3685665719
WYKŁADY PLENARNE I ZAPROSZONE
AZABICYCLIC NATURAL PRODUCT ANALOGUES VIA DYNAMIC CYANIDE INDUCED RING CLOSURE
Christian V. Stevens
SynBioC Research Group, Department of Sustainable Organie Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure links 653, B-9000 Gent, Belgium. E-mail: Chris.Stevens@UGent.be
A variety of azabicyclic natural product analogues have been synthesized using a one pot three component ring closure comprising a substituted ketone, an aminę and acetone cyanohydrine. The dynamie addition of cyanide to the corresponding imine followed by intramolecular ring closure leads to the complete conversion of the intermediate cyano aminę to the azabicyclic compound.
This strategy has been developed for the synthesis of 2,4-methanoproline, a non-proteinogenic amino acid isolated from the seeds of Ateleia herbert smithii Pittier. Methanoproline stabilizes the trans configuration during protein folding and it was suggested to possess anti-feedant activity. This was postulated sińce the seeds of the plant are denied by morę than 100 seed predators. This anti-feedant activity initiated our intrest to study the synthesis and the activity of 2,4-methanoproline and its derivatives containing the 2-azabicyclo[2.1.1 ]hexane skeleton. Ali analogues have been tested on their anti-feedant activity towards the larvae of Spodoptera littoralis.
The dynamie ring closure could also be used for the synthesis of 7-azabicyclo[2.2.1]heptanes. This skeleton is present in epibatidine, a very active analgesic compound isolated tfom the skin of the Ecuadorian łfog Epipedobates tricolor. Its potency was proven to be 200-fold higher than morphine, however epibatidine cannot be used clinically because of its high toxicity.
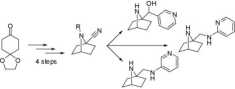
Our group has been developing different classes of epibatidine analogues trying to minimize toxicity while maintaining activity.
Next to the 7-azabicyclo[2.2.1]heptanes, the method proved also useful for the synthesis of 2-azabicyclo-[3.1.1]heptanes and 2-azabicyclo[2.2.2]octanes.
A number of compounds showed good in vitro activity on the alfa4,beta2 nicotinic acetylcholine receptor. Novel generations of azabicyclic compounds are currently under development in order to improve in vivo activity of the epibatidine analogues.
[ 1 ] T. Rammeloo, C.V. Stevens, Chem. Commun.. 250 - 251 (2002). A new and short Melhod for the Synthesis of 2,4-Methanoproline
[2] T. Rammeloo, C.V. Stevens, N. De Kimpe, J. Org. Chem., 67, 6509 - 6513 (2002). Synthesis of 2.4-Methanoproline derivatives via an addition intramolecular substitution Sequence
[3] C. V, Stevens, T. Rammeloo. G. Smagghe, N. De Kimpe. J. Agric. Food. Chem.. 53-1945 1948 (2005). Insect repellent/antifeedant Activity of 2,4-Methanoproline and Derivatives against a leaf- and seed feeding Pest Insect
Van der Jeught. K. Masschelein. C.V. Stevens. Eur. J. Org. Chem. 1017-1020 (2010). A straightforward Entry to 7-Azabicyclo[2.2.l]heptane-l-carbonitriles in the synthesis of novel Epibatidine Analogues.
[5] A. De Blieck, C.V. Stevens. Synlett. 1748 - 1752 (2011). Synthesis of a variety of 2-Alkyl-2-azabicyclo|3.1.1 jheptane-1 -carbonitriles via a dynamie addition-intramolecular substitution [61 C.V. Stevens, A. De Blieck. T. Heugebaert, PCT WO 2011/054885 Al, Internat. Publ. Datę: 12 May 2011. Fdingdatę:
03.11.2010. Priority: US/04.11/09/ USA 612452. GB/04.11.09/GBA 0919325. 1-Substituted 2-Azabicyck>[3.l .1 Iheptyl derivatives useful as nicotinic acetylcholine receptor modulator, for treating neurological
60
Wyszukiwarka
Podobne podstrony:
WYKŁADY PLENARNE I ZAPROSZONESP-PS1 Prezentacja Złotego Sponsora WITKO Sp. z o.o. Al. Piłsudskiego 1
WYKŁADY PLENARNE I ZAPROSZONESP-WZ4DESIGN AND PROPERTIES OF NANOMATERIALS BASED ON PHOSPHORUS DENDRI
WYKŁADY PLENARNE I ZAPROSZONESP-WP1 FIFTY YEARS OF HETEROCYCLIC CHEMISTRY AT MANCHESTER UNIVERSITY J
WYKŁADY PLENARNE I ZAPROSZONESP-WP2 TOWARD TRUE CARBAPORPHYRINOIDS Lechosław
WYKŁADY PLENARNE I ZAPROSZONESP-WP4 STAN BADAN CIEKŁOKRYSTALICZNYCH FAZ STACJONARNYCH Zygfryd
WYKŁADY PLENARNE I ZAPROSZONE CONQUERING ONE OF THE LAST BASTIONS IN ORGANIC SYNTHESES — CATALYTIC A
WYKŁADY PLENARNE I ZAPROSZONESP-WP6 RÓWNOWAGI FAZOWE-POMIARY I MODELOWANIE Urszula
WYKŁADY PLENARNE I ZAPROSZONE W POSZUKIWANIU AKTYWNOŚCI BIOLOGICZNEJ - PEREGRYNACJE
WYKŁADY PLENARNE I ZAPROSZONESP-WZ1 MACROMOLECULAR CRYSTALLOGRAPHY IN THE YEAR OF CRYSTALLOGRAPHY Ma
WYKŁAD PLENARNYFizyka materiałów i powierzchni z pierwszych zasad*Adam Kiejna Instytut Fizyki
Wykłady plenarne Plenary Lectures 9 September 2007, Sunday Opening Ceremony NCU AULA Chair person
Produkcja preparatów enzymatycznych Izolacja enzymu z hodowli jego naturalnego producenta Produkcja
10.15-11.15 Wykłady plenarne !Przewodniczący - Jan “Bręborozoicz, “Radzisław ‘Kordek 10.15
WYKŁADY PLENARNE Rola badań molekularnych w personalizacji terapii chorób nowotworowych. Marek
WYKŁADY WYKŁADY PLENARNE Rola badań molekularnych w personalizacji terapii chorób
PROGRAM SZCZEGÓŁOWY CZWARTEK 25 września 2008 r. PROGRAM SZCZEGÓŁOWY Wykład plenarny IV
PROGRAM OGOLNYCZWARTEK 25 września 2008 r. PROGRAM OGOLNY Wykład plenarny VI - godz. 9.00-9.45
więcej podobnych podstron