams is their dependence on thick
egg white proportion and quality. Foamability increases with egg white natural
liquefaction during its storage (Sauveur et al., 1979; Thapon, 1981; Baldwin,
1986), whereas foam stability decreases (Nau et al., 1996). Egg white foaming
properties can also be improved by the addition of sucrose (effect on foam
stability) and sodium chloride (effect on foamability), as suggested by Raikos et
al. (2007a).
14.5 Gels
14.5.1 Basic principles
A gel consists of polymers linked through covalent and/or non-covalent
interactions, to create a three-dimensional network. In whole egg as well as in
white and yolk, proteins are responsible for the gelling properties. Gelation
occurs when the protein stability in solution is modified, i.e., when the
equilibrium between attractive (Van der Waals) and repulsive (electrostatic,
steric) interactions is disrupted. The electrostatic repulsions vary with the net
charge of the proteins, that means with the ionisable protein groups and with the
physico-chemical characteristics of the solvent (pH, ionic strength). The
treatments that decrease the repulsive interactions, such as adjustment of pH
at proteins pI or addition of salts, induce destabilisation and thus can result in the
formation of aggregates or gels.
Moreover, some treatments can modify the protein structure, with
consequences for the repulsive and attractive interactions mentioned above.
This is especially the case during heat treatments, which are the major techno-
logical treatments used in the food industry for egg white and yolk gelation.
Heat-induced gelation of egg conforms completely with the model of heat
gelation of globular proteins. It is a two-step phenomenon: in the first stage,
unfolding of native proteins occurs, disrupting the well-defined secondary and
tertiary structures and producing denatured proteins exposing their inner
hydrophobic regions; following unfolding, the denatured proteins interact to
form high molecular weight aggregates that can further interact with each other
to result in a three-dimensional gel (Clark et al., 2001). The unfolding and
aggregation steps depend on many factors (protein concentration, ionic strength,
pH, presence of sucrose, etc.) that can modify the number and/or the kind of
interactions, with final consequences on the gel rheology. In the heat-induced
Egg proteins 373

gels of egg proteins, the interactions involved are predominantly hydrophobic
and electrostatic, but some highly energetic interactions can be observed
(disulfide bridges); thiol and amine groups are indeed very reactive, especially
in alkaline conditions.
14.5.2 Egg yolk gels
Yolk undergoes a gelation when it is subjected to a freezing-thawing process or
a heat treatment. LDL are responsible for yolk gelation, while the other
constituents of yolk do not participate directly (Kojima and Nakamura, 1985;
Kurisaki et al., 1981; Nakamura et al., 1982; Tsustui, 1988; Wakamatu et al.,
1982; Le Denmat et al., 1999).
Freezing-thawing gelation of yolk appears for a temperature below ÿ6 ëC
(Lopez et al., 1954). This gelation is undesirable because it makes yolk difficult
to handle. Freezing-thawing gelation is influenced by rate and temperature of
freezing and thawing, and length and temperature of storage (Kiosseoglou,
1989). Rapid freezing and thawing results in less gelation than slow freezing and
thawing. Freezing-thawing gelation is partially reversible and the initial
viscosity of yolk is recovered after heating for 1 h at 45±55 ëC.
The mechanism of freezing-thawing gelation of LDL remains hypothetical.
Freezing causes the formation of ice crystals which mobilise water (at ÿ6 ëC,
about 80% of the water in yolk is in ice crystals) and causes a dehydration of
apoproteins of LDL. This dehydration favours a rearrangement of apoproteins of
LDL, interactions between their amino acid residues and an aggregation which
leads to gelation.
LDL is the constituent of yolk responsible for heat-induced gelation of yolk
(Saari et al., 1964). LDL solution (4% w/v) start denaturing at 70 ëC and form
gels at 75 ëC (Tsutsui, 1988). LDL solutions, heated at 80 ëC for 5 min, form
more stable gels than ovalbumin and BSA (Kojima and Nakamura, 1985).
Unlike ovalbumin and BSA, LDL present a heat-induced gelation in a large
range of pH (4±9) with a minimal value around their pHi (Nakamura et al.,
1982). Between pH 6 and 9, LDL solutions form coagulum gels (opaque)
whereas LDL solutions form translucents gels for extreme pH (4±6 and 8±9)
(Kojima and Nakamura, 1985; Nakamura et al., 1982).
Heat-induced gelation of yolk is governed by the unfolding of proteins during
heating. Then the functional groups are exposed and attracted to one another
through hydrophobic bonds resulting in a gel (Nakamura et al., 1982).
The primary stage of the two phenomena (freezing-thawing and heat-induced
gelation) is the disruption of the LDL structure (Kurisaki et al., 1981). This
disruption is favoured by dehydration in the case of freezing-thawing, or by
unfolding under heating. Lipid-protein interactions are disrupted under freezing or
heating and interactions between proteins are increased. These interactions are both
principally of non-polar nature because a LDL gel is solubilised by SDS which
interacts with the hydrophobic residues of apoproteins (Mahadevan et al., 1969).
The aggregation product of LDL certainly contains lipids included in the structure
374 Handbook of hydrocolloids

(Tsutsui, 1988). Apoproteins of LDL present a large proportion of hydrophobic
amino acids and, consequently, they have a high ability to form such gels.
More recently, Le Denmat et al. (2000) have measured the critical concen-
trations (Cg) for heat gelation of dispersions of yolk, plasma and granules in pH
and NaCl ranges of respectively 3±7 and 0.15±0.55 M. In all cases, the domain Cg
for plasma is 12±28 mg protein/ml, whereas it is 26±120 mg protein/ml for
granules. For yolk solution Cg is comprised between 16 and 39 mg protein/ml.
This confirms the preponderant influence of LDL, the major compound of
plasma, in the heat gelation of yolk. This underlines the excellent capacity of
granules to resist to heat treatments, that could be used for industrial applications.
14.5.3 Egg white gels
The heat gelation of egg white is used in many food applications involving a
cooking step. Except ovomucin and ovomucoõÈd, all the egg white proteins
coagulate when heated (Johnson and Zabik, 1981b). But the heat sensitivity of
egg white proteins varies significantly: the temperature of denaturation at pH 7
in egg white is 84.5, 74 and 65 ëC for ovalbumin, lysozyme and ovotransferrin,
respectively (Donovan et al., 1975). Ovotransferrin is then the more heat-
sensitive, which is why it is generally considered as the gelation initiator, and
finally as a limiting factor considering the gelling properties. Therefore,
ovotransferrin elimination has been suggested to improve egg white gelling
properties (Kusama et al., 1990). However, ovotransferrin is more stable at
alkaline pH, at high ionic strength, and when metal ions are bound on it. Thus,
the gelation temperature of egg white can also be significantly increased by
modification of these parameters, and especially by Fe
3+
or Al
3+
addition
(Cunningham and Lineweaver, 1965).
The extent and the kind of the interactions between the denatured proteins
depend on the protein structure, that means on the extent of unfolding at the end
of the denaturation step. Indeed, the unfolding governs the more or less
important exposure of reactive groups or regions on the protein molecule. The
interactions also depend on the physico-chemical conditions that can be either
limiting or favouring, resulting in an increase or a decrease of aggregation rate
respectively, and then in a decrease or an increase of the denaturation extent
before interactions take place (Totosaus et al., 2002).
These mechanisms have been extensively studied for egg white and
ovalbumin heat-gelation, with a focus on ionic strength effect on the structure
and characteristics of the gels (Holt et al., 1984; Woodward and Cotterill, 1986;
Woodward, 1990; Croguennec et al., 2002; Raikos et al., 2007b). Heat
denaturation induces an increase of the protein surface hydropbobicity. When
heating occurs at high ionic strength, the protein charges are screened, inducing
a shielding effect on the repulsive forces between proteins, thus favouring the
hydrophobic interactions (Doi, 1993). In these conditions, random aggregates of
slightly denatured proteins appear, corresponding to opaque gels, with low
rigidity, elasticity and water retention capacity. On the other hand, at low ionic
Egg proteins 375

strength, the electrostatic repulsions are so high that it delays the aggregation
(Raikos et al., 2007b), favouring denaturation. Finally, the further aggregation
involves some specific area (hydrophobic regions), and induces linear polymeric
aggregates. Once placed in higher ionic strength conditions, these aggregates
can interact to form performing gels. Thus, a two-step heating process has been
proposed to produce translucent gels from egg white, very firm and elastic, and
with an exceptional water retention capacity (Kitabatake et al., 1988a). As well
as ionic strength influences the net charge of the proteins, pH is another major
parameter for egg white gelation control. Close to their pI, the proteins tend to
form random aggregates, similar to those obtained at high ionic strength. This
phenomenon explains the minimal rheological properties of the egg white gels
around pH 5. In contrast, at alkaline pH, egg white offers the best gelling
properties (Ma and Holme, 1982; Kitabatake et al., 1988b). The higher reactivity
of the thiol groups in these pH conditions probably also contributes to the
improvement of the gelling properties, because of disulfide bridges taking place.
On the other hand, at acidic pH (2.0), the limited protein solubility would be
responsible for the low gelation temperature and the low rheological properties
observed (Raikos et al., 2007b).
To improve the gelling properties of egg white, Kato et al. (1989) proposed
an original approach consisting of an extensive denaturation of proteins while
preventing aggregation. Such conditions can be obtained by heating of egg white
powder at high temperatures (80 ëC) for a long time (up to 10 days). This
treatment increase protein flexibility and exposure of reactive groups that can
further interact to strengthen the gel formed when previously dry-heated egg
white is solubilised and heated in solution. The efficiency of this process can be
improved by pH control (Mine, 1996, 1997). The dry-heating process is today
the basis for mass production practices of high-gel egg white powders.
14.6 Conclusion
Hen egg contains very high functional proteins, lipids and lipoproteins. These
functionalities are due partly to their chemical composition and structure, and
partly to the supramolecular assemblies they form naturally or under the action
of thermo-mechanical treatments during industrial processes. One of the major
challenges for the future is the control of the design of these assemblies and the
understanding of their functionalities (interfacial, emulsifying, foaming, phase
separation, etc.) to enhance the quality of existing products or to conceive
innovative products.
14.7 References
ALUKO RE, KEERATIURAI M
and
MINE Y
(1998), `Competitive adsorption between egg yolk
lipoproteins and whey proteins on oil-in-water interfaces', Colloids Surf B:
Biointerfaces, 10, 385±393.
376 Handbook of hydrocolloids

ANTON M
and
GANDEMER G
(1997), `Composition, solubility and emulsifying properties of
granules and plasma of hen egg yolk', J Food Sci, 62 (3), 484±487.
ANTON M, MARTINET V, DALGALARRONDO M, BEAUMAL V, DAVID-BRIAND E
and
RABESONA H
(2003) `Structural and chemical characterization of low-density lipoprotein
purified from hen egg yolk', Food Chem, 83, 175±183.
BALDWIN RE
(1986), `Functional properties of eggs in foods', in Stadelman WJ and
Cotterill OJ, eds, Egg Science and Technology, London, The Haworth press.
BENJAMINS J
and
LUCASSEN-REYNDERS EH
(1998), `Surface dilational rheology of proteins
absorbed air/water interfaces', in MoÈbius D and Miller R, Proteins at Liquid
Interfaces, Amsterdam, Elsevier, 341±384.
BENJAMINS J
and
VAN VOORST VADER F
(1992), `The determination of the surface shear
properties of adsorbed protein layers', Colloids Surf B: Biointerfaces, 65, 161±174.
BURLEY RW
(1975), `Recent advances in the chemistry of egg yolk', CSIRO Food Res
Quaterly, 35, 1±5.
BURLEY RW
and
COOK WH
(1961), `Isolation and composition of avian egg yolk granules
and their constituents - and -lipovitellins', Canadian J Biochem Physiol, 39,
1295±1307.
CAUSERET D, MATRINGE E
and
LORIENT D
(1991), `Ionic strength and pH effects on
composition and microstructure of yolk granules', J Food Sci, 56, 1532±1536.
CHANG CM, POWRIE WD
and
FENNEMA O
(1977), `Microstructure of egg yolk', J Food Sci,
42, 1193±1200.
CHANG S-H, CHEN L-Y
and
CHEN W-Y
(2005), `The effects of denaturants on protein
conformation and behavior at air/solution interface', Colloids Surf B:
Biointerfaces, 41, 1±6.
CLARK AH, KAVANAGH GM
and
ROSS-MURPHY SB
(2001) `Globular protein gelation ± theory
and experiment', Food Hydrocolloids, 15, 383±400.
COOK WH
and
MARTIN WG
(1969), `Egg lipoproteins', in Tria E and Scanu AM, eds,
Structural and Functional Aspects of Lipoproteins in Living Systems, New York,
Academic Press.
CROGUENNEC T, NAU F
and
BRULEÂ G
(2002), `Influence of pH and salts on egg white
gelation', J. Food Sci, 67, 608±614.
CROGUENNEC T, RENAULT A, BEAUFILS S, DUBOIS J-J
and
PEZENNEC S
(2007), `Interfacial
properties of heat-treated ovalbumin', J Colloids Interfaces Sci, 315, 627±636.
CUNNINGHAM FE
and
LINEWEAVER H
(1965), `Stabilization of egg white proteins to
pasteurizing temperatures above 60 ëC', Food Technol, 19, 136±141.
DAMODARAN S, ANAND K
and
RAZUMOVSKY L
(1998), `Competitive adsorption of egg
white proteins at the air water interface: direct evidence for electrostatic complex
formation between lysozyme and other egg proteins at the interface', J Agric Food
Chem, 46, 872±876.
DAUPHAS S, BEAUMAL V, RIAUBLANC A
and
ANTON M
(2006), `Hen egg yolk low density
lipoproteins film spreading at the air-water and oil-water interfaces', J Agric Food
Chem, 54 (10), 3733±3737.
DAVIS JP
and
FOEGEDING EA
(2007), `Comparisons of the foaming and interfacial
properties of whey protein isolate and egg white proteins', Colloids Surf B:
Biointerfaces, 54, 200±210.
DE FEIJTER JA
and
BENJAMINS J
(1987), `Adsorption kinetics of protein at the air-water
interface', in Dickinson E and Stainsby G, eds, Food Emulsions and Foams,
London, Royal Society of Chemistry.
DE FEIJTER JA, BENJAMINS J
and
VEER FA
(1978), `Ellipsometry as a tool to study the
Egg proteins 377

adsorption behavior of synthetic and biopolymers at the air-water interface',
Biopolymers, 17, 1759±1772.
DICKINSON E
(1996), Les colloõÈdes alimentaires, Paris, Dunod.
DOI E
(1993) `Gels and gelling of globular proteins', Trends in Food Sci. & Technol., 4,
1±5.
DONOVAN JW, MAPES CJ, DAVIS J
and
GARIBALDI J
(1975), `A differential scanning calori-
metric study of the stability of egg white to heat denaturation', J Sci Food Chem,
26, 73±83.
DYER-HURDON JN
and
NNANNA IA
(1993), `Cholesterol content and functionality of plasma
and granules fractionated from egg yolk,' J Food Sci, 58, 1277±1281.
EVANS RJ, BAUER DH, BANDEMER SL, VAGHEFI SB
and
FLEGEL CJ
(1973), `Structure of egg
yolk very low density lipoprotein: polydispersity of the very low density
lipoprotein and the role of lipovitellenin in the structure', Arch Biochem Biophys,
154, 493±500.
FOEGEDING EA, LUCK PJ
and
DAVIS JP
(2006), `Factors determining the physical properties
of protein foams', Food Hydrocolloids, 20, 284±292.
GUEÂRIN-DUBIARD C, PASCO M, MOLLEÂ D, DEÂSERT C, CROGUENNEC T
and
NAU F
(2006),
`Proteomic analysis of hen egg white', J Agric Food Chem, 54, 3901±3910.
HATTA H, OZEKI M
and
TSUDA K
(1997), `Egg yolk antibody IgY and its application', in
Yamamoto T, Juneja L, Hatta H, Kim M, eds, Hen Eggs: Their Basic and Applied
Science, CRC Press, Boca Raton, FL, pp. 151±178.
HOLT DL, WATSON MA, DILL CW, ALFORD ES, EDWARDS RL, DIEHL KC
and
GARDNER FA
(1984)
`Correlation of the rheological behaviour of egg albumen to temperature, pH, and
NaCl concentration', J Food Chem, 49, 137±141.
JOHNSON TM
and
ZABIK ME
(1981a), `Egg albumen proteins interactions in an angel food
cake system', J Food Sci, 46, 1231±1236.
JOHNSON TM
and
ZABIK ME
(1981b), `Gelation properties of albumen proteins, singly and
in combination', Poultry Sci, 60, 2071±2083.
KATO A, IMOTO T
and
YAGISHITA K
(1975), `The binding groups in ovomucin-lysozyme
interaction', Agric Biol Chem, 39, 541±544.
KATO A, IBRAHIM HR, WATANABE H, HONMA K
and
KOBAYASHI K
(1989), `New approach to
improve the gelling and surface functional properties of dried egg white by heating
in dry state', J Agric Food Chem, 37, 433±437.
KATO A, IBRAHIM HR, NAKAMURA S
and
KOBAYASHI K
(1994), `New methods for improving
the functionality of egg white proteins', in Sim JS and NakaõÈ S, eds, Egg Uses and
Processing Technologies. New Developments, Oxon, CAB International.
KIM G, GURAU M, KIM J
and
CREMER PS
(2002), `Investigations of lysozyme adsorption at
the air/water and quartz/water interfaces by vibrational sum frequency
spectroscopy', Langmuir, 18, 2807±2811.
KINSELLA JE
(1976), `Functional properties of proteins in foods: a survey', Crit Rev in
Food Sci and Nutrition, 219±276.
KIOSSEOGLOU VD
(1989), `Egg yolk', in Charalambous G and Doxastakis G, eds, Food
Emulsifiers: Chemistry, Technology, Functional Properties and Applications,
London, Elsevier.
KITABATAKE N, SHIMIZU A
and
DOI E
(1988a), `Preparation of transparent egg white gel
with salt by two-step heating method', J Food Sci, 53, 735±738.
KITABATAKE N, SHIMIZU A
and
DOI E
(1988b), `Preparation of heat-induced transparent gels
from egg white by the control of pH and ionic strength of the medium', J Food Sci,
53, 1091±1095.
378 Handbook of hydrocolloids

KOJIMA E
and
NAKAMURA R
(1985), `Heat gelling properties of hen's egg yolk low density
lipoprotein (LDL) in the presence of other protein', J Food Sci, 50, 63±66.
KUDRYASHOVA EV, MEINDERS MBJ, VISSER AJWG, VAN HOEK A
and
DE JONGH HHJ
(2003),
`Structure and dynamics of egg white ovalbumin adsorbed at the air±water
interface', Eur Biophys J, 32, 553±562.
KURAKAWA H, MIKAMI B
and
HIROSE M
(1995), `Crystal structure of differic hen
ovotransferrin at 2.4 AÊ resolution', J Mol Biol, 254, 196±207.
KURISAKI J, YAMAUCHI K, ISSHIKI H
and
OGIWARA S
(1981), `Differences between - and -
lipovitellin from hen egg yolk', Agric Biol Chem, 45, 699±704.
KUSAMA T, YOSHIDA K
and
HONMA K
(1990), Japan Patent No. HEI.2-257857.
LE DENMAT M, ANTON M
and
GANDEMER G
(1999), `Protein denaturation and emulsifying
properties of plasma and granules of egg yolk as related to heat treatment', J Food
Sci, 64 (2), 194±197.
LE DENMAT M, ANTON M
and
BEAUMAL V
(2000), `Characterisation of emulsion properties
and of interface composition in oil-in-water emulsions prepared with hen egg yolk,
plasma and granules', Food Hydrocolloids, 14, 539±549.
LE FLOCH-FOUEÂREÂ C, PEZENNEC S, LECHEVALIER V, BEAUFILS S, DESBAT B, PEÂZOLET M
and
RENAULT A
(2009), `Synergy between ovalbumin and lysozyme leads to non-
additive interfacial and foaming properties of mixtures', Food Hydrocolloids, 23,
352±365.
LECHEVALIER V, CROGUENNEC T, PEZENNEC S, GUEÂRIN-DUBIARD C, PASCO M
and
NAU F
(2003), `Ovalbumin, ovotransferrin, lysozyme: three model proteins for structural
modifications at the air±water interface', J Agric Food Chem, 51, 6354±6361.
LECHEVALIER V, CROGUENNEC T, PEZENNEC S, GUEÂRIN-DUBIARD C, PASCO M
and
NAU F
(2005), `Evidence for synergy in the denaturation at the air±water interface of
ovalbumin, ovotransferrin and lysozyme in ternary mixture', Food Chem, 92, 79±
87.
LI-CHAN ECY
and
NAKAI S
(1989), `Biochemical basis for the properties of egg white',
Critical Rev Poultry Biol, 2, 21±57.
LI-CHAN ECY, POWRIE WD
and
NAKAI S
(1995), `The chemistry of eggs and egg products',
in Stadelman WJ and Cotterill OJ, eds, Egg Science and Technology, 4th edn, New
York, Food Product Press, chap. 6.
LIN LN, MASON AB, WOODWORTH RC
and
BRANDTS JF
(1994), `Calorimetric studies of serum
transferring and ovotransferrin. Estimates of domain interactions and study of the
kinetic complexities of ferric ion binding', Biochem, 33, 1881±1888.
LINEWEAVER H
and
MURRAY CW
(1947), `Identification of the trypsin inhibitor of egg
white with ovomucoid', J Biol Chem, 171, 565±581.
LOPEZ A, FELLERS CR
and
POWRIE WD
(1954), `Some factors affecting gelation of frozen
egg yolk', J Milk Food Tech, 17, 334.
LUCASSEN-REYNDERS EH
and
BENJAMINS J
(1999), `Interfaces, interactions and stability', in
Dickinson E and Rodriguez Patino JM, eds, Food Emulsions and Foams,
Cambridge, Royal Society of Chemistry, 195.
MA C
and
HOLME J
(1982), `Effect of chemical modifications on some physicochemical
properties and heat coagulation of egg albumen', J Food Sci, 47, 1454±1459.
MAHADEVAN S, SATYANARAYANA T
and
KUMAR SA
(1969), `Physico-chemical studies on
the gelation of hen's egg yolk separation of gelling components from yolk plasma',
J Agric Food Chem, 17, 767±771.
MANN K
(2007), `The chicken egg white proteome', Proteomics, 7, 3558±3568.
Egg proteins 379

MARTIN WG, AUGUSTYNIAK J
and
COOK WH
(1964), `Fractionation and characterization of
the low-density lipoproteins of hen's egg yolk', Biochim Biophys Acta, 84, 714±
720.
MARTINET V, SAULNIER P, BEAUMAL V, COUTHAUDON JL
and
ANTON M
(2003), `Surface
properties of hen egg yolk low-density lipoproteins spread at the air-water
interface', Colloids Surf B: Biointerfaces, 31, 185±194.
MATRINGE E, PHAN TAN LUU R
and
LORIENT D
(1999), `Functional properties of milk-egg
mixtures', J Food Sci, 64, 787±791.
M
C
BEE LE
and
COTTERILL OJ
(1979), `Ion-exchange chromatography and electrophoresis of
egg yolk proteins', J Food Sci, 44, 657±666.
M
C
RITCHIE F
(1991), `Air/water interface studies of protein', Anal Chim Acta, 249, 241±
245.
MINE Y
(1995), `Recent advances in the understanding of egg white protein functionality',
Trends Food Sci Technol, 61, 225±232.
MINE Y
(1996), `Effect of pH during the dry heating on the gelling properties of egg white
proteins', Food Res Int, 29, 155±161.
MINE Y
(1997), `Effect of dry heat and mild alkalin treatment on functional properties of
egg white proteins', J Agric Food Chem, 45, 2924±2928.
MINE Y
(1998), `Adsorption behavior of egg yolk low-density-lipoproteins in oil-in-water
emulsions', J Agric Food Chem, 46, 36±41.
MINE Y
and
KEERATIURAI M
(2000), `Selective displacement of caseinate proteins by hens
egg yolk lipoproteins at oil-in-water interfaces', Colloids Surf B: Biointerfaces, 18,
1±11.
MIZUTANI R
and
NAKAMURA R
(1984), `Emulsifying properties of egg yolk low density
lipoprotein (LDL): comparison with bovine serum albumin and egg lecithin',
Lebensmittel Technol, 17, 213±216.
NAKAMURA R
(1963), `Studies on the foaming property of chicken egg white. Spread
monolayer of the protein fraction of the chicken egg white', Agric Biol Chem, 27,
427.
NAKAMURA R, FUKANO T
and
TANIGUSHI M
(1982), `Heat induced gelation of hen's egg
yolk LDL', J Food Sci, 47, 1449±1453.
NAU F, GESTIN L, PROTAIS J, AWADE A
and
THAPON JL
(1996), `Etude compareÂe des
proprieÂteÂs fonctionnelles et de la composition des fractions eÂpaisse et liquide du
blanc d'úuf de poule', Ind Alim Agr, 113, 5±10.
PERNELL CW, FOEGEDING EA, LUCK PJ
and
DAVIS JP
(2002), `Properties of whey and egg
white protein foams', Colloids and Surfaces A: Physicochemical Engineering
Aspects, 204, 9±21.
PERRIMAN AW
and
WHITE JW
(2006), `Kinetics of adsorption of lysozyme at the air±water
interface and the role of protein charge', Physica B, 385±386, 716±718.
PEZENNEC S, GAUTHIER F, ALONSO C, GRANER F, CROGUENNEC T, BRULE G
and
RENAULT A
(2000), `The protein net electric charge determines the surface rheological
properties of ovalbumin adsorbed at the air±water interface', Food Hydrocolloids,
14, 463±472.
POOLE S, WEST SI
and
WALTERS CL
(1984), `Protein±protein interactions: their importance
in the foaming of heterogeneous protein systems', J Sci Food Agric, 35, 701±711.
POSTEL C, ABILLON O
and
DESBAT B
(2003), `Structure and denaturation of adsorbed
lysozyme at the air±water interface', J Colloid Interface Sci, 266, 74±81.
POWRIE WD
and
NAKAI S
(1986), `The chemistry of eggs and egg products', in Egg Science
and Technology, 3rd edn. Westport, CN, AVI Publishing Co., 105±175.
380 Handbook of hydrocolloids

RAIKOS V, CAMPBELL L
and
EUSTON SR
(2007a), `Effects of sucrose and sodium chloride on
foaming properties of egg white proteins', Food Res Int, 40, 347±355.
RAIKOS V, CAMPBELL L
and
EUSTON SR
(2007b), `Rheology and texture of hen's egg protein
heat-set gels as affected by pH and the addition of sugar and/or salt', Food
Hydrocolloids, 21, 237±244.
RAZUMOVSKY L
and
DAMODARAN S
(2001), `Incompatibility of mixing of proteins in
adsorbed binary protein films at the air±water interface', J Agric Food Chem, 49,
3080±3086.
RENAULT A, PEZENNEC S, GAUTHIER F, VIEÂ V
and
DESBAT B
(2002), `The surface rheological
properties of native and S-ovalbumin are correlated with the development of an
intermolecular -sheet network at the air±water interface', Langmuir, 18, 6887±
6895.
ROBERTS SA, KELLAWAY IW, TAYLOR KMG, WARBURTON B
and
PETERS K
(2005), `Combined
surface pressure-interfacial shear rheology study of the effect of pH on the
adsorption of proteins at the air±water interface', Langmuir, 21, 7342±7348.
SAARI A, POWRIE WD
and
FENNEMA O
(1964), `Isolation and characterization of low-density
lipoproteins in native egg yolk plasma', J Food Sci, 29, 307±315.
SAUVEUR B, ZYBKO A
and
COLAS B
(1979), `ProteÂines alimentaires et qualite de l'úuf. 1.
Effet de quelques proteÂines sur la qualite interne de l'úuf et les proprieÂteÂs
fonctionnelles', Ann Zootech, 28, 271±295.
SIRVENTE H
(2007), `FonctionnaliteÂs des fractions du jaune d'oeuf dans les emulsions
alimentaires huile-dans-eau alleÂgeÂes en matieÁre grasse: impact des diffeÂrents
niveaux de structuration', Ph D thesis, University of Nantes, France.
SHENTON AJ
(1979), `Membrane composition and performance of food emulsions', PhD
thesis, University of London, UK.
SMITH LJ
and
BACK JF
(1965), `Studies on ovalbumin. The formation and properties of S-
ovalbumin, a more stable form of ovalbumin', Aus J Biol Sci, 21, 539±545.
STEVENS L
(1991), `Mini-review: egg white proteins', Comp Biochem Physiol, 100, 1±9.
THAPON JL
(1981), `Effets de divers facteurs sur les proprieÂteÂs physico-chimiques et
fonctionnelles du blanc d'úuf', PhD thesis, ENSAR ± Universite Rennes 1.
THAPON JL
(1994) `Evolution de l'uf au cours de la conservation', In Thapon JL,
Bourgeois CM, eds, L'úuf et les ovoprodutis, Paris, Tec et Doc Lavoisier, pp. 84±
94.
THAPON JL
and
BOURGEOIS CM
(1994), L'úuf et les ovoproduits, Paris, Tec et Doc
Lavoisier, chap. 1.
TOTOSAUS A, MONTEJANO JG, SALAZAR JA
and
GUERRERO I
(2002), `A review of physical
and chemical protein-gel induction', Int J Food Sci Technol, 37, 589±601.
TOWNSEND A
and
NAKAI S
(1983), `Relationship between hydrophobicity and foaming
characteristics of food proteins', J Food Sci, 48, 588±594.
TRZISZKA T
(1993), `Protein aggregation during whipping of egg white and its effect on
the structure and mechanical properties of foams', Arch GefluÈgelkd, 57, 27±34.
TSUTSUI T
(1988), `Functional properties of heat-treated egg yolk low density lipoprotein',
J Food Sci, 53, 1103±1106.
VAN DER PLANCKEN I, VAN LOEY A
and
HENDRICKX ME
(2007), `Foaming properties of egg
white proteins affected by heat or high pressure treatment', J Food Eng, 78, 1410±
1426.
VANI B
and
ZAYAS JF
(1995), `Foaming properties of selected plant and animal proteins', J
Food Sci, 60, 1025±1028.
Egg proteins 381

WAKAMATU T, SATO Y
and
SAITO Y
(1982), `Identification of the component responsible
for the gelation of egg yolk during freezing', Agric Biol Chem, 46, 1495±1503.
WOODWARD SA
(1990), `Egg protein gels', in Harris R, ed., Food Gels, London, Elsevier
Applied Science, pp. 175±199.
WOODWARD SA
and
COTTERILL OJ
(1986), `Texture and microstructure of heat-formed egg
white gels', J Food Sci, 51, 333±339.
XU S
and
DAMODARAN S
(1993), `Mechanism of adsorption of lysozyme at the air±water
interface', in Schwenke KD and Mothes R, eds, Food Proteins ± Structure and
Functionality, Wiley, New York.
YAMASAKI M, TAKAHASHI N
and
HIROSE M
(2003), `Crystal structure of S-ovalbumin as a
non-loop-inserted thermostabilized serpin form', J Biol Chem, 278, 35524±35530.
382 Handbook of hydrocolloids
Document Outline
- Front Matter
- Table of Contents
- 14. Egg Proteins
- Index
Wyszukiwarka
Podobne podstrony:
wyklad 14
Vol 14 Podst wiedza na temat przeg okr 1
Metoda magnetyczna MT 14
wyklad 14 15 2010
TT Sem III 14 03
Świecie 14 05 2005
2 14 p
i 14 0 Pojecie administracji publicznej
Wyklad 14 2010
14 Zachowanie Przy Wypadkach 1 13
Wyklad 14 PES TS ZPE
14 Ogniwa słoneczne
Wyklad 14
Wykład z fizyki 14
1 Wprowadzenie do psychologii pracy (14)id 10045 ppt
ZO NST 14 ĆW1CZ 1, 2 STUD F F3
więcej podobnych podstron