3685665730
WYKŁADY PLENARNE I ZAPROSZONE
TOWARD TRUE CARBAPORPHYRINOIDS
Lechosław Latos-Grażyński
Department of Chemistry, University of Wrocław, POLAND
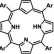
Carbaporphyrinoids realize the original concept of macrocyclic ligand construction by fusing the structural motifs of porphyrin and suitable carbon donor(s). An entrapment of metal ions in a coordination core of carbaporphy-rinoids creates a very efficient protection of the metal-carbon bond, and allows to stabilize extremely rare oxidation/electronic States of metal ions in an organometallic environment. A replacement of a single pyrrole of -tetraarylporphyrin by cyclopentadiene
moiety seems to be a notion of the very first choice for the creation of true carbaporphyrins which ideally conserve the fundamental motif of porphyrin The true carbaporphyrins prompt developments in carbaporphyrinoid and organometallic chemistry building on specific reactivity of cyclopentadienyl moiety.
[1] Szyszko,B.; L. Latos-Grażyński, L.: Szterenberg, L.
Angew.Chem.lnt.Ed. 2011, 50,6587-6591.
[2] Szyszko, B.; Kupietz. K.; Szterenberg. L.; Latos-Grażyński. L.
Chem. Eur. J. 2014,20,1376-1382.
[3] Berlicka, A.; Dutka, P.; Szterenberg, L.; Latos-Grażyński. L.
Angew. Chem. Int. Ed. 2014,53,4885-4889.
HYDROGEN BONDING - THE BRIDGE
Poul Erik Hansen
Department of Science, Systems and Models, Roskilde University, DK-4000 Roskilde, Denmark. E-mail: poulerik@ruc.dk
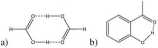
The talk will concentrate on hydrogen bonding, both intra and intermolecular and related to that tautomerism. The talk will be a safari through a number of fundamental subjects in chemistry, biology and pharmacy. The key techniques are a combination of NMR, IR and theoretical calculations. The NMR experiments are often aimed at determining deuterium isotope effects on Chemical shifts. One of the key questions regarding hydrogen bonding is the difference between inter and intra-molecular hydrogen bonds or rather between hydrogen bonds with variable heavy atom distance (a) and those of fixed distance (b) as illustrated in Fig.
The latter situation is often referred to as resonance assisted hydrogen bonding (RAHB). Deuterium isotope effects on l3C or l5N Chemical shifts may tell about the strength of the hydrogen bond. The importance of RAHB was early on illustrated in enaminones. Fig. 2.

The cousins to enaminones , the o-hydroxy Schiff bases are discussed both in terms of neutral vs. zwitter ionic structure, tautomerism and factor analysis. Tautomerism, a fundamental property in relation to reactivity and biological function, is often quite difficult to establish. Deuterium isotope effects on Chemical shifts are often a strong tool as demonstrated in P-thioxoketones.
The use of hyphenated techniques, laser irradiation and NMR detection is demonstrated in p-thioxoketones and the results are compared with matrix isolation IR experiments.
Intermolecular hydrogen bonding is discussed in thioamides leading to new understanding of isotope effects on Chemical shifts and demonstrating that the latter can detect long rangę effects.
The structure of protic ionic liquids is also discussed and the importance of hydrogen bonding is emphasized. In addition, the degree of proton transfer as well as the composition is elucidated.
In pharmacy, structure activity relationships are important. Equally important is of course to know the correct structure. An example is uśnie acid. The correct structure is arrived at using deuterium isotope effects on Chemical shifts.
52
Wyszukiwarka
Podobne podstrony:
WYKŁADY PLENARNE I ZAPROSZONESP-PS1 Prezentacja Złotego Sponsora WITKO Sp. z o.o. Al. Piłsudskiego 1
WYKŁADY PLENARNE I ZAPROSZONESP-WZ4DESIGN AND PROPERTIES OF NANOMATERIALS BASED ON PHOSPHORUS DENDRI
SP-WZ6 WYKŁADY PLENARNE I ZAPROSZONE AZABICYCLIC NATURAL PRODUCT ANALOGUES VIA DYNAMIC CYANIDE INDUC
WYKŁADY PLENARNE I ZAPROSZONESP-WP1 FIFTY YEARS OF HETEROCYCLIC CHEMISTRY AT MANCHESTER UNIVERSITY J
WYKŁADY PLENARNE I ZAPROSZONESP-WP4 STAN BADAN CIEKŁOKRYSTALICZNYCH FAZ STACJONARNYCH Zygfryd
WYKŁADY PLENARNE I ZAPROSZONE CONQUERING ONE OF THE LAST BASTIONS IN ORGANIC SYNTHESES — CATALYTIC A
WYKŁADY PLENARNE I ZAPROSZONESP-WP6 RÓWNOWAGI FAZOWE-POMIARY I MODELOWANIE Urszula
WYKŁADY PLENARNE I ZAPROSZONE W POSZUKIWANIU AKTYWNOŚCI BIOLOGICZNEJ - PEREGRYNACJE
WYKŁADY PLENARNE I ZAPROSZONESP-WZ1 MACROMOLECULAR CRYSTALLOGRAPHY IN THE YEAR OF CRYSTALLOGRAPHY Ma
WYKŁAD PLENARNYFizyka materiałów i powierzchni z pierwszych zasad*Adam Kiejna Instytut Fizyki
Wykłady plenarne Plenary Lectures 9 September 2007, Sunday Opening Ceremony NCU AULA Chair person
10.15-11.15 Wykłady plenarne !Przewodniczący - Jan “Bręborozoicz, “Radzisław ‘Kordek 10.15
WYKŁADY PLENARNE Rola badań molekularnych w personalizacji terapii chorób nowotworowych. Marek
WYKŁADY WYKŁADY PLENARNE Rola badań molekularnych w personalizacji terapii chorób
PROGRAM SZCZEGÓŁOWY CZWARTEK 25 września 2008 r. PROGRAM SZCZEGÓŁOWY Wykład plenarny IV
PROGRAM OGOLNYCZWARTEK 25 września 2008 r. PROGRAM OGOLNY Wykład plenarny VI - godz. 9.00-9.45
PROGRAM OGÓLNY PIĄTEK 26 września 2008 r. PROGRAM OGÓLNY Wykład plenarny VII - godz 9.00-9.45 Wykła
- wykłady na zaproszenie oraz staż w trakcie Olimpiady Przedszkolaków o dr In
więcej podobnych podstron