3685665729
WYKŁADY PLENARNE I ZAPROSZONE
FIFTY YEARS OF HETEROCYCLIC CHEMISTRY AT MANCHESTER UNIVERSITY
John A. Joule
School of Chemistry, The University of Manchester, UK
This lecture will tracę my career from North Wales to Manchester, then to Princeton and Stanford in the USA, and back to Manchester. Various aspects of Heterocyclic
Chemistry1 will be illustrated,
extracted from my research from 1961.
Early studies involved isolation and structure
determination of indole alkaloids: pseudakuammigine from the West African tree Picralima nitida; then ellipticine, uleine and congeners, and apparicine from various Brazilian Aspidosperma species, working with Carl Djerassi in Stanford.
Synthetic studies on lycorane-related compounds working with Richard K. Hill in Princeton were based on Diels-Alder chemistry.


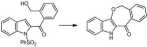
Our total synthesis of apparicine depended on Mannich chemistry to form the eight-membered ring.
There are in excess of forty molybdenum and tungsten enzymes, occurring throughout the entire rangę of organisms in the biosphere, that catalyse oxygen atom transfer. They all have the same cofactor, known as
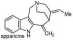
The concept that an enamine (conjugated) is morę stable than its isomeric allylamine was central to our synthesis of uleine.
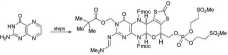
molybdopterin, that coordinates the metal centre. We synthesized the cofactor, a pteridine derivative, in protected form.
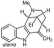
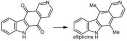
Our total synthesis of ellipticine, a potent antineoplastic agent, allows the preparation of analogues by proceeding through ellipticine quinone.
3-Oxidopyraziniums act as 1,3-dipoles and undergo cycloadditions generating 3,8-diazabicyclo[3.2.1]octanes.
11 ] 'Heterocyclic Chemistry', 1“ Edition, J. A. Joule & G. F. Smith, Van Nostrand Reinhold Co., 1972; 5'" Edition, J. A. Joule & K. Mills, Wiley, 2010. 'Heterocyclic Chemistiv at a Glance'. 2nd Edition, J. A. Joule & K. Mills, Wiley, 2013.
Initial attempts to synthesise apparicine were unsuccessful but led to the discovery of indole (5-nucleophilic substitution processes, which proved to be generał for the construction of a variety of fused indoles.
51
Wyszukiwarka
Podobne podstrony:
WYKŁADY PLENARNE I ZAPROSZONESP-WZ4DESIGN AND PROPERTIES OF NANOMATERIALS BASED ON PHOSPHORUS DENDRI
WYKŁADY PLENARNE I ZAPROSZONE CONQUERING ONE OF THE LAST BASTIONS IN ORGANIC SYNTHESES — CATALYTIC A
WYKŁADY PLENARNE I ZAPROSZONESP-WZ1 MACROMOLECULAR CRYSTALLOGRAPHY IN THE YEAR OF CRYSTALLOGRAPHY Ma
WYKŁADY PLENARNE I ZAPROSZONESP-PS1 Prezentacja Złotego Sponsora WITKO Sp. z o.o. Al. Piłsudskiego 1
SP-WZ6 WYKŁADY PLENARNE I ZAPROSZONE AZABICYCLIC NATURAL PRODUCT ANALOGUES VIA DYNAMIC CYANIDE INDUC
WYKŁADY PLENARNE I ZAPROSZONESP-WP2 TOWARD TRUE CARBAPORPHYRINOIDS Lechosław
WYKŁADY PLENARNE I ZAPROSZONESP-WP4 STAN BADAN CIEKŁOKRYSTALICZNYCH FAZ STACJONARNYCH Zygfryd
WYKŁADY PLENARNE I ZAPROSZONESP-WP6 RÓWNOWAGI FAZOWE-POMIARY I MODELOWANIE Urszula
WYKŁADY PLENARNE I ZAPROSZONE W POSZUKIWANIU AKTYWNOŚCI BIOLOGICZNEJ - PEREGRYNACJE
Publications The Institute of Political Science at the University of Wrocław publishes
00449 ?6b2c81a17b7f60430d45ea04acefc6 454 Russell Figurę 13. This univariate phase map movie extrac
WYKŁAD PLENARNYFizyka materiałów i powierzchni z pierwszych zasad*Adam Kiejna Instytut Fizyki
S20C 409120813420 portrait scarf Rer.rnih/, J resumed loeituing—nfłer many years of not Juiring acce
Uimmagine digitale in diagnostica per immagini PDF eBooks Download this way, but years of being pure
Insłitute of Political ScienceDear Sir/Madam, Having over 40 years of history, the In-stitute of Pol
więcej podobnych podstron