
Journal of Basic Microbiology 2011, 51, 33 – 39
33
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Research Paper
Effects of Piriformospora indica and Sebacina vermifera
on growth and yield of essential oil in fennel
(Foeniculum vulgare) under greenhouse conditions
Hossein Kari Dolatabadi
1
, Ebrahim Mohammadi Goltapeh
1
, Kamkar Jaimand
2
, Neda Rohani
3
and
Ajit Varma
4
1
Department of Plant Pathology, Faculty of Agriculture, Tarbiat Modarres University, Tehran, Iran
2
Phytochemistry Group, Department of Medicinal plants & By-products, Research Institute of Forest
and Ranglands, Tehran, Iran
3
Department of Plant Protection, Sari Agricultural and Natural Resources University. Sari, Iran
4
Amity Institute of Microbial Technology, Amity University Uttar Pradesh Sector 125 New Super Highway,
Noida, UP, India
Fennel (Foeniculum vulgare) is a very important plant in the family of Apiaceae. Effects of inocu-
lation of two endophytic fungi (Piriformospora indica and Sebacina vermifera) in growth, yield and
composition of the essential oil of fennel (F. vulgare) were evaluated in pot cultures. Dry fruits
were ground with an electric grinder and oil was extracted by hydrodistillation, and their
composition was determined by GC/MS. In pot experiment, the maximum dry weight of the
green tissue and root and plant height were obtained with P. indica, and maximum number of
umbels per plant and dry weight of 1000 fruits were produced with S. vermifera. The P. indica
and S. vermifera inoculation significantly increased oil yield as compared to non-inoculated
control plants. GC and GC/MS studies revealed that the level of anethole was increased with
P. indica and S. vermifera.
Keywords: Piriformospora indica / Sebacina vermifera / Fennel / Apiaceae
Received: June 27, 2010; accepted: September 24, 2010
DOI 10.1002/jobm.201000214
Introduction
*
Fennel (Foeniculum vulgare) a member from the family
Apiaceae is one of the most important aromatic plants
widely applied in culinary and medicinal preparations.
It is generally considered indigenous to the Mediterra-
nean area, but it is also cultivated elsewhere (Russia,
India, China, and Japan) [1, 2]. Fennel is used against
digestive disorders such as spasmodic gastrointestinal
complaints and bloating [3]. It may be an effective di-
uretic and a potential drug for the treatment of hyper-
tension [4, 5], nervous disturbances [6], pediatric colic
and some respiratory disorders due to its antispasmodic
effects. It is also a galactogogue [7]. Essential oils are
mainly concentrated in the fruits and provide their
unique aroma and taste. Anethole and
fenchone are
Correspondence: E. Mohammadi Goltapeh, Department of Plant Pa-
thology, Faculty of Agriculture, Tarbiat Modarres University, Tehran, Iran
E-mail: emgoltapeh@yahoo.com or emgoltapeh@modares.ac.ir
the most important volatile components
of F. vulgare
essential oil [8].
Arbuscular mycorrhizal fungi (AMF), obligate bio-
trophs belonged to the phylum Glomeromycota [10]
form symbiotic relationships with roots of about 90%
of land plants in natural and agricultural ecosystems
[9]. AM fungi provide several benefits to their host
plants, including increased uptake of phosphorus (P)
and other nutrients, increased resistance to abiotic
stress and increased resistance disease [11–14]. In con-
trast to most mycorrhizal fungi, Piriformospora indica
[15] and Sebacina vermifera [16] are cultivable fungi and
can grow on synthetic or complex media in the absence
of their plant hosts [17, 18]. Being of a wide host range,
P. indica is capable of colonizing the roots of numerous
mono- and dicotyledonous plants [19, 20]. Many authors
have reported that P. indica can improve the growth
rate of various host plants [21–25]. In this study Piri-
formospora indica and Sebacina vermifera have been tested

34
H. K. Dolatabadi et al.
Journal of Basic Microbiology 2011, 51, 33 – 39
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
for their effects on the growth and essential oil yield
Fennel (Foeniculum vulgare) under green house condi-
tions.
Materials and methods
Cultivation of P. indica and S. vermifera
Fungi were grown in Petri dishes on a Kaefer’s medium
[26]. The plates were inoculated with the fungi and
incubated at 25 ± 1 °C in an incubator for a week.
Liquid culture of P. indica and S. vermifera
Broth cultures were prepared in 500 ml flasks contain-
ing 200 ml of autoclaved KM liquid medium through
inoculation with four mycelia disks from 10 d old agar
culture of P. indica and S. vermifera. Flasks were kept on
a shaker (140 rpm) at the 25 ± 1 °C for 15 d till dense
mycelial suspensions were generated. Then, the broth
cultures were stored at 4 °C for pot culture experi-
ments.
Pot culture experiments
Seed of F. vulgare were obtained from a farm of the In-
stitute of Medicinal Plants & Natural Products Research,
Karaj, Iran. Pot culture experiments were conducted
following a completely randomized design in three
replications in 2009. Seed of F. vulgare were surface-
sterilized soaking them in 1% sodium hypochlorite for
1 min then the seed were rinsed three times in sterile
distilled water and placed in sterile perlite for germina-
tion. After 12–16 d when the plumule and radicle ap-
peared, two germinated seeds were transferred to each
pot culture and grown in a 2:1:1 sterile mixture of
sand, peat, and perlite, with the following chemical
properties: pH 7.12, EC 0.9 ds/m, organic carbon 0.7%,
total N 0.3%, available phosphorus 4.2 mg/kg and po-
tassium 185 mg/kg in green house at 24: 18 °C tempera-
ture and a photoperiod of 16 h light: 8 h dark. For in-
oculation, inoculum (1% w/v) of crushed mycelium of
P. indica and S. vermifera was added to germlings. Efforts
were made to keep the root system in direct contact
with the fungal inocula as Kumari, Kishan, Bhoon, and
Varma and Varma and Schuepp’s method of inocula-
tion [24, 27]. The controls were also maintained without
inocula and only with sterile distilled water. Plants
grown in pots were analyzed after 150 d. Roots were
washed thoroughly under running tap water and cut
into 1 cm pieces for microscopic observations. Seg-
ments were stained following the techniques described
in literature [28, 29]. The root-pieces were examined
under microscope at the magnification of 10–40 X.
Isolation of essential oil
According to the type of culture, 10–20 g dry fruits
were crushed in electric grinder and submitted to hy-
drodistillation in a Clevenger-type device for two hours
in order to extract the oil. Samples were dried with
anhydrous sodium sulfate and kept in vials at 4 °C until
chromatographic analysis.
Essential oil analysis
The chemical composition of oil samples were analyzed
on a Thermo-UFM (Ultera Fast Model) gas chromato-
graph equipped with a flame ionization detector (FID)
and a Ph-5 capillary column (5% Dimethylsiloxane
phenyl, 10 m length, 0.1 mm in diameter and 0.25 μm
film thickness). Analyses were run under the following
conditions: helium carrier gas flow: 0.5 ml/min, injec-
tion temperature 280 °C, the detector’s temperature
290 °C, and the oven temperature programmed from
60 °C to 285 °C at the rate of 80 °C/min.
GC-MS analyses were performed on a Varian 3400 gas
chromatograph attached to a Saturn II mass spectro-
meter operating in electron impact ionization mode at
70 eV. The DB-5 capillary column (30 m length, 0.25 mm
in diameter and 0.25 μm film thickness) was initially at
40 °C, increased to 250 °C at a rate of 4 °C/min. The
injector was held at 260 °C and the MS transfer line
was held at 270 °C. The components were identified
through the comparison of their Kovats indices (KI)
relative to C7-C25 n-alkanes and mass spectra with au-
thentic standards and with spectral data from library
files and literature [30–32].
Statistical analysis
The collected data were statistically computed using
software SAS 6.12. Data were subjected to analyses of
variance and treatment means were compared by an
approximate Duncan’s multiple-range tests and main
effective interaction was found significant at P < 0.05.
Results
Both P. indica and S. vermifera significantly increased
growth of the inoculated of fennel plants in compari-
son to non-inoculated control plants (Fig. 1). To assess
the influence of P. indica and S. vermifera on plant mor-
phology after 150 d, plant height, number of umbels
per plant, dry weight of 1000 fruits and dry weight
(shoot, root) were analyzed. The mean plant height
ranged from 92.67 to 107.67 cm. The highest plants
were those inoculated with P. indica, followed by S. ver-
mifera and controls (Fig. 2a). The Number of umbels per
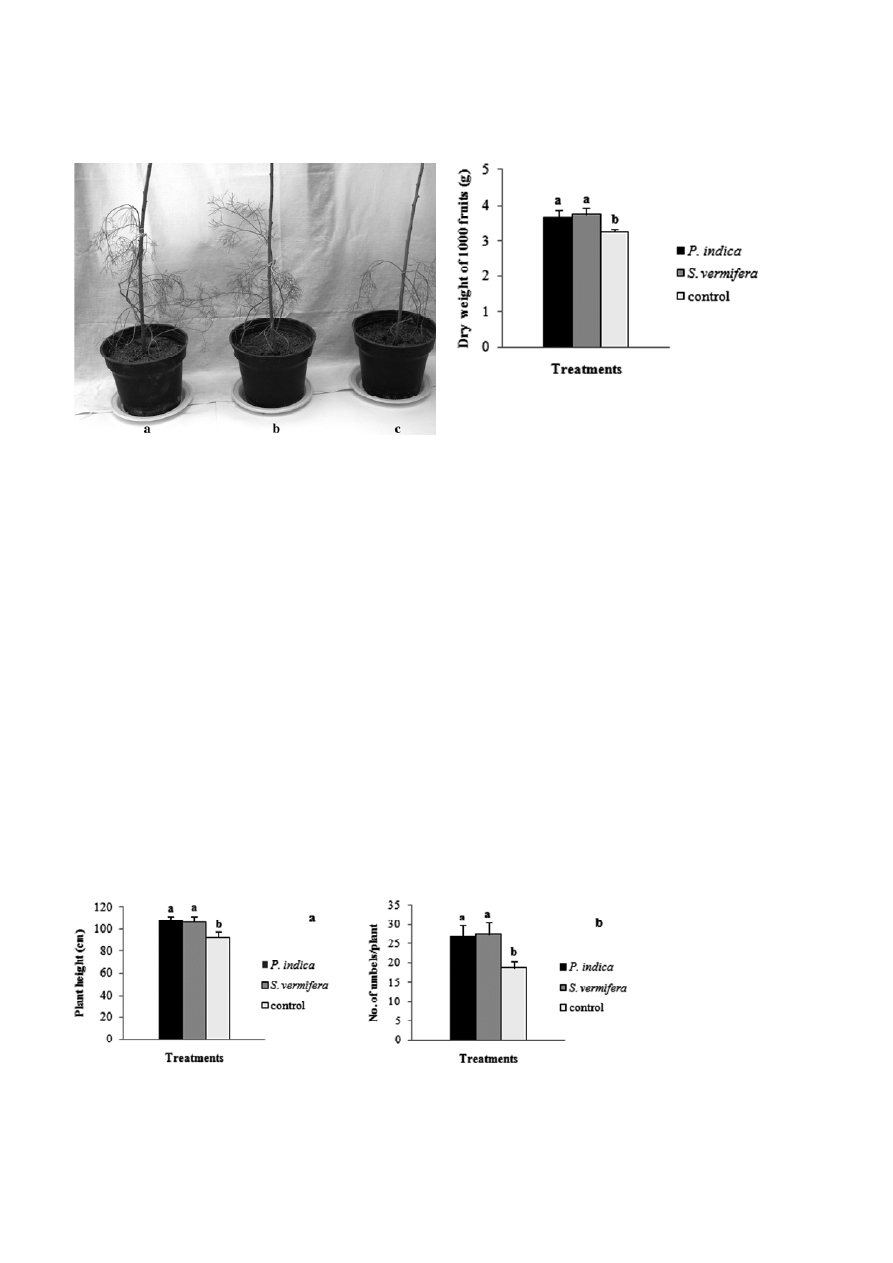
Journal of Basic Microbiology 2011, 51, 33 – 39
Effect of endophytic fungi on essential oil production in fennel
35
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 1. Effect of endophytic fungi on plant growth in Foeniculum
vulgare. a: Piriformospora indica, b: Sebacina vermifera and c:
control.
plant inoculated with S. vermifera was 47% higher than
that of non-inoculated control plants (Fig. 2b).
Both
P. indica and S. vermifera significantly increased
dry weight of 1000 fruits in comparison to controls.
Dry weight of 1000 fruits in plants inoculated with
S. vermifera was 16% higher than that of controls and
3% higher than that of plants inoculated with P. indica
(Fig. 3).
In addition, the mean shoot dry weight ranged from
3.02 to 3.66 g. The highest shoot dry weight was pro-
duced by the plants inoculated with P. indica, and the
lowest shoot dry weight was determined with control
plants. Similarly, the root dry weight ranged from 0.98
to 1.31 g, where the highest root dry weight was re-
corded with the plants inoculated with P. indica and the
lowest root dry weight was measured with the control
plants. The dry weight of the aerial parts of the plants
inoculated with P. indica or S. vermifera increased by 21
and 19%, and those of the root by 34 and 21% in com-
Figure 3. Effect of P. indica and S. vermifera on dry weights of
1000 fruits (g) in F. vulgare. The upward error bars within the
column represents standard deviation (SD). Histograms with a
differing letter are significantly different by Duncan’s multiple range
test (P < 0.05).
parison to control plants (Fig. 4a and b). Microscopic
inspection of roots inoculated with P. indica and S. ver-
mifera detected heavy colonization and abundant pro-
duction a large number of chlamydospores in root cells
(Fig. 5).
The concentration of essential oil increased in pot
cultures inoculated with P. indica and S. vermifera in
comparison to controls (Fig. 6). The response of P. indica-
colonized plants was better than that of S. vermifera-
colonized plants. The highest (2.46% w/w) and the low-
est (1.83% w/w) essential oil yields were obtained with
P. indica- inoculated plants and control, respectively.
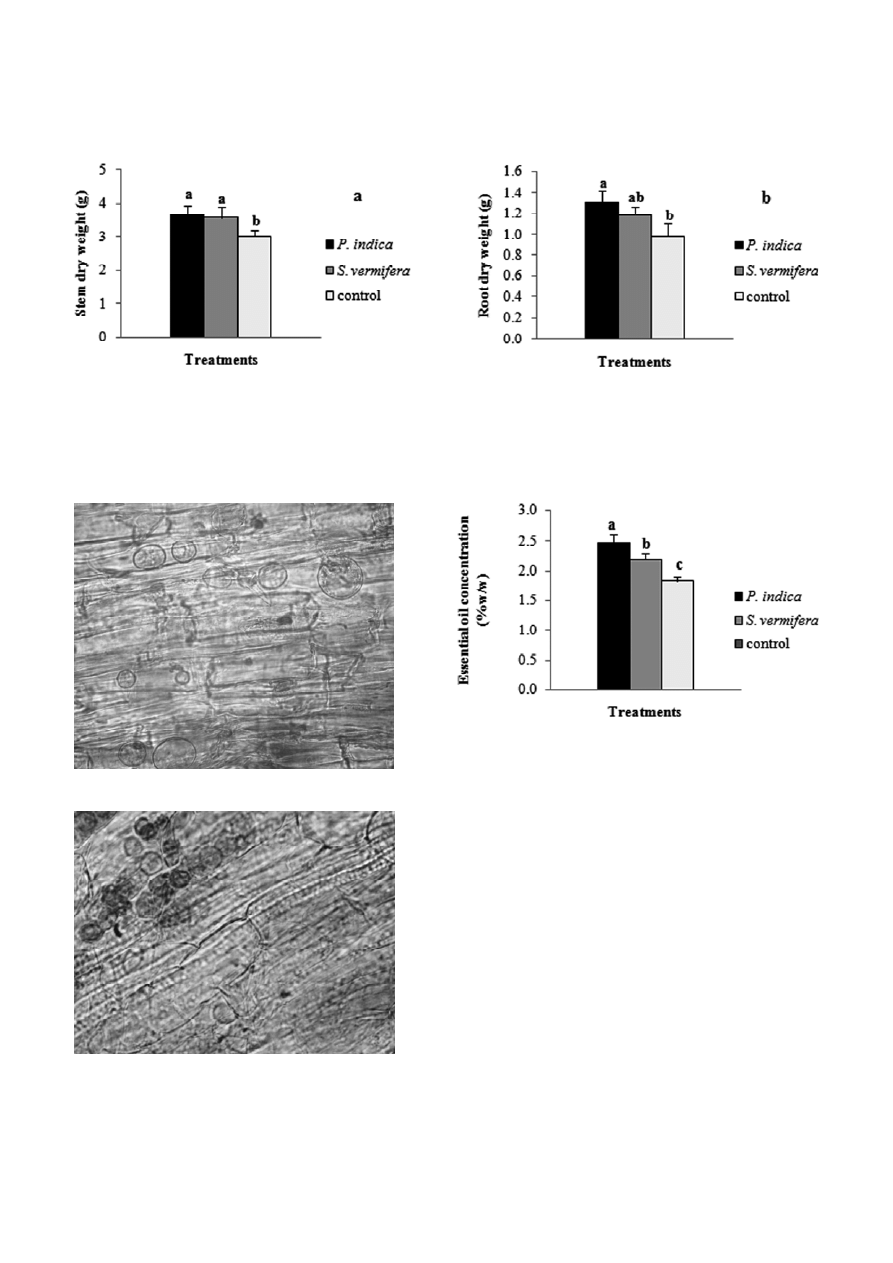
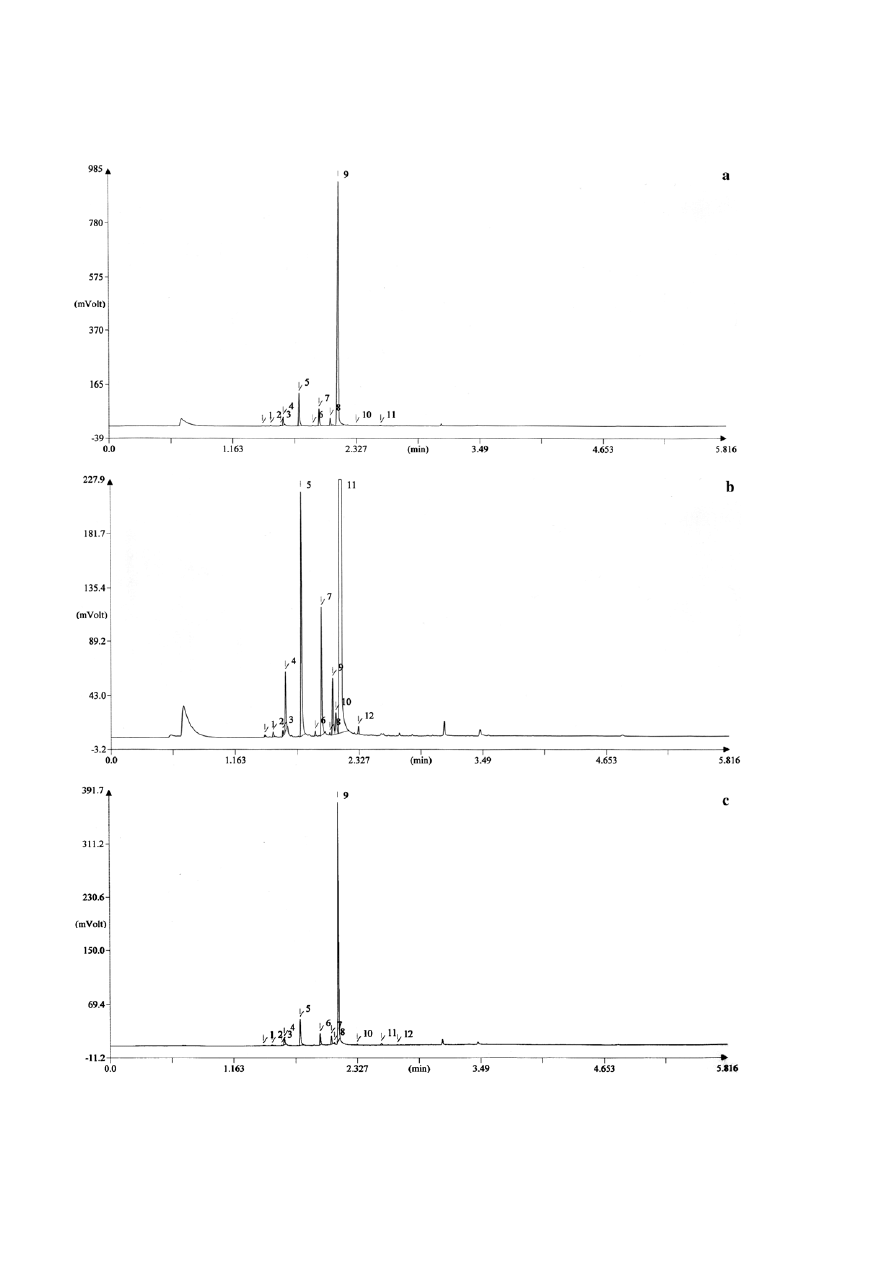
The gas chromatographs and essential oil composi-
tion obtained have been shown in Fig. 7 and Table 1.
The major constituents of the oil in P. indica-inoculated,
S. vermifera-inoculated and control plant were E-ane-
thole (83.3, 82.1 and 77.4%, respectively), fenchone (8.4,
8.3 and 11%, respectively), Methyl chavicol (3.8, 3.8 and
3.6%, respectively), trans-carvone oxide (1.7, 2 and
3.3%, respectively) and γ-terpinene (2.0, 1.8 and 2.4%,
respectively) (Table 1).
Figure 2. (a) Effect of P. indica and S. vermifera on plant height (cm) in F. vulgare. (b) Effect of P. indica and S. vermifera on number of
umbels per plant in F. vulgare. The upward error bars within the column represents standard deviation (SD). Histograms with a differing
letter are significantly different by Duncan’s multiple range test (P < 0.05).
36
H. K. Dolatabadi et al.
Journal of Basic Microbiology 2011, 51, 33 – 39
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 4. (a) Effect of P. indica and S. vermifera on stem dry weight (g) in F. vulgare. (b) Effect of P. indica and S. vermifera on root dry
weight in F. vulgare. The upward error bars within the column represents standard deviation (SD). Histograms with a differing letter are
significantly different by Duncan’s multiple range test (P < 0.05).
a
b
Figure 5. Detection of chlamydospores of endophytic fungi in root
cells of Foeniculum vulgare. a: Piriformospora indica, b: Sebacina
vermifera.
Figure 6. Effect on P. indica and S. vermifera on essential oil con-
centration (% g/g dry weight). The upward error bars within the
column represents standard deviation (SD). Histograms with a
differing letter are significantly different by Duncan’s multiple range
test.
Discussion
Data on growth and oil yield clearly showed that
P. indica and S. vermifera inoculation had a stimulating
effect on the growth. Arbuscular mycorrhizal fungi
enhance plant growth by increasing nutrients and wa-
ter uptake, explored soil volume 100× greater, and pre-
vent heavy metal toxicity, pathogenic infection, and
improve soil structure [33–38]. It is well known that
Arbuscular mycorrhizal-like fungi enhance growth and
biomass production in symbiotic plants [21–25]. This
study showed that P. indica and S. vermifera inoculation
could increase plant height compared to controls. Ghi-
mire et al. [39] reported that S. vermifera increased plant
height, root length, and biomass production in Switch-
grass (Panicum virgatum L). Rai et al. [23] also reported
Journal of Basic Microbiology 2011, 51, 33 – 39
Effect of endophytic fungi on essential oil production in fennel
37
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 7. Effect of endophytic fungi in chromatogram for fennel oil (Foeniculum vulgare). a: Piriformospora indica, b: Sebacina vermifera
and c: control.

38
H. K. Dolatabadi et al.
Journal of Basic Microbiology 2011, 51, 33 – 39
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Table 1. Effect of P. indica and S. vermifera inoculation on con-
centration (%) of various constituents in fennel oil after 150 d.
Treatments Components
Piriformospora
indica
Sebacina
vermifera
control
γ
-terpinene
2.0
1.8
2.4
fenchone
8.4
8.3
11.0
Methyl chavicol
3.8
3.8
3.6
trans-carvone oxide
1.7
2.0
3.3
cis-verbenyl acetate
–
1.0
0.5
E-anethole
83.3
82.1
77.4
Germacrene B
0.2
–
0.8
that shoot and root length, biomass, basal stem, leaf
area, overall size, number of inflorescences, flowers
and seed production in Spilanthes calva and Withania
somnifera were increased in the presence of the P. indica.
Maximum shoot and root dry weight were observed
with P. indica. Varma et al. [21] reported that inoculation
of P. indica promotes plant growth and biomass produc-
tion. Plants inoculated with S. vermifera and P. indica
produced higher number of umbels per plants, more
dry weight of 1000 fruits and also increased level of
anethole content. Kapoor et al. [12] reported that AM
(Glomus macrocarpum and Glomus fasciculatum) inocula-
tion led to the significantly increased shoot biomass,
number of umbels per plant, dry weight of 50 fruits
and level of anethole in fennel plants. P. indica produces
low amounts of auxins, and relatively high levels of
cytokinins, and the cytokinin levels are higher in colo-
nized roots compared with that of uncolonized controls
[40]. Sirrenberg et al. [41] suggested that auxin produc-
tion affecting root growth is responsible for, or at least
contributes to, the beneficial effect of P. indica on its
host plants. P. indica was shown to produce IAA (indole
acetic acid) in liquid culture [41]. In presents study, our
observation and data showed that P. indica and S. vermif-
era could affect growth and increase essential oil levels
of F. vulgare.
Acknowledgement
This study was supported by the Department of Plant
Pathology, Faculty of Agriculture, Tarbiat Modarres
University. We thank the Research Institute of Forests
and Rangelands, Tehran, Iran for GC and GC-mass
analyses.
References
[1] Samuelsson, G., 1992. Drugs of Natural Origin, Swedish
Pharmaceutical Press, Stockholm, p. 94.
[2] Evans, W.C., 1989. Trease and Evans’ Pharmacognosy,
Bailliea re Tindall, London, p. 440.
[3] Bilia, A.R., Fumarola, M., Gallori, S., Mazzi, G., Vincieri,
F.F., 2000. Identification by HPLC-DAD and HPLC-MS ana-
lyses and quantification of constituents of fennel teas and
decoctions. J. Agric. Food. Chem., 48, 4734–4738.
[4] Wright, C.I., Van-Buren, L., Kroner, C.I., Koning, M.M.,
2007. Herbal medicines as diuretics: a review of the scien-
tific evidence. J. Ethnopharmacol., 114(1), 1–31.
[5] El Bardai, S., Lyoussi, B., Wibo, M., Morel, N., 2001. Phar-
macological evidence of hypotensive activity of Marrubium
vulgare and Foeniculum vulgare in spontaneously hyperten-
sive rat. Clin. Exp. Hypertens., 23(4), 329–43.
[6] Jahromi, B.N., Tartifizadeh, A., Khabnadideh, S., 2003.
Comparison of fennel and mefenamic acid for the treat-
ment of primary dysmenorrhea, Int. J. Gynecol. Obstet.,
80, 153.
[7] Crellin, J.K., Philpott, J., Tommie Bass, A.L., 1989. A Refer-
ence Guide to Medicinal Plants Herbal Medicine Past and
Present. Duke University Press, pp. 207–208.
[8] Sim’andi, B., De’ak, A., R’onyai, E., Yanxiang, G. et al.,
1999. Supercritical carbon dioxide extraction and frac-
tionation of fennel oil, J. Agric. Food Chem., 47, 1635.
[9] Brundrett, M.C., 2002. Coevolution of roots and my-
corrhizas of land plants. New Phytol., 154, 275–304.
[10] Schussler, A., Schwarzott, D., Walker, C., 2001. A new
fungal phylum, the Glomeromycota: phylogeny and evo-
lution. Mycol. Res., 105, 1413–1421.
[11] Brundrett, M., Bougher, N., Dell, B., Grove, T., Malajczuk,
N., 1996. Working with mycorrhiza in forestry and agri-
culture, ACIAR Monograph 32, 374 pp.
[12] Kapoor, R., Giri, B., Mukerji, K.G., 2004. Improved growth
and essential oil yield and quality in Foeniculum vulgare
Mill. on mycorrhizal inoculation supplemented with P-
fertilizer. Bioresour. Technol., 93, 307–311.
[13] Kapoor, R., Chaudhary, V., Bhatnagar, A.K., 2007. Effects
of arbuscular mycorrhiza and phosphorus application on
artemisinin concentration in artemisia annua L. Mycor-
rhiza, 17, 581–587.
[14] Sharma, D., Kapoor, R., Bhatnagar, A.K., 2008. AM fungi
help in conservation of curculigo orchioides gaertn – a vul-
nerable anticancerous plant. World J. Microbiol. Biotech-
nol., 24, 395–400.
[15] Verma, S., Varma, A., Rexer, K.H., Kost, G. et al., 1998. Piri-
formospora indica, gen. et sp. nov., a new root-colonizing
fungus. Mycologia, 95, 896–903.
[16] Warcup, J.H., 1988. Mycorrhizal associations of isolates of
Sebacina vermifera. New Phytol., 110, 227–231.
[17] Varma, A., Singh, A., Sudha Sahay, N., Sharma, J. et al.,
2001. Piriformospora indica: A cultivable mycorrhiza-like
endosymbiotic fungus. In: Mycota IX, Springer Series, Ger-
many, pp. 123–150.
[18] Peskan-Berghoefer, T., Shahollaria, B., Giong, P.H., Hehl,
S. et al., 2004. Association of Piriformospora indica with Ara-
bidopsis thaliana roots represents a novel system to study
beneficial plant–microbe interactions and involves early
plant protein modifications in the endoplasmatic reticu-
lum and at the plasma membrane. Physiol. Plant, 122,
465–477.

Journal of Basic Microbiology 2011, 51, 33 – 39
Effect of endophytic fungi on essential oil production in fennel
39
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
[19] Pham, G.H., Singh A., Kumari, R., Malla, R. et al., 2004.
Interactive of Piriformospora indica with diverse microor-
ganisms in plants. In: Varma A., Abbott L., Werner D. and
Hampp R. (eds.). Plant Surface Microbiology. Springer-
Verlag, Berlin, pp. 237–265.
[20] Verma, R.K., Arya, I.D., 1998. Effect of arbuscular my-
corrhizal fungal isolates and organic manure on growth
and mycorrhization of micropropagated Dendrocalamus
asper plantlets and on spore production in their rhi-
zosphere. Mycorrhiza, 8, 113–116.
[21] Varma, A., Verma, S., Sudha Sahay, N., Bütehorn, B.,
Franken P., 1999. Piriformospora indica, a cultivable plant
growth-promoting root endophyte. Appl. Environ. Micro-
biol., 65, 2741–2744.
[22] Sahay, N.S., Varma, A., 1999. Piriformospora indica: a new
biological hardening tool for micropropagated plants.
FEMS Microbiol. Lett., 181, 297–302.
[23] Rai, M., Acharya, D., Singh, A., Varma, A., 2001. Positive
growth responses of the medicinal plants Spilanthes calva
and Withania somnifera to inoculation by Piriformospora in-
dica in a field trial. Mycorrhiza, 11, 123–128.
[24] Kumari, R., Kishan, H., Bhoon, Y.K., Varma, A., 2003.
Colonization of cruciferous plants by Piriformospora indica.
Curr. Sci., 85, 1672–1674.
[25] Waller, F., Achatz, B., Baltruschat, H., Fodor, J. et al.,
2005. The endophytic fungus Piriformospora indica repro-
grams barley to salt-stress tolerance, disease resistance,
and higher yield. Proc. Natl. Acad. Sci. USA, 38, 13386–
13391.
[26] Kaefer, E., 1977. Meiotic and mitotic recombination in
Aspergillus and its chromosomal aberrations. Advances in
Genetic, 19, 33–131.
[27] Varma, A., Schuepp, H., 1995. Mycorrhization of the
commercially important micropropagated plants. Crit.
Rev. Biotechnol., 15, 313–328.
[28] Dickson, S., Mandeep and Smith, S.M., 1998. Evaluation of
vesicular arbuscular mycorrhizal colonization by stain-
ing. In: Mycorrhiza Manual (Varma, A., ed.), pp. 77–84.
Springer-Verlag, Berlin.
[29] Phillip, J.M., Hayman, D.S., 1970. Improved procedures
for clearing roots and staining parasitic and VAM fungi
for rapid assessment of infection. Trans. Br. Mycol. Soc.,
55, 158–161.
[30] Adams, R.P., 1989. Identification of Essential Oils by Ion
Trap Mass Spectroscopy. Academic Press: New York.
[31] Shibamoto, T., 1987. Retention indices in Essential oil
analysis. In: Capillary Gas Chromatography in Essential
oils analysis (P. Sandra and C. Bicchi, eds.), pp. 259–274,
Dr. Alferd Huethig Verlag, New York.
[32] Davies, N.W., 1990. Gas chromatographic retention index
of monoterpenes and sesquiterpenes on methyl silicon
and carbowax 20M phases. Chromatogr., 503, 1–24.
[33] Marschner, H., Dell, B., 1994. Nutrient uptake in my-
corrhizal symbiosis. Plant and Soil, 159, 89–102.
[34] Cooper, K.M., 1984. Physiology of VA mycorrhizal associa-
tions. In: VA Mycorrhiza (C.L. Powell and D.J. Bagyaraj,
eds.), pp. 155–186. CRC Press, Inc., Boca Raton, Florida.
[35] Huang, R.S., Smith, W.K., Yost, R.E., 1985. Influence of
vesicular arbuscular mycorrhizae on growth, water rela-
tion and leaf orientation in Lcucaena Icucocephala (Lam.) de
wit. New Phytol., 99, 229–243.
[36] Ellis, J.R., Larsen, H.J., Boosalis, M.G. 1985. Drought re-
sistance of wheat plants inoculated with vesicular my-
corrhizae. Plant and Soil, 86, 369–378.
[37] Dela Cruz, R.E., 1991. Status of Bio-reforestation in the
Philippines. Paper presented during the pre-Workshop on
Bio-Reforestation sponsored by IUFRO Japan, Bogor, Indo-
nesia March 25–29.
[38] Abbott, I.K., Robson, A.D., 1982. The role of vesicular-
arbuscular mycorrhizal fungi in agriculture and the se-
lection of fungi for inoculation. Aust. J. Res., 33, 389–408.
[39] Ghimire, S.R., Charlton, N.D., Craven, K.D., 2009. The
mycorrhizal fungus, Sebacina vermifera, enhances seed
germination and biomass production in switchgrass (Pani-
cum virgatum L). Bioenerg. Res., 2, 51–58.
[40] Vadassery, J., Ritter, C., Venus, Y., Camehl, I. et al., 2008.
The role of auxins and cytokinins in the mutualistic in-
teraction between Arabidopsis and Piriformospora indica.
Molecular Plant-Microbe Interactions, 21(10), 1371–1383.
[41] Sirrenberg, A., Gobel, C., Grond, S., Czempinski, N. et al.,
2007. Piriformospora indica affects plant growth by auxin
production. Physiologia Plantarum, 131, 581–589.
((Funded by
• Department of Plant Pathology, Faculty of Agriculture, Tarbiat Modarres University))
Wyszukiwarka
Podobne podstrony:
jobm 201000013
jobm 201000298
jobm 201000191
jobm 201000321
jobm 201000018
jobm 201000067
jobm 201000037
jobm 201000074
jobm 201000280
jobm 201000385
jobm 201000198
jobm 201000458
jobm 201000147
jobm 201000520
jobm 201000327
jobm 201000342
jobm 201000420
jobm 201000364
jobm 201000317
więcej podobnych podstron