
Journal of Basic Microbiology 2011, 51, 523 – 530
523
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Research Paper
Root colonization of a rice growth promoting strain
of Enterobacter cloacae
Manoharan Shankar, Paramasivan Ponraj, Devaraj Ilakkiam and Paramasamy Gunasekaran
Department of Genetics, Centre for Excellence in Genomic Sciences, School of Biological Sciences,
Madurai Kamaraj University, Madurai, India
Enterobacter cloacae GS1 was isolated by in-planta enrichment of a rice rhizoplane bacterial
community. It displayed strong seed adherence ability (2.5 × 10
5
cfu/seed) and colonized rice
roots reaching up to 1.65 × 10
9
cfu/g of fresh root weight in a gnotobiotic root colonization
system. E. cloacae GS1 was motile, able to solubilize tricalcium phosphate, and produced indole
acetic acid like substances (15 μg/ml). As an introduced bioinoculant in non-sterile soil,
E. cloacae GS1 colonized rice roots and significantly improved the fresh weight, root length,
shoot length, and nitrogen content in inoculated rice seedlings as compared to uninoculated
controls. This isolate was tagged with green fluorescent protein and various stages of root
colonization in gnotobiotic hydroponic environment and non-sterile soil environment were
followed by fluorescence microscopy. Owing to its effective root colonizing ability and growth
promoting potential, Enterobacter cloacae
GS1 is a promising symbiotic bioinoculant for rice.
Keywords: Enrichment / Root colonization / Seed adherence / Green fluorescent protein / Bioinoculant / Growth
promotion
Received: August 27, 2010; accepted: February 22, 2011
DOI 10.1002/jobm.201000342
Introduction
*
Plant growth promoting rhizobacteria are the future
hope for organic agriculture both in terms of growth
promotion and biocontrol. They maintain a close asso-
ciation with plants, control pathogens and help in nu-
trient acquisition. Growth promotion by rhizobacteria
can be attributed to one or more of factors such as
nitrogen fixation, mineral solubilization, nutrient scav-
enging and release of plant growth hormones [1]. Colo-
nization of plant roots by bacteria is an important step
in the interaction between beneficial bacteria and the
host plant. Bacteria found effective in growth promo-
tion and biocontrol under laboratory conditions some
times fail due to their inability to colonize plant roots
and the rhizosphere [2]. Root colonization is a multifac-
tor dependant process initiated by coordinated motility
Correspondence: Prof. Paramasamy Gunasekaran, Department of Ge-
netics, Center for Excellence in Genomic Sciences, School of Biological
Sciences, Madurai Kamaraj University, Madurai – 625021, Tamil Nadu,
India
E-mail: gunagenomics@gmail.com
Phone: +914522458478
Fax: +914522459873
of bacteria towards the roots in response to plant
chemo-attractants followed by adhesion of bacterial
cells to the root surface [3]. Thus, the analysis of root
colonization by beneficial bacteria under gnotobiotic
conditions and soil environments would provide in-
sights into the behaviour of the microbe in simple and
complex ecosystems, the knowledge of which is essen-
tial for the development of effective bioinoculants.
There has always been a constant need to identify
and study new and native plant growth promoting
bacteria from various agro-ecological niches [4]. Being
the staple food for over 40% of the world’s population,
rice has been the interest of several investigations prob-
ing beneficial rhizospheric bacterial communities. The
role of diazotrophic enterobacteria in the rice rhizo-
sphere have been well reviewed [5]. Fatty acid methyl
ester (FAME) profiling and BOX PCR analysis also re-
vealed that the Enterobacteriaceae are the most diverse
growth promoting population of Gram-negative bacte-
ria associated with rice seeds, among which Pantoea
spp. and Enterobacter spp. are dominating populations
[6]. Conventional screening methods for plant growth
promoting rhizobacteria include detection of known

524 M.
Shankar
et al.
Journal of Basic Microbiology 2011, 51, 523 – 530
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
plant growth promoting traits such as N-fixation, P-so-
lubilization, plant growth hormone production, secre-
tion of antimicrobial substances, tolerance to stresses,
production of ACC deaminase etc. Use of methods
which allow enrichment of microbes that are beneficial
to plants under in-vivo conditions is an attractive alter-
native to above methods as they demonstrate the eco-
logical success of the microbe on plant hosts. For ex-
ample, an enrichment technique applying in-planta
selection has been used in the identification of a plant
and bacterium for rhizostimulation of Polycyclic Aro-
matic Hydrocarbon degrading bacteria [7] and the selec-
tion of bacteria with enhanced root colonization ability
from a rhizospheric bacterial suspension [8].
We report here, the application of this in-planta en-
richment strategy for identification of an effective root
colonizer from a rice rhizospheric bacterial population.
The ability of the bacteria to colonize rice roots under
gnotobiotic and soil environments was examined. The
potential of the root colonizing PGPR as a bioinoculant
was also explored.
Materials and methods
Gnotobiotic colonization assay and direct
enrichment of root colonizing bacteria
Surface sterilization and germination of rice seeds was
carried out as described earlier [9]. Three day old ger-
minated seedlings were transplanted to glass tubes
(30 × 200 mm) containing 60 ml of half strength nutrient
solution [10]. Seedlings were supported by a thin disc of
polyurethane foam as the inert support and tubes were
covered by placing other tubes on top of them. Gnotobi-
otic plants were then grown at 25 ± 1 °C with 16 h day,
8 h night cycle. Four day old seedlings were inoculated
with 1 ml of a suspension of bacterial culture in sterile
10 mM MgSO
4
(~10
8
cfu) for root colonization assays
whereas control plants received sterile 10 mM MgSO
4
.
Enrichment of bacteria was carried out as described
earlier [7] with modifications. The choice of field to be
sampled was made based on the healthy and nourished
appearance of plants in the selected field as compared to
other fields in the area. Healthy rice plants (cv. IR20)
were uprooted along with adherent soil from the select-
ed field in Madurai, transferred to sterile plastic bags and
transported to the laboratory. Loose soil was removed by
five washes with sterile distilled water. Ten grams of root
material with tightly adhering soil particles was trans-
ferred to an Erlenmeyer flask containing 50 ml saline
and shaken at 200 rpm for 20 min. Thoroughly washed
root material hypothesized to contain only irreversibly
attached microflora was shaken in 50 ml of saline con-
taining 50 glass beads (0.3 mm diameter) at 200 rpm for
20 min to dislodge tightly adhered cells. The supernatant
containing the tightly adhered microbial flora (rhizo-
spheric suspension) was supplied as inoculum (1 ml) to
gnotobiotic rice seedlings grown hydroponically for en-
richment of effective root colonizers in-planta.
Phenotyping of the selected isolate
Carbohydrate utilization profile of the isolate was ob-
tained using HiCarbohydrate
TM
kit (Himedia laborato-
ries, Mumbai, India) and compared against previous
documentation [11]. The total IAA like substances in
the 24 h culture supernatant was estimated as de-
scribed earlier [12]. Phosphate solubilization ability of
the isolate was judged by appearance of a clear zone
around the colony on Pikovskaya’s agar medium [13].
Swimming and swarming motility was examined by
spotting 1 μl of an exponentially growing broth culture
of the organism on the surface of LB soft agar (0.3 and
0.7% respectively) as described earlier [14].
Identification and phylogenetic analysis
Using standard molecular biology protocols [15], the
16S rDNA was amplified from the genomic DNA
of isolate GS1 using bacterial primers 8F
(5′-AGAGTTTGATC(AC)TGGCTCAG-3′) and 1522R
(5′-AAGGAGGTGATCCA(AGCT)CC(AG)-3′) [16] and ligated
with pTZ57R/T (Fermentas, Opelstrasse, Germany).
Escherichia coli DH5α was transformed with the ligation
mix and the recombinant plasmid was isolated from
the transformants and sequenced at Macrogen, Seoul,
South Korea. The sequence was analysed with BLASTN
[17]. Related Enterobacteriaceae sequences obtained from
the database were used to construct a phylogenetic tree
using the Phylogeny inference Package [18].
Seed and root colonization studies
Bacterial seed adhesion was estimated by immersion of
surface sterilized, germinated rice seeds (cv. IR64) in
a bacterial suspension (1.7 × 10
8
cfu/ml) prepared in
10 mM MgSO
4
while control seeds were immersed in
sterile 10 mM MgSO
4
. Seeds were recovered at different
time points (1, 10, 20, 30 min) and washed by vortexing
in saline for 1 min to remove reversibly adhered cells
[19–21]. Washed seeds were transferred to a fresh tube
containing 5 ml of saline and 5 glass beads (3 mm di-
ameter) and vortexed to dislodge tightly adhered cells
followed by estimation of bacterial count by plating of
an appropriate dilution on LB agar. Triplicates were
processed for each time point and all values reported
are means of these measurements. To estimate root

Journal of Basic Microbiology 2011, 51, 523 – 530
Rice root colonization by Enterobacter cloacae GS1
525
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
colonizing ability, hydroponically grown rice plants pre-
viously inoculated with a ~10
8
cfu/ml suspension of GS1
were recovered at 2 h and between 24–192 h post in-
oculation at 24 h intervals. Bacterial count on each root
surface was estimated by vortexing root systems in the
presence of glass beads and plating appropriate dilutions
of the suspension on LB agar. Normalization of counts
was carried out based on the root weight and cfu were
expressed as log (cfu/g fresh root weight). This value was
then plotted against time to observe the trend of bacte-
rial population on rice roots. Three different plants were
processed for each time point and values shown are
means of these measurements. Negative controls which
received sterile 10 mM MgSO
4
instead of bacterial sus-
pension were processed in parallel at each time point.
Plant growth promotion assay
Rice seeds (cv. IR64) previously surface sterilized and
germinated for 3 d on moist filter paper were sown in
plastic pots each containing ~500 g of non-sterile field
soil. Two seeds were sown per pot, and 10 such repli-
cates were included for both control and treated
groups. Plants were adequately watered with sterile
distilled water once a day in the evening. One ml sus-
pension (OD
600
= 0.2) of E. cloacae GS1 prepared in
10 mM MgSO
4
(~10
8
cfu/ml) was applied as a soil drench
to the treated group immediately after watering on the
second day after sowing whereas control group received
sterile 10 mM MgSO
4
. Thirty two days post inoculation,
both groups of plants were uprooted, washed to remove
adhering soil and blotted dry. Fresh weight, root length
and shoot length were measured for both control and
treated plants. Shoots of individual control and inocu-
lated plants were dried completely at 80 °C for 48 h.
The dried shoot samples were ground to fine powder
and used for determination of N, P and K content. Ni-
trogen content of plant material was determined using
a CHN analyser (Perkin Elmer-2400) while P and K were
estimated as described earlier [10] by spectrophotome-
try (Shimadzu UV-2600) and atomic absorption spec-
troscopy (Perkin Elmer AAnalyst 400) respectively. Two
independent experiments were conducted and the data
presented was obtained from one of the experiments.
Results were analysed by one way analysis of variance
(p = 0.05) (MATLAB, Mathworks inc.). The null hypothe-
sis stated that the inoculation of E. cloacae GS1 did not
have any effect on the growth of rice seedlings based on
the parameters measured.
Tagging E. cloacae GS1 with GFP
The plasmid pGB5, previously reported construct carry-
ing gfpmut2 and stably maintained in Pseudomonas sp.
during root colonization [22] was used for tagging
E. cloacae GS1. Electrocompetent cells of E. cloacae GS1
were electrotransformed with pGB5 (Biorad Gene Pulser
Xcell
TM
; 25 μF, 200 ohm, and 1800 V). Transformants
were screened for resistance to tetracycline (10 μg/ml)
and fluorescence under UV transillumination.
Plasmid stability and fitness in the rhizosphere
To test plasmid stability in the rhizosphere in the ab-
sence of selection pressure, four day old gnotobiotic
seedlings of rice were inoculated with ~10
8
cfu of
E. cloacae GS1 (pGB5). Ten days after inoculation; bacte-
ria on the root surface were isolated on LB agar. Tripli-
cates were included and 100 colonies from each plant
were replicated onto LB agar and LB agar containing
tetracycline (10 μg/ml) to determine the percentage of
cells retaining the plasmid after 10 d in the rhizoplane.
In order to detect the effect of pGB5 carriage on root
colonization, E. cloacae GS1 lacking and containing pGB5
were used to inoculate separate plants in triplicates.
Ten days post inoculation populations of E. cloacae GS1
and E. cloacae GS1 (pGB5) on the root surface were esti-
mated by plate count and compared.
Microscopy
Rice plants (cv. IR64) grown hydroponically or in non-
sterile soil were inoculated with E. cloacae GS1 (pBG5)
suspensions prepared in 10 mM MgSO
4
(~10
8
cfu/ml) in
duplicates and roots were recovered at specified time
intervals and washed twice in sterile distilled water to
remove loosely adhered cells and tightly adhering soil
particles. Wet mounts of roots in 10 mM MgSO
4
were
prepared and micrographed under epifluorescence mode
(FITC filter Excitation: 494/Emission: 518) in an inverted
microscope (Eclipse-Ti, Nikon, Japan). Negative controls
which received sterile 10 mM MgSO
4
on the day of inocu-
lation were simultaneously imaged to ensure absence of
green fluorescent bacteria in the soil used.
Results
Direct enrichment of and phenotyping
of root colonizing bacteria
The sampled rhizoplane bacterial population (2.73 ×
10
7
cfu/ml) had atleast 7 distinguishable colony mor-
phologies. After enrichment, by colonization on plant
roots, one bacterial isolate GS1 appeared at a density of
10
5
cfu/root. This isolate was a Gram negative, oxidase
negative rod exhibiting swimming and swarming motil-
ity and able to utilize several carbon sources such as
lactose, xylose, maltose, fructose, dextrose, galactose,
526 M.
Shankar
et al.
Journal of Basic Microbiology 2011, 51, 523 – 530
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
raffinose, trehalose, melibiose, sucrose, arabinose, man-
nose, glycerol, sorbitol, mannitol, α-methyl-D-glucoside,
ribose, rhamnose, cellobiose, and citrate. It produced
indole acetic acid like substances (15 μg/ml) in 24 h LB
broth culture in addition to solubilization of tricalcium
phosphate.
Identification and phylogenetic analysis
BLASTN analysis of the 1534 bp long 16S rDNA se-
quence of the isolate GS1 revealed 99% identity with
Enterobacter sp. XW122 (EU545406.1). A UPGMA-dis-
tance based dendrogram was constructed including
several representative organisms from Enterobacteriaceae
obtained as hits in BLASTN. Isolate GS1 clustered with
E. cloacae strain An20-1. Thus, the isolate GS1 was iden-
tified as Enterobacter cloacae based on its 16S rDNA se-
quence similarity and carbohydrate utilization pattern.
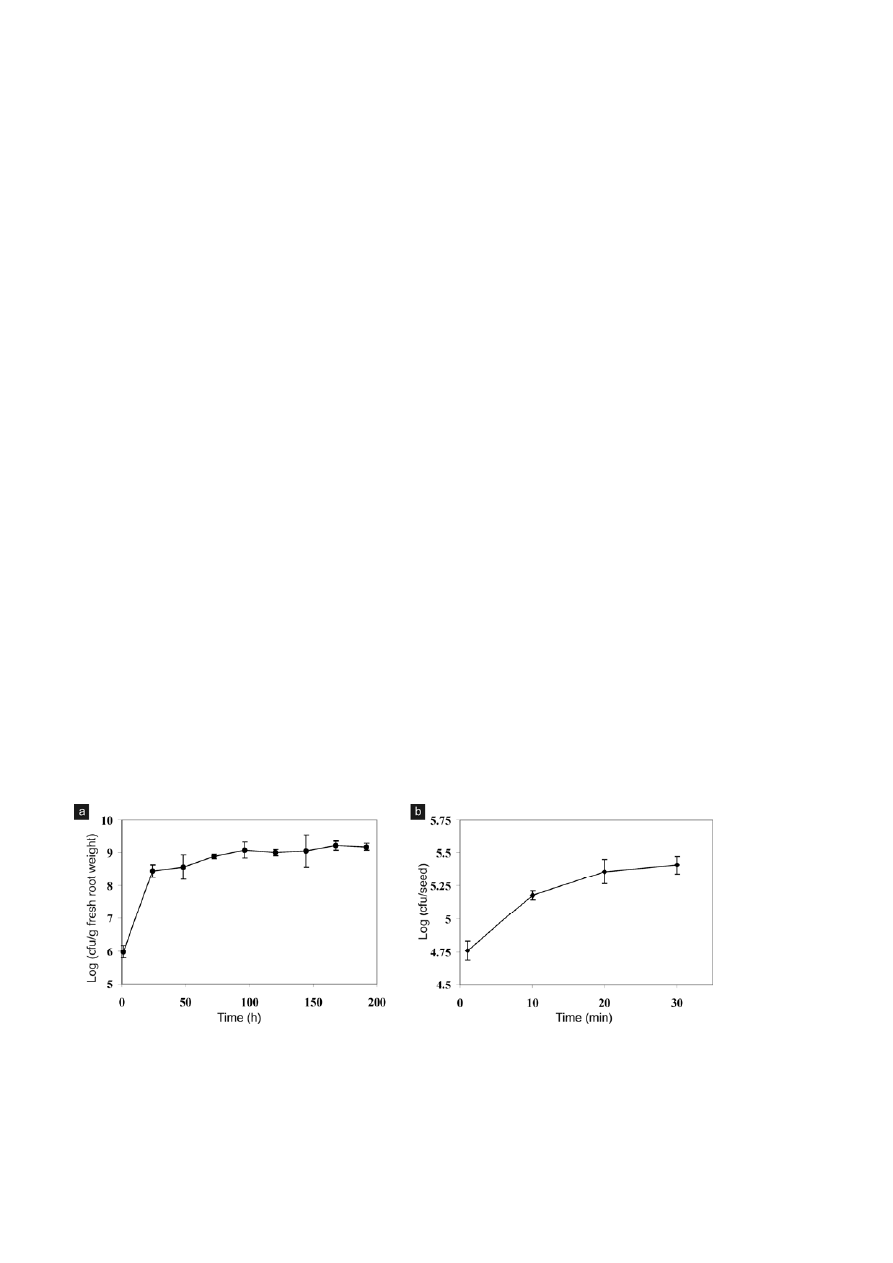
Root colonization
The population dynamics of E. cloacae GS1 on rice root
was studied for an 8 d period (Fig. 1a). E. cloacae GS1
cells colonizing rice roots increased dramatically from
5.98 ± 0.18 to 8.45 ± 0.19 log (cfu/g fresh root weight)
24 h after inoculation. A gradual increase in bacterial
counts was seen between 24–96 h beyond which no
drastic changes in bacterial population was observable.
This observation highlights that E. cloacae GS1 responds
immediately to the presence of its host by quickly colo-
nizing rice roots until permissible populations are
achieved on the root surface.
Seed adhesion
Treatment of germinated rice seeds with a suspension
of E. cloacae GS1 (~10
8
cfu/ml) for 1 min resulted in the
adhesion of 5.8 × 10
4
bacteria per seed. The time of
exposure to bacterial suspension was varied between
1–30 min and the number of bacterial cells irreversibly
adhered to seeds was plotted against time in minutes
(Fig. 1b). Within 10 min the bacterial population ad-
hered per seed was 1.51 × 10
5
. Bacteria adhering to
germinated seeds increased proportionally with the
time of exposure reaching a plateau of 2.32 × 10
5
and
2.58 × 10
5
CFU per seed in 20 and 30 min respectively.
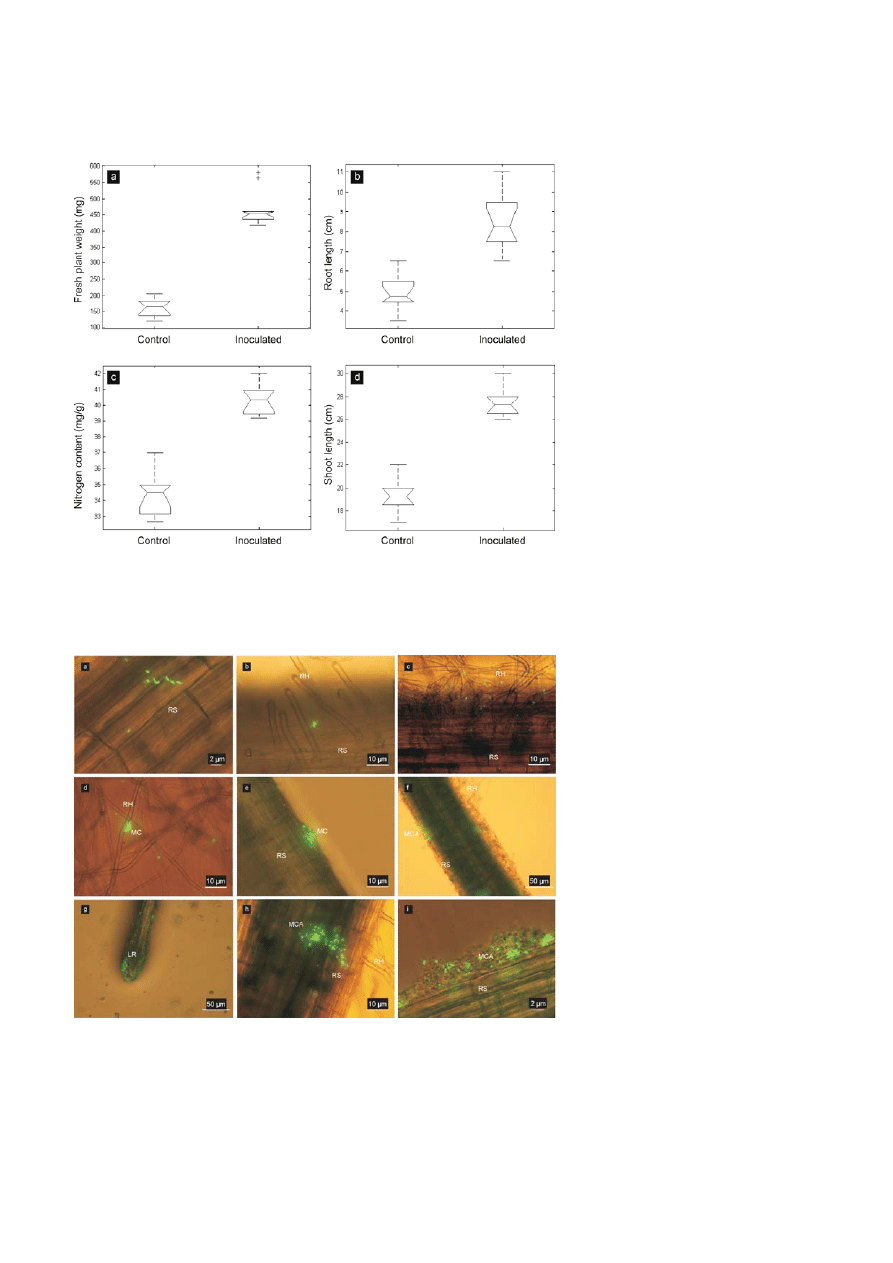
Plant growth promotion
Thirty two days post inoculation, average fresh plant
weight, root length, shoot length, and N-content of
seedlings bacterized with E. cloacae GS1 were signifi-
cantly higher than that of the values recorded for
uninoculated controls (Fig. 2). No significant difference
in P and K-content was detectable between control and
inoculated groups [data not shown].
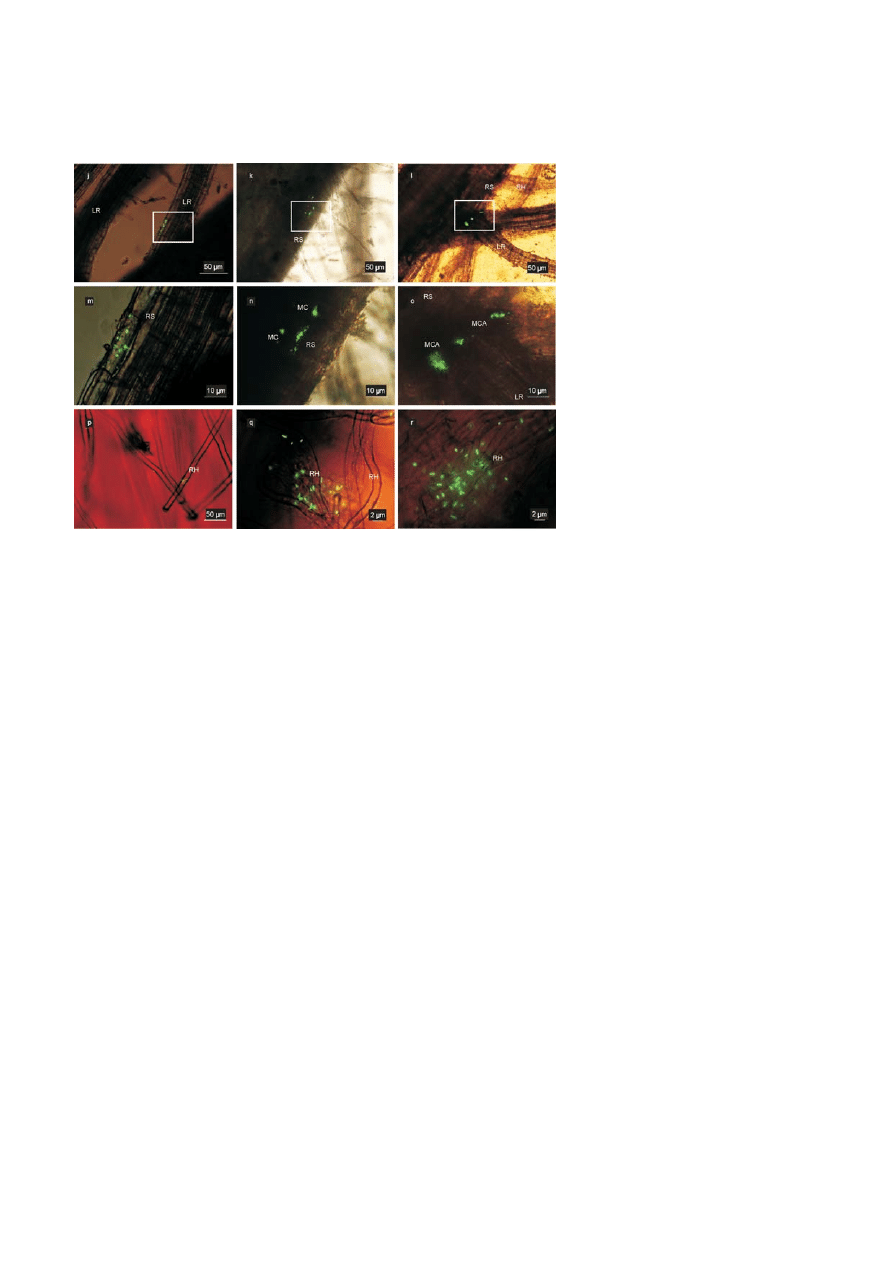
Root colonization by E. cloacae GS1 in a gnotobiotic
hydroponic environment
Our time scale root colonization studies under hydro-
ponic conditions suggested the observation of early
events during colonization (1–48 h post inoculation)
during which a rapid increase in bacterial population
on the roots was seen. Single cells of E. cloacae GS1 were
seen adhered onto the root surface 2 h after inoculation
(Fig. 3a and b). Extensive adhesion of cells to root hair
surfaces was visible after 12 h (Fig. 3c). After 24 h, mi-
crocolonies were established on root hairs (Fig. 3d) and
well established colonies were seen at 36 h on the root
surface (Fig. 3e). Multicellular aggregates of E. cloacae
GS1 were observed on certain regions of the roots
at 48 h indicating sites of increased rhizodeposition
Figure 1. (a) Time course of E. cloacae GS1 colonization on rice roots. Four day old seedlings were inoculated with ~ 10
8
cells and the
bacterial count on the root was estimated at intervals. The cfu/g of fresh root weight was transformed to log values and plotted against time.
Values shown are means of triplicates that were processed independently with error bars indicating standard deviation (SD). (b) Effect of
exposure time on seed adhesion by E. cloacae GS1 Germinated rice seeds were treated with a bacterial suspension (1.7
× 10
8
cfu/ml). After
removal of loosely adhered cells, the number of cells adhered per seed was estimated, transformed to log values and plotted against time.
Values indicated are means of triplicate measurement with error bars indicating SD.
Journal of Basic Microbiology 2011, 51, 523 – 530
Rice root colonization by Enterobacter cloacae GS1
527
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 2. Box plots showing significant difference between medians of parameters measured as indices of rice growth promotion and
enhanced nutrition across control and E. cloacae GS1 inoculated groups using one way analysis of variance (Matlab-2007): (a) fresh plant
weight (mg), (b) root length (cm), (c) nitrogen content (mg/g dry shoot material), (d) shoot length (cm).
Figure 3. Root colonization by GFP tagged E. cloacae GS1 in a simple gnotobiotic hydroponic environment: Images (a) through (i) show
the sequential events during root colonization by E. cloacae GS1 on rice plants grown hydroponically. [RH-Root hair, RS-Root surface, MC-
Microcolony, MCAMulticellular aggregate, LR-Lateral root]. Embedded bars are scales as indicated in individual images.
528 M.
Shankar
et al.
Journal of Basic Microbiology 2011, 51, 523 – 530
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 4. Root colonization behaviour of E. cloacae GS1 in a complex non-sterile soil environment: Images (j) through (r) show the patterns
of root colonization by E. cloacae GS1 on rice plants grown in nonsterile soil. Regions highlighted in images (j), (k) and (l) are shown at a
higher magnification in (m), (n) and (o), respectively. [RH-Root hair, RS-Root surface, MC-Microcolony, MCA-Multicellular aggregate, LR-
Lateral root]. Embedded bars are scales as indicated in individual images.
(Fig. 3f). Extensive coloniziation of a rice lateral root tip
by E. cloacae GS1 (pGB5) was seen at 4 d (Fig. 3g). Seven
days post inoculation, multicellular aggregates were
found on the root surface (Fig. 3h) showing cells em-
bedded in an extracellular matrix cemented to the root
surface (Fig. 3i).
Pattern of root colonization by E. cloacae GS1
in a non-sterile soil environment
E. cloacae GS1 (pGB5) introduced into non-sterile soil was
seen bound to rice roots 24 h post inoculation (Fig. 4j,
m and p). Microcolonies and multicellular aggregates
were visible after 48 h (Fig. 4k and n). It was noted that
multicellular aggregates were clustered around the
base of a lateral root (Fig. 4l and o) at 5 d after inocula-
tion. No single cells were seen bound to the root sur-
face as in the case of hydroponic root colonization.
E. cloacae GS1 however established itself successfully on
roots and root hairs of the inoculated plant 15 d post
inoculation (Fig. 4q and r) which was comparatively
slower than hydroponic colonization where similar
levels of colonization were seen within 48 h.
Discussion
This study describes the rice root colonizing and
growth promoting potentials of E. cloacae GS1 isolated
from rice rhizoplane by direct enrichment. E. cloacae is
known to be a versatile plant associated bacterium able
to colonize and benefit different plant hosts. Factors
affecting cucumber seed colonization and canola root
colonization by E. cloacae have been well investigated
[23–26]. However, very little information is available on
rice root colonization by this bacterium though it has
been known to have desirable characteristics such as
nitrogen fixation, P-solubilization, and IAA production
[5]. We used fluorescent protein as a reporter which
allows easy detection of tagged bacteria in a non-
destructive manner and does not require the addition
of extraneous substrates [27]. Root colonization by En-
terobacter agglomerans in rice and wheat has been stud-
ied earlier by electron microscopy [28]. Our study re-
veals the contrast in the pattern of root colonization
between E. cloacae GS1 and E. agglomerans as no sym-
plasmata were visible throughout the observation pe-
riod. The classical stages involved in colonization of
host roots were clearly discernable. Initial adhesion to
the root and seed surface was strong enough as not to
be dislodged by vortexing. Preferential colonization of
root hairs was seen in the early stages of colonization.
Root hairs are known to be sites of increased rhizode-
position and have been suspected to be involved in
eliciting chemotaxis and specific attachment [29, 30].
Aggregates of bacteria and microcolonies visible on the

Journal of Basic Microbiology 2011, 51, 523 – 530
Rice root colonization by Enterobacter cloacae GS1
529
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
root surface appeared to be cemented by an extracellu-
lar matrix. This has been hypothesized to be exopoly-
saccharides produced either by the plant or the bacte-
rial partner [31]. Since the enrichment technique does
not exclude isolation of an endophytic colonizer, we
made several attempts to detect E. cloacae GS1 endo-
phytically, We found no conclusive evidence that the
organism invades plant tissues. The pattern of root
colonization exhibited in nonsterile soil was analysed
and compared with that obtained under gnotobiotic
conditions. It was seen that E. cloacae GS1 was signifi-
cantly slower in establishing itself on rice roots grown
in non-sterile soil as compared to hydroponic gnotobi-
otic environment. This could be attributed to competi-
tion for nutrients with other soil microbiota. Further
more, in gnotobiotic conditions, (1 ml bacterial suspen-
sion OD
600
= 0.2) ~ 10
8
cells were introduced into 60 ml
of plant nutrient solution while the same inoculum
was supplied into 500 g of nonsterile soil. This com-
paratively lesser bacterial inoculum as in the case of
soil inoculation could explain the delay in proficient
colonization of rice roots by E. cloacae GS1. Nevertheless;
E. cloacae GS1 was able to form a good number of mi-
crocolonies and multicellular aggregates in due time
suggesting that E. cloacae GS1 is successful in coloniza-
tion of rice roots by competing with native microflora.
Direct in-planta enrichment of root colonizing bacteria
allows selection of efficient root colonizers that are
ecologically successful from a diverse microbial popu-
lation. Performance of bacteria that are identified
through conventional in-vitro screening methods under
laboratory conditions may not correlate with their
performance under in-vivo soil conditions. E. cloacae GS1
has been isolated from a rice rhizoplane bacterial
community by in-planta selection placing emphasis on
the rhizosphere competence of the isolate. Previous
investigations [6, 32] support this finding as they report
that Enterobacteriacae are most predominant in the
rice rhizosphere. Successful bacterial inoculants should
be able to compete with native microflora and colonize
host plants [33]. When applied as a bioinoculant for
rice, E. cloacae GS1 could easily compete with the native
microflora, colonize rice roots and promote plant
growth. The observed increase in root length of inocu-
lated plants could be attributed to IAA produced by
E. cloacae GS1 as IAA is known to improve root growth
[34]. Enhanced root length implies an increase in sur-
face area for nutrient uptake and hence better nutri-
tion and yield. We assessed the performance of E. clo-
acae GS1 as an introduced bioinoculant. Nitrogen
content of inoculated plants were 17.6% higher than
uninoculated control plants throwing light on the abil-
ity of the isolate to assist in nitrogen nutrition of inocu-
lated plants. The observed increment in fresh weight
correlated well with higher nitrogen content in inocu-
lated plants. The presence of such growth promoting
traits in an isolate obtained by in-planta enrichment
highlights the efficacy of the enrichment technique in
selection of plant beneficial bacteria from a given eco-
logical niche. Owing to its efficient root colonizing
nature and growth promotion ability, this strain would
be a suitable candidate for field trials and development
as bioinoculant. We are currently involved in under-
standing plant induced gene expression in this bacte-
rium.
Acknowledgements
This work was financially supported by the Indian
Council for Agricultural Research (NBAIM/AMAAS/2007-
2012/MG (5)/PG/BG/3), India. Support facility from Cen-
ter for Excellence in Genomic Sciences and UGC-
Networking Resource Center in Biological Sciences is
acknowledged. CHN analysis was performed at the
Central Instrumentation Facility, CECRI, Karaikudi,
India. Plasmid pGB5 was a gift from Dr. Guido V.
Bloemberg, Leiden University. MS gratefully acknowl-
edges technical assistance from Mr. Lalrammawia, Mr.
Ganesh Babu and constructive discussions with Dr.
Manoharan, Dr. Chitralekha and Mr. Madhankumar.
References
[1] Kloepper, J.W., 1997. Plant growth–promoting rhizobac-
teria (other systems). In: Okon, Y. (ed.), Azospirillum⁄Plant
Associations. CRC Press, Boca Raton, pp. 137–166.
[2] Benizri, E., Baudoin, E., Guckert, A., 2001. Root coloniza-
tion by inoculated plant growth promoting rhizobacteria.
Biocontrol. Sci. Techn., 11, 557–574.
[3] Urgel, M.E., Kolter, R., Ramos, J.L., 2002. Root coloniza-
tion by Pseudomonas putida: love at first sight. Microbiol.,
148, 341–343.
[4] Selvakumar, J., Mohan, M., Kundu, S., Gupta, A.D. et al.,
2007. Cold tolerance and plant growth promotion poten-
tial of Serratia marcescens strain SRM (MTCC8708) isolated
from flowers of summer squash (Cucurbita pepo). Lett.
Appl. Microbiol., 46, 171–175.
[5] Barraquio, W.L., Segubre, E.M., Gonzalez, M.A.S., Verma,
S.C. et al., 2001. Diazotrophic enterobacteria: What is
their role in the rhizosphere of rice? In: Ladha, J.K., Red-
dy, P.M. (eds.), The Quest for Nitrogen Fixation in Rice. In-
ternational Rice Research Institute, Los Baños, Philip-
pines, pp. 93–118.
[6] Cottyn, B., Regalado, E., Lanoot, B., De Cleene, M., et al.,
2001. Bacterial populations associated with the rice seed

530 M.
Shankar
et al.
Journal of Basic Microbiology 2011, 51, 523 – 530
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
in the tropical environment. Phytopathology, 91, 282–
292.
[7] Kuiper, I., Bloemberg, G.V., Lugtenberg, B.J.J., 2001. Selec-
tion of a plant-bacterium pair as a novel tool for
rhizostimulation of polycyclic aromatic hydrocarbon-
degrading bacteria. Mol. Plant. Microbe. Interact., 14,
1197–1205.
[8] Kamilova, F., Validov, S., Azarova, T., Mulders, I., Luten-
berg, B., 2005. Enrichment for enhanced competitive
plant root tip colonizers selects for a new class of bio-
control bacteria. Environ. Microbiol., 7, 1809–1817.
[9] Reiders, H., Bonnecarre’re, V., Rainey, P.B., Hamonts, K.
et al., 2003. Development and application of a dapB-based
in vivo expression technology system to study coloniza-
tion of rice by the endophytic nitrogen-fixing bacterium
Pseudomonas stutzeri A15. Appl. Environ. Microbiol., 69,
6864–6874.
[10] Yoshida, S., Forno, D.A., Cock, J.H., Gomez, K.A. (eds.),
1976. Laboratory Manual for Physiological Studies of Rice.
International Rice Research Institute, Los Banos, Philip-
pines.
[11] Bergey, D.H. (ed.), 1930 Bergey’s Manual of Determinative
Bacteriology, 9
th
edn. The Williams and Wilkins Co., Bal-
timore, Md.
[12] Gordon, S.A., Weber, R.P., 1951. Colorimetric estimation
of Indoleacetic acid. Plant. Physiol., 26, 192–195.
[13] Pikovskaya, R.I., 1948. Mobilization of phosphorous in
soil in connection with the vital activity of some micro-
bial species. Mikrobiologiya, 17, 362–370.
[14] Millikan, D.S., Ruby, E.G., 2002. Alterations in Vibrio fi-
scheri motility correlate with a delay in symbiosis initia-
tion and are associated with additional symbiotic coloni-
zation defects. Appl. Environ. Microbiol., 68, 2519–2528.
[15] Sambrook, J., Fritsch, E.F., Maniatis, T., 1989. Molecular
Cloning: A Laboratory Manual. Cold Spring Harbor Lab
Press, Cold Spring Harbor.
[16] Giovannoni, S.J., 1991. The polymerase chain reaction. In:
Stackebrandt, E., Goodfellow, M. (eds.), Nucleic Acid
Techniques in Bacterial Systematics. John Wiley and Sons,
New York, pp. 177–201.
[17] Altschul, S.F., Gish, W., Miller, W., Myers, E.W., Lipman,
D.J, 1990. Basic local alignment search tool. J. Mol. Biol.,
215, 403–410.
[18] Felsenstein, J., 1989. PHYLIP – Phylogeny Inference Pack-
age (Version 3.2). Cladistics, 5, 164–166.
[19] Urgel, M.E., Ramos, J.L., 2004. Cell density dependant
gene contributes to efficient seed colonization by Pseudo-
monas putida KT2440. Appl. Environ. Microbiol., 70, 5190–
5198.
[20] Urgel, M.E., Salido, A., Ramos, J.L., 2000. Genetic analysis
of functions involved in adhesion of Pseudomonas putida to
seeds. J. Bacteriol., 182, 2363–2369.
[21] DeFlaun, M.F., Marshall, B.M., Kulle, E.P., Levy, S.B., 1994.
Tn5 insertion mutants of Pseudomonas fluorescens defective
in adhesion to soil and seeds. Appl. Environ. Microbiol.,
60, 2637–2642.
[22] Bloemberg, G.V., O’Toole, G.A., Lutenberg, B.J.J., Kolter,
R., 1997. Green fluorescent protein as a marker for Pseu-
domonas spp. Appl. Environ. Microbiol., 63, 4543–4551.
[23] Roberts, D.P., Dery, P.D., Yucel, I., Buyer, J.S., 2000. Im-
portance of pfkA for rapid growth of Enterobacter cloacae
during colonization of crop seeds. Appl. Environ. Micro-
biol., 66, 87–91.
[24] Lohrke, S.M., Dery, P.D., Li, W., Reedy, R., et al., 2002.
Mutation of rpiA in Enterobacter cloacae decreases seed and
root colonization and biocontrol of damping-off caused by
Pythium ultimum on cucumber. Mol. Plant. Microbe. Inter-
act., 8, 817–825.
[25] Roberts, D.P., McKenna, L.F., Hu, X., Lohrke, S.M. et al.,
2007. Mutation in cyaA in Enterobacter cloacae decreases
cucumber root colonization. Arch. Microbiol., 187, 101–
115.
[26] English, M.M., Coulson, T.J.D., Horsman, S.R., Patten, C.L.,
2010. Overexpression of hns in the plant growth-
promoting bacterium Enterobacter cloacae UW5 increases
root colonization. J. Appl. Microbiol., 108, 2180–2190.
[27] Rochat, L., Tarr, M.P., Baehler, E., Maurhofer, M., Keel, C.,
2010. Combination of fluorescent reporters for simulta-
neous monitoring of root colonization and antifungal ge-
ne expression by a biocontrol Pseudomonad on cereals
with flow cytometry. Mol. Plant. Microbe. Interact., 23,
949–961.
[28] Achouak, W., Heulin, T., Villemin, G., Balandreau, J.,
1993. Root colonization by symplasmata forming Entero-
bacter agglomerans. FEMS Microbiol. Ecol., 13, 287–294.
[29] Zhu, G.Y., Dobbelaere, S., Vanderleyen, J., 2002. Use of
green fluorescent protein to visualize rice root coloniza-
tion by Azospirillum irakense and A. brasilense. Funct. Plant.
Biol., 29, 1279–1285.
[30] Kim, C., Kecskés, M.L., Deaker, R.J., Gilchrist, K. et al.,
2005. Wheat root colonization and nitrogenase activity by
Azospirillum isolates from crop plants in Korea. Can. J.
Microbiol., 51, 948–956.
[31] Poonguzhali, S., Madhaiyan, M., Yim, W.J., Kim, K.A., Sa,
T.M., 2008. Colonization pattern of plant root and leaf
surfaces visualized by use of green-fluorescent-marked
strain of Methylobacterium suomiense and its persistence in
rhizosphere. Appl. Microbiol. Biotechnol., 78, 1033–1043.
[32] Stoltzfus, J.R., Bruijn, F.J., 2000. Evaluating diazotrophy,
diversity, and endophytic colonization ability of bacteria
isolated from surface-sterilized rice. In: Ladha, J.K., Red-
dy, P.M., (eds.), The Quest for Nitrogen Fixation in Rice.
International Rice Research Institute, Los Baños, Philippi-
nes, pp. 63–91.
[33] Schiemann, K.B., Schmid, M., Schreiner, K., Welzl, G.,
Hartmann, A., 2010. Root colonization by Pseudomonas
sp. DSMZ 13134 and impact on the indigenous rhizosphe-
re bacterial community of barley. Microb. Ecol., 60, 381–
393.
[34] Khalid, A., Arshad, M., Zahir, Z.A., 2004. Screening plant
growth promoting rhizobacteria for improving growth
and yield of wheat. J. Appl. Microbiol., 96, 473–480.
((Funded by
• Indian Council for Agricultural Research, India; grant number: NBAIM/AMAAS/2007-2012/MG (5)/PG/BG/3))
Wyszukiwarka
Podobne podstrony:
jobm 201000013
jobm 201000298
jobm 201000191
jobm 201000321
jobm 201000018
jobm 201000214
jobm 201000067
jobm 201000037
jobm 201000074
jobm 201000280
jobm 201000385
jobm 201000198
jobm 201000458
jobm 201000147
jobm 201000520
jobm 201000327
jobm 201000420
jobm 201000364
jobm 201000317
więcej podobnych podstron