381
A HibUl. IM1U.1 .Vvu r ), buui :uO
ISBN D4H1II t-7. © l>. »N TOS »*}
12 5 POTENCJAŁY STANDARDOWE I SZEREG NAPIĘCIOWY PIERWIASTKÓW 381
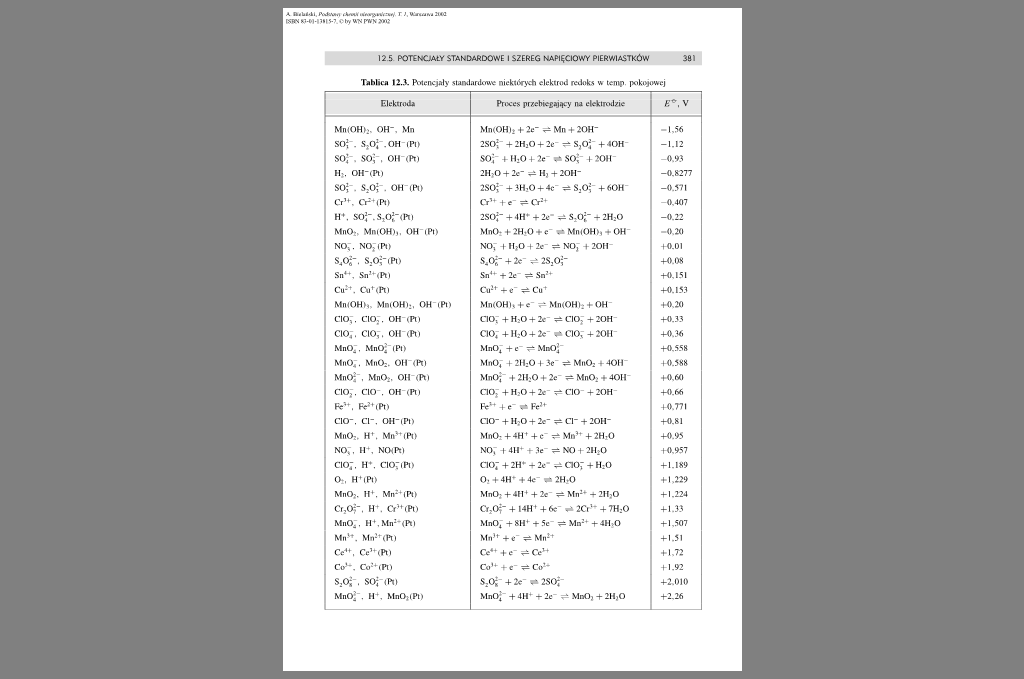
Tablica 12.3. Potencjały ttoadanleiwe niektórych elektrod redoks w icnip pokojowej
|
Elektroda |
Proces pr/chiefąj^ey na elektrodzie |
£*■. V |
|
MnlOHl;. OH'. Mn |
Mn(OH). + 2c- ^ Mn + 20H* |
—1,56 |
|
SO{ ■ SjOj .OH iPll |
2SOi + 2H.O + 2e- ^ S,Oj + 40H |
-1.12 |
|
SOi~. SO; • OH (Pt) |
SO;’ f H:O ł 2e~ *SO; 3 2011 * |
-0.93 |
|
H,. OH" (Pt) |
2HjO + 2e- H, + 20H- |
-0.8277 |
|
soi . s:oi-. oh (Po |
2S0i + 3H.O + 4C s S.oi + 60H |
-0.571 |
|
Cr’4. Cr-iPD |
Cr- ~ Cr- |
-0.407 |
|
h\ soi-.s,o;-(pti |
2SOj- + 4H4 + 2e- ^ S,0;“ + 2H:0 |
-0.22 |
|
MnO;. MmOH),. OH iPi> |
MnO- + 2H.0 + c MmOH), ~ OH |
-0.20 |
|
NO,. NOjiPt) |
NO, 1 HjO + 2c- = NO, + 20H |
+0.01 |
|
s,or. S.Oi-iPD |
S40j,- + 2c‘ ^ 2S.0-- |
+0.08 |
|
Sn“. Sn5’ (Pt) |
Sn‘* + 2e- s±Sn- |
+0.151 |
|
Cu;\ Cu’(Ptl |
Cu— + e" — Cu’ |
+0.153 |
|
MnlOHli. MmOH):. OH iPl) |
Mn(OHi, + e Mn(OH): + OH |
+0.20 |
|
CIO,. CIO.. OH (Pt) |
CIO, + H;0 + 2e = CIO, + 20H ’ |
+0.33 |
|
CIO;. CIO,. OH (PU |
CIO, + H:0 + 2c CIO, + 20H |
+0.36 |
|
MnO.. MnO;-(Pt) |
MnO, + e- ^ MnO; |
+0.558 |
|
MnO;. MnO:. OH (PU |
MnO, + 2H;0 1 3 f s± MnO- f 4011 |
+0.588 |
|
MnO: . MnO.. OH (Pu |
MnOj- + 2H>0 + 2c ^ MnO. + 4011 |
+0.60 |
|
CIO,. CIO . OH (Pu |
no, +H;0+2f-e: CIO + 20H’ |
+0.66 |
|
Fe‘”. Fe— (Pt) |
Fe- + e- * Fe- |
+0.771 |
|
CIO-. CI-. OH-iPl) |
CIO- + H;0 + 2e- 5= Cl- + 20H- |
+0.81 |
|
MnO;. H\ Mn-iPo |
MnO- + 4H ’ + c Mn- + 2H,0 |
+0.95 |
|
NO,. H*. NO(Pti |
NO,- -1 411- + 3e" - NO + 211,0 |
+0.957 |
|
cio;. H*. ClOriPl) |
cio; + 2H- + 2«- ^ cto; + h;o |
+ 1.189 |
|
0;. H * lPt> |
0. +4H* +4c- ar 2H.0 |
+ 1.229 |
|
MnO... H*. Mn-lPO |
MnO; +411* + 2e- Mn- + 2lljO |
+ 1.224 |
|
Cr.O*;-. H-. Cr1-(PU |
Cr.O-;- + 14H ’ + 6c' ** 2Cr- + 7H.-0 |
+ 1.33 |
|
MnO.. H\Mn: (PH |
MnO, + SHł + Se- 5= Mn- +4H.O |
+ 1.507 |
|
Mn*. Mm-(PU |
Mn’* +«- sMn:’ |
+ 1.51 |
|
Cc4*. Cc-(PU |
Cc4* + e- Ceł* |
+ 1,72 |
|
Co**. Co-(Pl) |
Co’4 + e“ -ee Co- |
+ 1.92 |
|
s,oJ-. soj i Pu |
S.O; + 2© 2S0; |
+2.010 |
|
MnO*’. H*. MnO iPl) |
MnO*- + 411* + 2e- ^ MnO, + 2H,0 |
+2.26 |
Wyszukiwarka
Podobne podstrony:
A HibUl. IM1U.1 .Vvu .•»•»»«.--u, r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS »*} 7 12 TEORIA PAS
A HibUl. IM1U.1 .Vvu r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >*} 7 12 TE
A HibUl. IM1U.1 .Vvu :u, r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >«} 12
A HibUl. IM1U.1 .Vvu :u, r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >«} 12
A HibUl. IM1U.1 .Vvu :i>, r ), buui :uO ISBN D4H1II 1-7. © l>. »N TOS »*} 12
A HibUl. IM1U.1 .Vvu r.., r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >«} 34
A HibUl. IM1U.1 ,Vvu --u, r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS »*} 2
A HibUl. IM1U.1 ,Vvu r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >«} 2 a SZY
A HibUl. IM1U.1 ,Vvu r.», r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >«} 2
A HibUl. IM1U.1 .Vvu --u, r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >«} 37
A HibUl. IM1U.1 ,Vvu --u, r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS »*} 40 2
A HibUl. IM1U.1 .Vvu -u, r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >«} 2 1
A HibUl. IM1U.1 ,Vvu r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >*} 3 1 DWO
A HibUl. IM1U.1 .Vvu --u, r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >«} 3
A HibUl. IM1U.1 ,Vvu r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >«} 3 2 ZAS
A HibUl. IM1U.1 .Vvu r ), buui :uO ISBN D4H1II ł-Ż © l>. »N TOS »*} 3.2 ZASADA
A HibUl. IM1U.1 .Vvu r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >«} 54 3
A HibUl. IM1U.1 .Vvu -u, r ), buui :uO ISBN D4H1II ł-7. © l>. »N TOS >«} 3 E
więcej podobnych podstron