
Journal of Basic Microbiology 2010, 50, S5 – S17
S5
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Research Paper
Metamorphosis of Borrelia burgdorferi organisms – RNA,
lipid and protein composition in context
with the spirochetes’ shape
Samiya Al-Robaiy*
, 1, 2
, Hassan Dihazi*
, 3
, Johannes Kacza
4
, Johannes Seeger
4
, Jürgen Schiller
5
,
Daniel Huster
5
, Jens Knauer
1, 6
and Reinhard K. Straubinger
1, 7
1
Institute of Immunology, College of Veterinary Medicine, and Center for Biotechnology and Biomedicine,
University of Leipzig, Germany
2
Cardiothoracic Surgery, Martin Luther University Halle-Wittenberg, Halle/Saale, Germany
3
Department of Nephrology and Rheumatology, University Hospital Goettingen, Germany
4
Institute of Veterinary Anatomy, University of Leipzig, Germany
5
Institute of Medical Physics and Biophysics, Medical Department, University of Leipzig, Germany
6
Fraunhofer Institute for Cell Therapy and Immunology, Leipzig, Germany
7
Institute for Infectious Diseases and Zoonoses, Department for Veterinary Sciences,
Faculty of Veterinary Medicine, LMU Munich, Germany
Borrelia burgdorferi, the agent of Lyme borreliosis, has the ability to undergo morphological
transformation from a motile spirochetal to non-motile spherical shape when it encounters
unfavorable conditions. However, little information is available on the mechanism that enables
the bacterium to change its shape and whether major components of the cells – nucleic acids,
proteins, lipids – are possibly modified during the process. Deducing from investigations utilizing
electron microscopy, it seems that shape alteration begins with membrane budding followed by
folding of the protoplasmatic cylinder inside the outer surface membrane. Scanning electron
microscopy confirmed that a deficiency in producing functioning periplasmic flagella did not
hinder sphere formation. Further, it was shown that the spirochetes’ and spheres’ lipid
compositions were indistinguishable. Neither phosphatidylcholine nor phosphatidylglycerol were
altered by the structural transformation. In addition, no changes in differential protein
expression were detected during this process. However, minimal degradation of RNA and a
reduced antigen-antibody binding activity were observed with advanced age of the spheres. The
results of our comparisons and the failure to generate mutants lacking the ability to convert to
spheres suggest that the metamorphosis of B. burgdorferi results in a conditional reconstruction of
the outer membrane. The spheres, which appear to be more resistant to unfavorable conditions
and exhibit reduced immune reactivity when compared to spirochetes, might allow the B. burg-
dorferi to escape complete clearance and possibly ensure long-term survival in the host.
Abbreviations: phosphatidylcholine, PC; phosphatidylglycerol, PG; Barbour-Stoenner-Kelly II medium,
BSKII medium; Dark field microscopy, DFM; N-methyl-N-nitro-N-nitrosoguanidine, MNNG; Transmission
electron microscopy, TEM; Scanning electron microscopy, SEM; dihydroxybenzoic acid, DHB; trifluoro-
acetic acid, TFA; Acetonitrile, ACN; Formic acid, FA
Keywords: Borrelia burgdorferi / Spheres / Flagellin / Lipids
Received: February 26, 2010; accepted: June 21, 2010
DOI 10.1002/jobm.201000074
*
* Authors contributed equally to this work.
Correspondence: Prof. Dr. Reinhard K. Straubinger, Institute for Infectious
Diseases and Zoonoses, Faculty of Veterinary Medicine, LMU Munich,
Veterinaerstraße 13, 80539 Munich, Germany
E-mail: R.Straubinger@lmu.de
Phone: +49 (0)89 2180 2528
Fax: +49 (0)89 2180 99 2527

S6 Samiya
Al-Robaiy
et al.
Journal of Basic Microbiology 2010, 50, S5 – S17
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Introduction
Lyme borreliosis caused by Borrelia burgdorferi is a per-
sistent infection despite the fact that these organisms
encounter a strong host defense at the level of innate
and adaptive immunity [1, 2]. To prolong their own
survival these organisms developed different strategies
in order to escape the host’s defense mechanisms.
These are based, for example, on the suppression of
both the innate as well as the adaptive arms of the
immune system [3]. B. burgdorferi also utilizes antigenic
variations of outer surface proteins to escape host
immunity [4]. The ability of B. burgdorferi to change
its morphology from the spiral to a spherical form in
response to inappropriate conditions such as depletion
of metabolites [5–7], changes in pH [8], and exposure
to antibiotics [9, 10] was suggested to be another strat-
egy of B. burgdorferi to survive unfavorable condi-
tions.
In
vitro studies proved that morphological transfor-
mation to spherical forms could occur in body fluids
such as the cerebrospinal fluid [11]. A reversibility of
the spherical shape back to spirochetal form in rich
culture medium was reported by different research
groups [7, 9, 11, 12]. Furthermore, successful isolation
of motile spirochetes from mice inoculated with
spheres [12] and detection of the spherical shapes in a
tissue ex vivo infected with B. burgdorferi spirochetes
under controlled conditions [13] as well as in tissue
samples from patients with erythema migrans [14] or
with neurodegenerative disorders [15] could be inter-
preted as the possible existence of these spherical sur-
vival forms in vivo.
The long-term persistence of B. burgdorferi in tissues
of infected patients despite sufficient treatment with
antibiotics might be the cause for late complications
and a chronic course of the disease [9, 16, 17]. The in-
ability to resolve infection with antibiotics in some
patients could result from sphere formation [17, 18], in
contrast to a hypothesis that was formulated by Rave-
che et al. [19], who suggested that cross-reactive anti-
bodies induced by B. burgdorferi bind to host antigens
and consequently induce autoimmunity. According to
this hypothesis, autoimmune mechanism based on
molecular mimicry rather than the persistent infection
is thought to play a major role in persistent Lyme bor-
reliosis.
To understand the role of shape conversion in the
course of persistent Lyme borreliosis we studied bio-
chemical and structural cell elements that might be
important for the metamorphosis of B. burgdorferi or-
ganisms from spirochete to sphere.
Material and methods
Borrelia culture and induction of sphere formation
Wild-type B. burgdorferi sensu stricto B31 strain and B31
FlaB mutant (MC-1), which have been constructed and
characterized previously by Motaleb et al. [20] were used
in this study. The spirochetes were routinely main-
tained in liquid Barbour-Stoenner-Kelly (BSKII) medium
[21] supplemented with 6% (v/v) rabbit serum (PAA
laboratories, Austria) and incubated at 34 °C. For the
analysis of isolated colonies of B. burgdorferi, cells were
cultured on solid medium, which was prepared as de-
scribed by Kurtti et al. [22].
For the analysis of B. burgdorferi morphology, spiro-
chetes in exponential growth phase were centrifuged at
4,500 × g and 15 °C for 10 min and washed twice in
PBS. To induce sphere formation, spirochetes were re-
suspended and incubated either in distilled water or in
BSK-H medium (Sigma-Aldrich, Germany) lacking rabbit
serum [6]. Dark-field microscopy (DFM; Zeiss, Jena, Ger-
many) was used to examine bacterial cultures. To en-
sure that reconversion was not due to reproduction of
spirochetes that did not transform into spheres, we
tested for the absence of spirochetal, reproductive bor-
relia after sphere induction by filtration of the culture
medium through 0.45 μm filter as described [6, 8, 23].
Spirochetal organisms can migrate through the filter
and, if spirochetal borreliae are present in the flow-
through, they can multiply when passed into rich me-
dium. The filtration method is very sensitive, since one
single borrelia per ml will pass through the filter [6].
We did not detect any mobile spirochete even after
incubation for three months.
RNA isolation
Pellets of B. burgdorferi spirochetes and transformed
cells of different ages were digested for 3 min with
40 μg Lysozyme (Sigma-Aldrich, Germany) at room
temperature. RNA was isolated using the RNeasy Mini
Kit (Qiagen, Hilden, Germany).
Generation of B. burgdorferi mutants
To generate mutations of B. burgdorferi that might have
lost the ability to form spheres, the method of Newton
et al. [24] was used with minimal modifications. Mid-log
phase B. burgdorferi cells were exposed to 400 μg of
freshly-made solution of N-methyl-N-nitro-N-nitrosogu-
anidine (MNNG) (Fluka, Buchs, Germany) in 10 μl
DMSO. Spirochetes in DMSO only were used as controls.
The two preparations were incubated at 34 °C for
60 min, then diluted with 9 ml BSKII medium and in-
cubated over night at 34 °C. The bacterial cells were

Journal of Basic Microbiology 2010, 50, S5 – S17
Metamorphosis of Borrelia burgdorferi organisms S7
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
harvested, washed twice with PBS and re-suspended in
10 ml BSKII medium. B. burgdorferi cells were plated on
solid BSKII medium and incubated for 20–22 d at 34 °C
in a humidified incubator supplemented with 5% CO
2
.
To test whether the spirochetes were still able to trans-
form into spheres, borrelia colonies were picked and
cultivated for 5–7 d at 34 °C in 1 ml enriched culturing
BSKII medium. After growth to sufficient numbers,
sphere formation was induced in distilled water as
described previously.
Electron microscopy
For transmission electron microscopy (TEM) borrelia
spirochetes and spheres were fixed in 2.5% (v/v) glu-
taraldehyde and 4.0% (w/v) paraformaldehyde in PBS
(pH = 7.4) for 15 min at room temperature. Subse-
quently, cells were washed and re-suspended with dis-
tilled water. A drop of the fixed cells was mounted on
300 mesh copper grids and after drying postfixed in
1.0% (v/v) osmium tetroxide in PBS for 15 min. After
washing the grids in distilled water, the dried prepara-
tions were examined with a Zeiss EM 900 (Zeiss, Ober-
kochen, Germany).
For scanning electron microscopy (SEM), borrelia
spirochetes and spheres were fixed in a buffered mix-
ture of 2% (v/v) glutaraldehyde and 2% (w/v) paraform-
aldehyde for 2 h. The bacteria were washed with dis-
tilled water, loaded on pioloform-coated grids and
dehydrated in ethanol. Grids were then placed
on stubs
by means of self-adhering carbon tabs and sputtered
with gold (thickness 20 nm) using argon plasma (MED
020; Bal-Tec)
for 2 × 40 sec at 40 mA, with a probe–
target distance
of approximately 50 mm. The cells were
studied with a scanning electron microscope (Leo
vp1430, Zeiss, Oberkochen, Germany).
Lipid extraction
Lipids were extracted from pelleted spirochetes and
spheres according to the protocol of Blight and Dyer
[25] using a 1:1:0.9 (v:v:v) mixture of methanol, chlo-
roform and the aqueous fraction. After separation of
the organic and the aqueous layer, the chloroform
phase was used immediately for measurements.
Matrix assisted laser desorption/ionisation-time-of-
flight mass spectroscopy (MALDI-TOF-MS)
All chemicals (2,5-dihydroxybenzoic acid (DHB) and
trifluoroacetic acid (TFA)) and all solvents (chloroform
and methanol) for MALDI-TOF-MS were obtained from
Fluka. Selected phosphatidylcholines that were used for
means of comparison were purchased from AVANTI
Polar Lipids (Alabaster, Alabama, USA).
The above mentioned lipid extracts were mixed 1:1
(v/v) with the matrix solution (0.5 mol l
–1
2,5-DHB solu-
tion in methanol containing 0.1% (v/v) TFA [26]. Subse-
quently, 1 μl of each sample was brought onto a gold-
coated MALDI target and rapidly dried under a warm
stream of air for improved homogeneity of the matrix-
analyte-co-crystals in comparison to air drying [27].
All MALDI-TOF mass spectra were acquired on a
Bruker Autoflex mass spectrometer (Bruker Daltonics,
Leipzig, Germany). The system utilizes a pulsed nitro-
gen laser, emitting at 337 nm. The extraction voltage
was 20 kV and gated matrix suppression was applied to
prevent the saturation of the detector by matrix ions
[28]. One-hundred-twenty-eight single laser shots were
averaged for each mass spectrum. The laser strength
was kept about ten percent above threshold to obtain an
optimum signal-to-noise ratio. In order to enhance the
spectral resolution, all spectra were acquired in the reflec-
tor mode using delayed extraction conditions.
Protein extraction, precipitation and estimation
For a comparative analysis of the proteins expressed in
the two morphological forms of borrelia, spirochetes
and spherical organisms were harvested, re-suspended
in PBS containing 10 mM PMSF and sonicated 3× for
10 sec with a power of 70% (Bandelin Electronic, Berlin,
Germany). For two-dimensional gel electrophoresis and
SELDI-TOF-MS analysis the proteins were precipitated
over night at –20 °C in 3 volumes of acetone, centri-
fuged at 18,000 × g and the pellet was stored at –80 °C
until used.
Two-dimensional electrophoresis
The first and second dimensional electrophoresis for
the resulting protein pellet from the precipitation was
prepared as described previously by Dihazi et al. [29].
Gels were stained with colloidal Coomassie Brilliant
Blue G-250
as described previously [30] or with Fla-
mingo™ Fluorescent Gel Stain (Bio-Rad, Munich, Ger-
many) as recommended by the manufacturer. Image
analysis
was performed using the PDQuest system. To
account for experimental variation,
three gels were
prepared for each experiment. The gel spot pattern
of
each gel was summarized in a standard after spot
matching.
Thus, standard gel was obtained for each
experiment. These
standards were then matched to
yield information about down- and up-regulation of the
spots.
In-gel digestion and peptide sequence analysis
To permit identification of detected proteins, Coomas-
sie Blue stained gels were prepared using 400 μg of

S8 Samiya
Al-Robaiy
et al.
Journal of Basic Microbiology 2010, 50, S5 – S17
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
total protein. Spots were manually excised from the
gels and washed with distilled water for 15 min. After
destaining, in-gel digestion was performed as described
previously [29] and the resulting peptide mixture was
extracted using different concentrations of acetonitrile
(ACN) and TFA.
One microliter of sample was introduced using a
CapLC auto sampler (Waters, Eschborn, Germany) onto
a μ-precolumn
TM
Cartridge C18 pepMap (300 μm ×
5 mm; 5 μm particle size) and separated through a C18
pepMap100 nano Series
TM
(75 μm × 15 cm; 3 μm particle
size) analytical column (LC Packing, Amersham, Neth-
erlands). The mobile phase consisted of solution-A con-
taining 5% (v/v) ACN in 0.1% (v/v) formic acid (FA) and
solution-B (95% (v/v) ACN in 0.1% (v/v) FA). Total sample
run time was 60 min per sample analysis. Initially,
samples were injected into a precolumn and washed
with 0.1% (v/v) FA (30 μl) for 5 min. The washing step
was followed by an elution step with an exponential
gradient starting with 10% (v/v) and ending with 95%
(v/v) solution-B. The flow rate was decreased by a flow
splitter from 5 μl min
–1
to 0.25 nl min
–1
. The precolumn
was re-equilibrated with 0.1% (v/v) FA (20 μl min
–1
) for
5 min. After chromatographic separation, peptide se-
quencing was performed on a Q-TOF Ultima Global
mass spectrometer (Micromass, Manchester, UK)
equipped with a nanoflow electrospray ionization (ESI)
Z-spray source in positive ion mode. The nanospray
needle was held at 2 kV and the source temperature
was 40 °C. Multiple charged peptide parent ions were
automatically marked, selected in the quadruple frag-
mented in the hexapole collision cell and their frag-
ment patterns were analyzed by time of flight. The data
acquisition was performed using MassLynx (v 4.0) soft-
ware on a Windows NT PC, while data were further
processed on a Protein-Lynx-Global-Server (v 2.1), (Mi-
cromass, Manchester, UK).
Database search and protein identification
Raw data files were deconvoluted, deisotoped using
Max Ent™ lite algorithm and a file format was produc-
ed to search against Swissprot database 50.5 (230150
sequences, 84479584 residues) through the web based
Mascot search engine (MASCOT 2.1, Oxford, UK
http://www.matrixscience.com.search_form_select.html)
[31] using following parameters: trypsin as an enzyme,
monoisotopic, 1 possible missed cleavage, a peptide
mass tolerance of 100 ppm, fragment mass tolerance of
0.6 Da and carbamidomethyl and methionine oxidation
were considered as variable modifications. Results were
scored using Probability Based Mowse Score (Protein
score is-10*Log (p), where p is the probability that the
observed match is a random event. Individual ions
scores >26 indicate identity or extensive homology
(p < 0.05).
Protein profiling using ProteinChip arrays
For an analysis of differentially expressed proteins in B.
burgdorferi forms by surface-enhanced laser desorp-
tion/ionization (SELDI-TOF), reversed phase (H50) Pro-
teinChip arrays (Ciphergen Biosystems, Fremont, CA,
USA) were used.
Twenty microliters of the dissolved protein to a con-
centration of 2.5 μg μl
–1
in the rehydration buffer (8 M
urea, 1%
(w/v) CHAPS, 0.2% (v/v) ampholytes pH = 3–10,
15 mM DTT, and a
trace of bromphenol blue) were
mixed with 30 μl of a solution containing 8 M urea and
2% (w/v) CHAPS in 50 mM Tris/HCl buffer pH = 7.4 and
incubated for 15 min at 4 °C for protein denaturation.
Five microliters of the denatured samples were diluted
1:40 in binding buffer (1% (v/v) TFA). The denatured
protein samples were mixed directly to the binding
buffer (2 volumes binding buffer/one volume sample).
H50 Hydrophobic capture arrays were activated with
150 μl of binding buffer prior to sample loading. Hun-
dred microliters of the samples were applied to each
spot in duplicates on the ProteinChip arrays by a 96-
well bioprocessor (Ciphergen Biosystems, Fremont, CA,
USA). After 60 min incubation at room temperature on
a platform shaker, the arrays were washed three times
for 5 min in 150 μl of binding buffer before being
quickly rinsed twice with 100 μl of distilled H
2
O. The
arrays were air-dried and 1 μl of saturated sinapinic
acid matrix prepared in 0.1% (v/v) TFA with 50% (v/v)
ACN was added twice to each spot. Proteins bound to
the arrays were detected with a PBS II ProteinChip
Reader (Ciphergen Biosystems, Fremont, CA, USA) using
an automated data collection protocol. Instrument
settings were as follows: laser intensity was set to
200 U, detector sensitivity to 8, focus mass to 16,000 Da.
An average of 80 laser shots was collected per spot.
Data were externally calibrated with a peptide mixture
containing ACTH (18–39) ([M + H]
+
2465.19), bovine
insulin ([M + H]
+
5733.5), bovine ubiquitin ([M + H]
+
8564.8), bovine cytochrome C ([M + H]
+
12230) for the
lower molecular weight range and with myoglobin
([M + H]
+
16951.5), horse peroxidase ([M + H]
+
43240.0)
and bovine albumin ([M + H]
+
66433.0) for the high
molecular weight range.
Reproducibility was evaluated using two samples.
Each sample was spotted on all eight bait surfaces of
one ProteinChip array. All spectra were normalized and
analyzed using the ProteinChip Software version 3.0
(Ciphergen Biosystems, Fremont, CA, USA). The peak
Journal of Basic Microbiology 2010, 50, S5 – S17
Metamorphosis of Borrelia burgdorferi organisms S9
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
intensities were normalized to the total ion current
with mass-to-charge ratios (m/z) between 2,000 and
150,000. Qualified mass peaks (signal-to-noise ratio >5)
m
/z between 2,000 and 150,000 were autodetected.
Sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and immunoblotting
SDS-PAGE gels were performed as described [32].
Twenty-microliter-aliquots of protein extracts were
separated on 12% (v/v) vertical gels. Gels were stained
using the SilverQuest™ Silver Staining Kit (Invitrogen,
Karlsruhe, Germany) or transferred to nitrocellulose
membrane and incubated with sera of experimentally
infected dogs with B. burgdorferi sensu stricto N40 or
with the monoclonal antibody against B. burgdorferi
FlabB, which were a gift from Markus M. Simon (Max-
Plank-Institute, Freiburg, Germany).
Results and discussion
Properties of sphere-shaped spirochetes
The ability of B. burgdorferi to transform from mobile
spiral-shaped spirochetes to non-mobile spherical-shap-
ed organisms in BSK-H medium without rabbit serum
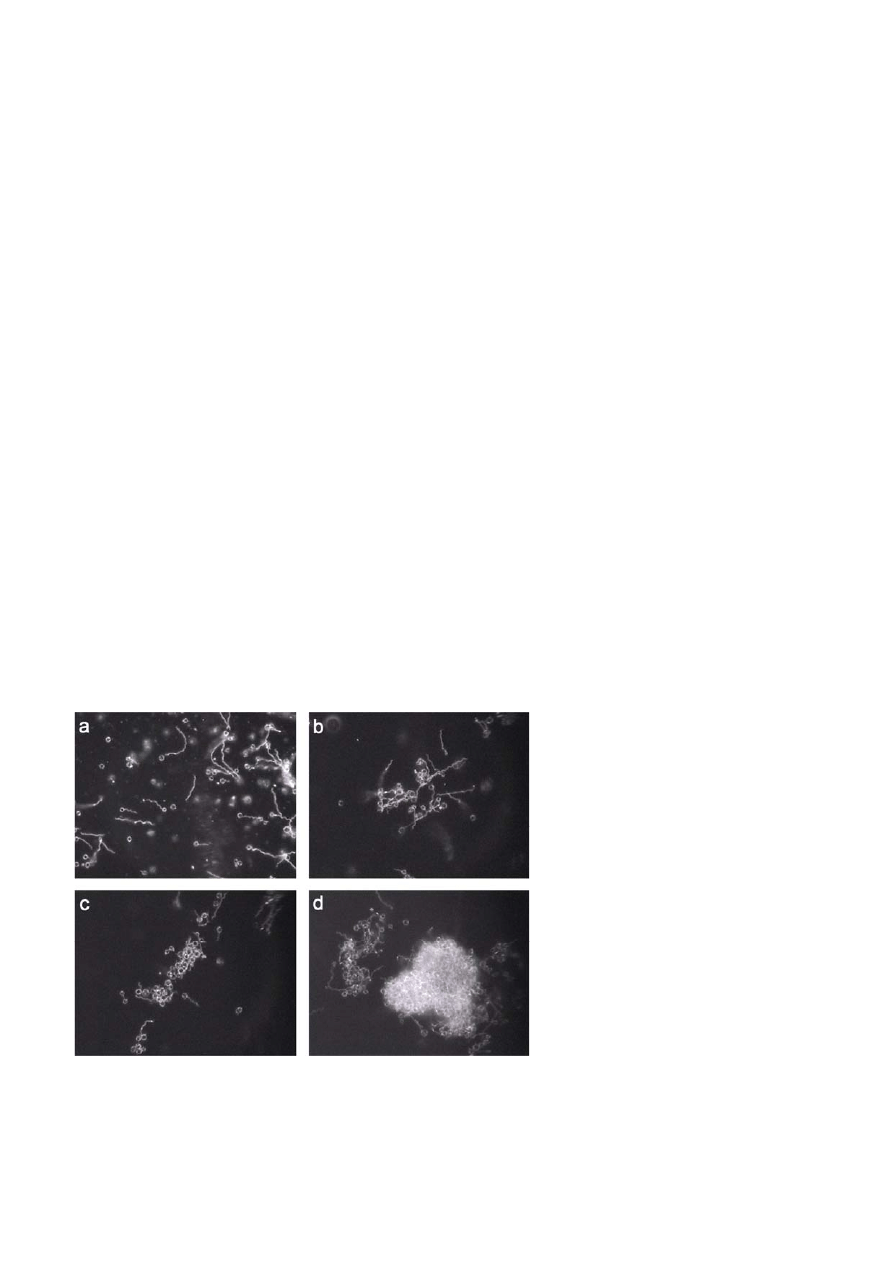
[6] or in distilled water [7, 8] was investigated. Observa-
tions made with DFM confirmed that a transfer of the
spirochetes into these media induces metamorphosis.
The rate of cell transformation was dependent on the
type of culture medium used for the experiment. In
distilled water more than 95% of the spirochetes trans-
formed to spheres within 2 to 3 h (Fig. 1), whereas 7 to
10 d were needed for the same process in BSK-H me-
dium lacking rabbit serum. A successful recovery of
spirochetes from sphere-shaped organisms in rich me-
dia also depends on the way of sphere induction and
their age. Mobile spirochetes arising from four-day-old
spheres produced in distilled water were detected after
an incubation period of 10 d in BSK II medium, whereas
22 d of incubation time were needed until mobile spi-
rochetes were detected that were recovered from seven-
day-old spheres produced in distilled water.
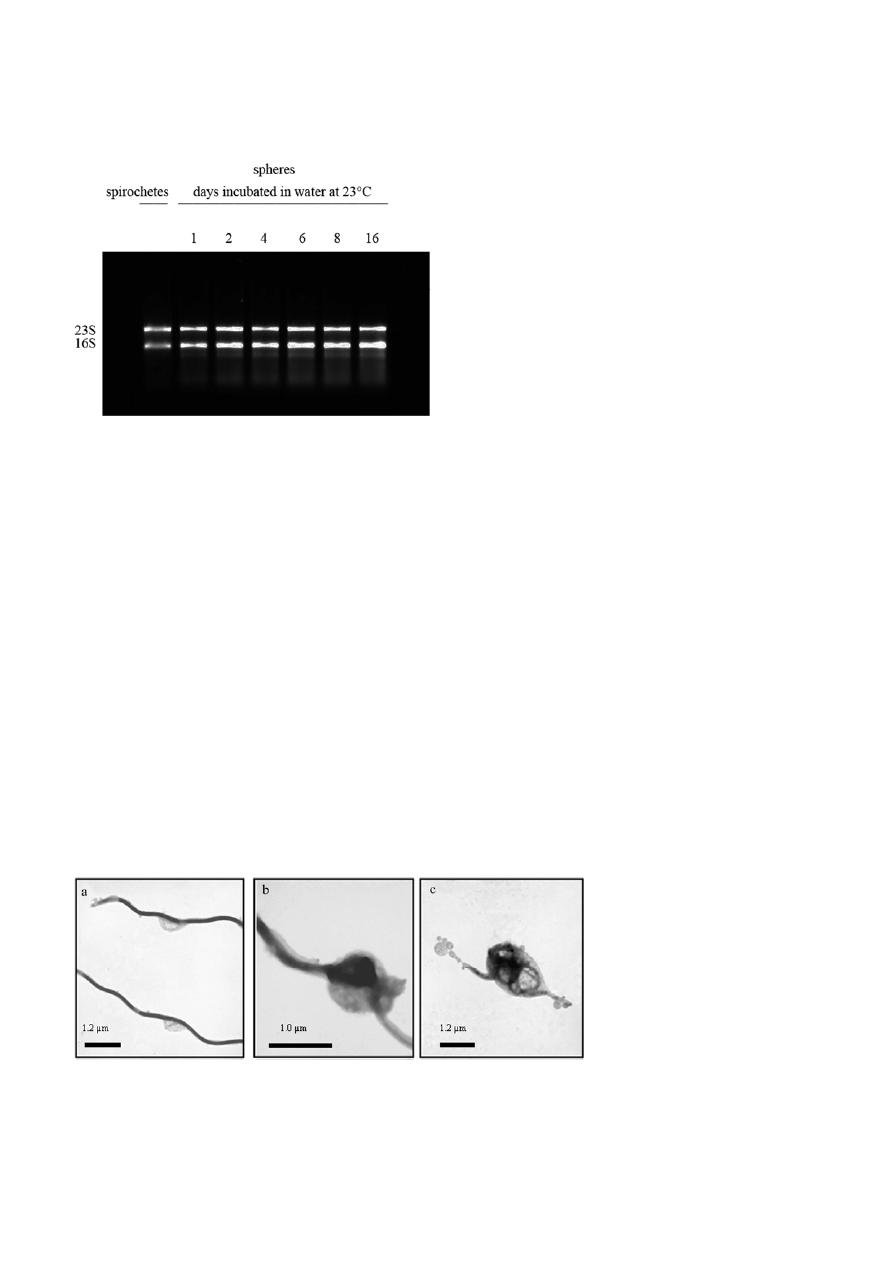
RNA isolation from spirochetes and spheres incu-
bated for 1, 2, 4, 6, 8, 16 d in distilled water at 23 °C
showed that the tRNA signals at 16S and 23S of the
converted cells were still intact with minimal degrada-
tion signs even after 16 day incubation under starva-
tion condition (Fig. 2). This confirms the results re-
ported by Brorson and Brorson [7], who used acridine
orange to demonstrate the presence of RNA in sphere-
shaped organisms that were incubated in water for
5 weeks. Therefore, the long-term stability of RNA in
spherical-shaped B. burgdorferi is a good indicator for a
population of viable transformed organisms rather
than for degenerated bacteria.
TEM and SEM were used to study the morphological
changes after B. burgdorferi transformation from the
spiral spirochete to sphere. It seems that the spiro-
Figure 1. B. burgdorferi spheres induced in distilled water over a time period of 2 h. a) – d) show sequential morphological transformation of
B. burgdorferi from the spirochete to spheres as observed through dark-field microscopy. More than 95% of the spirochetes transform to
spheres within the first 2 h (d). Magnification 200
×.
S10 Samiya
Al-Robaiy
et al.
Journal of Basic Microbiology 2010, 50, S5 – S17
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 2. RNA from B. burgdorferi spirochetes and spheres incu-
bated for 1, 2, 4, 6, 8 d in distilled water at 23
°C. Visualized 16S
and 23S tRNA on the 1% (w/v) agarose gel denatured with
formaldehyde show minimal signs of degradation.
chetes’ shape conversion begins with a stretching of the
membrane (Fig. 3a). This is followed by the folding of
the spirochete cylinder inside the budding membrane
(Fig. 3b). Our observations are consistent with those
described by Murgia and Cinco [8]. In general, the shape
of the transformed spirochete is globular, often with
one end of the protoplasmatic cylinder protruding from
the surface (Fig. 3c). In some cases it was possible to
detect blebs of the outer membrane at the end of the
protruding protoplasmatic cylinder as described by
Alban et al. and Charon et al. [5, 33].
No morphological differences were observed using
electron microscopy when spheres from different star-
vation media were compared (data not shown).
The role of flagellin in morphological transformation
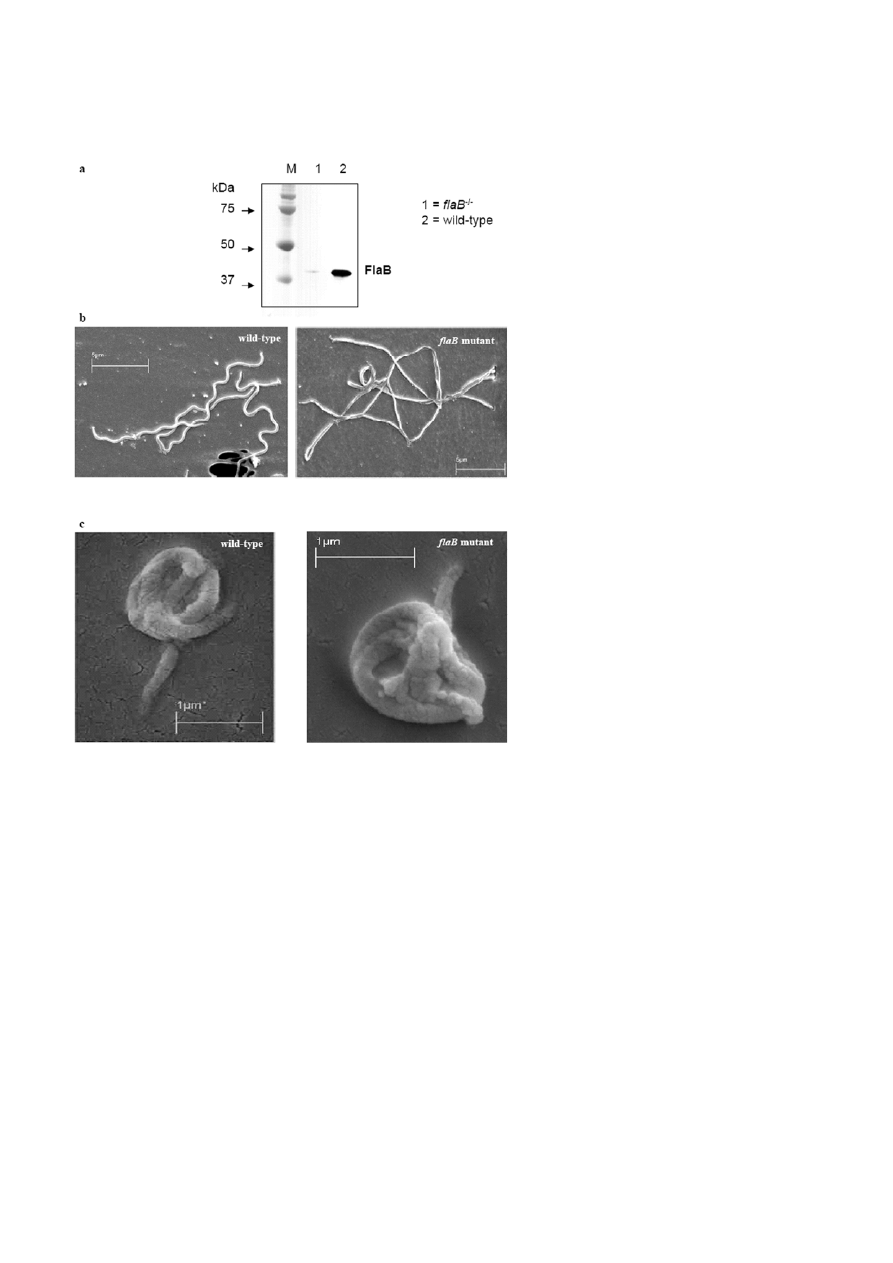
The role of the periplasmic flagellin was studied using a
B. burgdorferi mutant lacking the major flagllar protein
FlaB (Fig. 4a). Morphological examination of the mu-
tants with scanning electron microscopy showed that
these mutants had lost the spiral shape and resembled
long rods (Fig. 4b) [20]. As shown in Fig. 4c, borrelia
lacking flagellin B were still able to produce spheres.
These spheres were microscopically indistinguishable
from those originating from wild-type organisms. These
results indicate that flagellin B protein is not required
for shape conversion.
Generation of B. burgdorferi mutants
One of our hypothesis was that shape-conversion is a
gene-controlled process. Consequently, it should be
possible to produce mutants that lack the ability to
transform into spheres. In order to generate these mu-
tants, B. burgdorferi cells were exposed to the chemical
mutagen MNNG, which produces mainly single-base
substitutions. O
6
-methylguanine, which can substitute
adenine during replication and transcription is the
most important lesion
due to MNNG-induced mutage-
nesis [34]. This agent used at a concentration of
100 μg ml
–1
induces mutations at rates up to 8 × 10
–5
[35].
Dark-field microscopy examination of 280 B. burgdor-
feri colonies from 4 independent experiments plated on
BSKII solid medium after exposure to MNNG showed
that all colonies had preserved the ability to convert to
spheres. The failure to produce mutants incapable of
transformation into other shapes might have different
reasons. a) Most likely the number of examined colo-
nies was too small in order to find an appropriate mu-
tant (~500 mutations; ~1,280 genes in B. burgdorferi
sensu stricto [36]; b) the mutation might be lethal and a
viable phenotype is not accessible. Due to the small
number of mutants tested in this study the possibility
of gene regulation needs to be considered as a possible
trigger for shape transformation.
Figure 3. Transmission electron micrographs of B. burgdorferi organisms during sphere formation in distilled water. a) a spirochete showing
budding of the outer membrane; b) folding of the protoplasmatic cylinder inside the stretched membrane; c) a sphere containing the
periplasmatic cylinder inside the membrane: note the blebbing ends of the outer membrane at the end of the protruding cylinder.
Journal of Basic Microbiology 2010, 50, S5 – S17
Metamorphosis of Borrelia burgdorferi organisms S11
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 4. Wild-type B. burgdorferi and the flaB mutant organisms, which contain an inactivated flagellin B gene. a) Western blot analysis of
wild-type and flaB mutants. The blotting membrane that is coated with extracts of flaB mutant and wild-type B. burgdorferi organisms shows
a clear differing signal at 41 kDa where flagellin is expected. The blot was probed with a monoclonal antibody against mouse FlaB. b) Wild-
type and the flaB mutant organisms as observed by scanning electron microscopy. The flaB mutant cells appear as long rods. c) Scanning
electron micrographs of B. burgdorferi spheres from wild-type and flaB mutant organisms. The two strains produced identical spheres with
no obvious morphological differences.
Analysis of membrane lipids
Changes in bacterial membrane phospholipid composi-
tion have the potential to affect the activity of cyto-
plasmic and periplasmic proteins that may play a role
in host adaptation [37].
The fact that bacteria have the ability to adjust their
membrane phospholipid composition in response to
environmental changes [38] leads to the assumption
that stretching and budding of the membrane observed
in transformed B. burgdorferi spirochetes to spheres dur-
ing stress conditions is due to changes in phospholipid
composition of the bilayer-membrane. It is well known
that several lipid species can induce drastic morpho-
logical changes of the membrane and for instance pro-
duce structures with high curvature, inverse struc-
tures, or cubic phases. Therefore, it is reasonable to
investigate the occurrence of alterations in the phos-
pholipid composition between B. burgdorferi spirochetes
and spheres transitions.
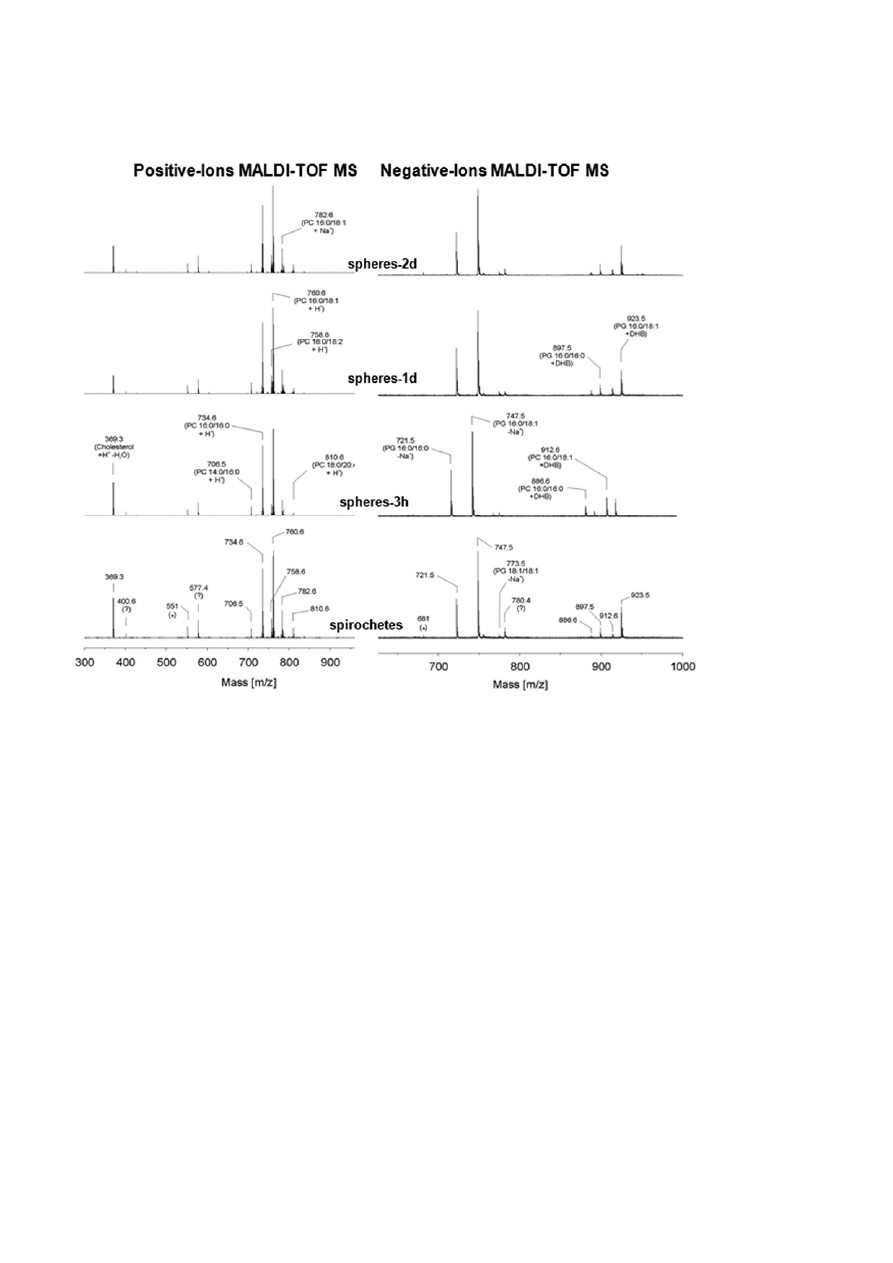
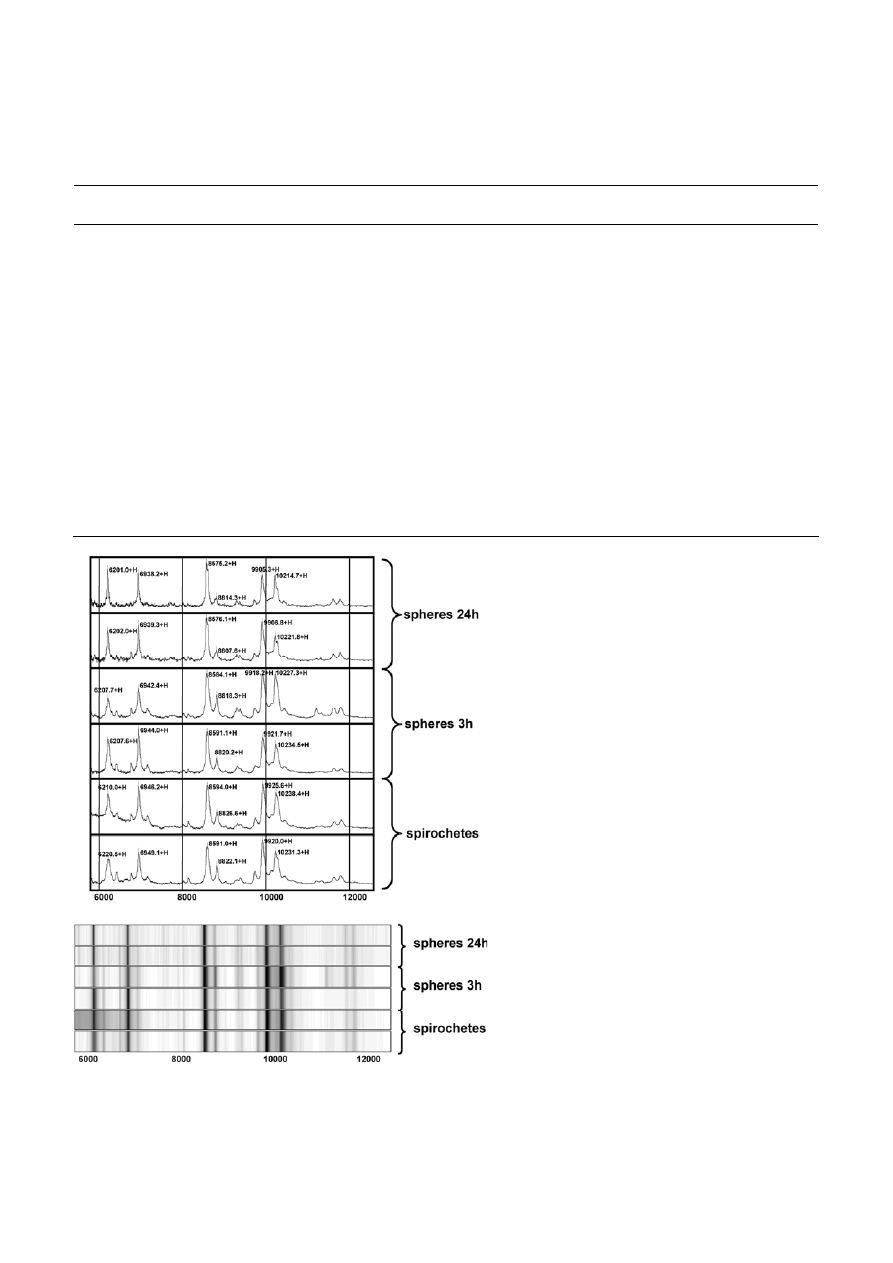
As shown in Fig. 5, lipid analysis of B. burgdorferi
spirochetes as well as spheres induced for 3, 24 and
48 h in water with MALDI-TOF-MS demonstrated that
phosphatidylcholine (PC) and phosphatidylglycerol (PG)
are the major phospholipids in the membrane of these
organisms. The results of early studies also identified
PC and PG as the main phospholipids in the membrane
of B. burgdorferi [39, 40]. The positive and negative ion
spectra revealed from the mass spectrometry of the
S12 Samiya
Al-Robaiy
et al.
Journal of Basic Microbiology 2010, 50, S5 – S17
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 5. B. burgdorferi lipid analysis with MALDI-TOF mass spectrometry in the positive and negative mode using 2,5-dihydroxybenzoic
acid solution in methanol containing 0.1% (v/v) trifluoroacetic acid as matrix. No differences in the spectra of spirochetes and 3 h, 1 and 2 d
old spheres can be observed.
extracted lipids from the spiral and the spherical
shapes of B. burgdorferi showed no differences (Fig. 5).
Neither the PC nor PG content of the membranes is
altered during the process of morphological conversion.
Further, there were no additional lipid species detected
that would influence the curvature of the membranes
such as phosphatidylethanolamine or lysolipids. These
results demonstrate that the phospholipids are not
directly involved in the process of shape transforma-
tion.
Protein analysis
B. burgdorferi is known to have the ability to change
their protein synthesis, gene expression and antigenic-
ity during the different stages of their life cycle and
during different environmental conditions [41, 42]. In
this study characterizing the process of sphere forma-
tion, it is necessary to investigate and to identify the
proteins that could play a role during the process of
morphological conversion of B. burgdorferi. For this pur-
pose protein profile analysis including two-dimensional
gels and SELDI analysis for the protein extracts of spi-
rochetes and spheres was accomplished. 2D image
analysis of the 2D maps generated from protein ex-
tracts revealed no differences between the extracts of
the spirochetes and the spheres induced for 3 and 24 h
in distilled water (Fig. 6a). Many protein spots visual-
ized on the 2D gel were identified by MALDI-TOF-MS
(Fig. 6b). As shown in Table 1 the analysis of these spots
revealed that many proteins that are known as immu-
norelevant antigens such as oligopeptide permease,
glyceraldehyde-3-phosphate dehydrogenase, heat shock
protein, membrane-associated protein 66, outer surface
protein B and flagellin [43] are represented in the ex-
tracts of spirochetes as well as the spherical borrelia
cells. With the help of 2D gel analysis we could not
confirm the results of Alban et al. [5] in which it was
shown that several proteins especially in the low mo-

Journal of Basic Microbiology 2010, 50, S5 – S17
Metamorphosis of Borrelia burgdorferi organisms S13
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 6.
Two-dimensional electrophoresis patterns of borrelia
spirochetes and spheres. a) Two-dimensional pattern of protein
extract from borrelia spheres. Cell extracts of borrelia spheres were
prepared as described in “Materials and method”, and the proteins
(400
μg) were separated by 2D SDS-PAGE based on their
differential pH value for the isoelectic point and molecular masses.
a) Overlapping of 2D map from borrelia spirochetes with 2D map
from berrelia spheres. The proteins were visualized with Flamingo
TM
Fluorescent Gel Stain (Bio-Rad). Green: borrelia spirochetes, red:
borrelia spheres, yellow: overlapping proteins. b) The protein spots
were visualized by colloidal Coomassie Brilliant Blue G-250. Num-
bered spots represents proteins identifies by mass spectrometry.
Numeric labelling of the spots corresponds to the number assigned
in Table 1.
lecular masses were consistently more reactive in cyst
preparation and that four proteins were up-regulated in
the spheres. We suggested that the high sensitivity of
their method might be the reason for these contradic-
tory results. To overcome this problem we profiled the
proteins in the extracts of spirochetes and spherical
shapes with the mass spectrometric-based SELDI-TOF-
MS approach. With this accurate method according to
mass, reproducibly and high output, the differential
protein expression as well as up- and down-regulated
proteins can be detected according to mass in a semi-
quantitative fashion. It has been recommended as an
appropriate technology for the study of bacterial pro-
teomics [44]. In our study, the profile of the proteins de-
tected with this technique ranged between 6–16 kDa.
With a high reproducibility it could be shown that the
protein profiles of the spiral forms and the spheres
induced for 3 and 24 h in distilled water are indistin-
guishable (Fig. 7). Neither differential protein expres-
sion nor an up-regulation of sphere proteins in com-
parison to those of the spirochetes could be detected.
Also Kersten et al. [10] showed on SDS gels that
B. burgdorferi exposed to antibiotics leading to spherical
shape formation have identical polypeptide distribution
as untreated spiral B. burgdorferi. Our results are also
consistent with those of Murgia et al. [45] where they
showed that the deficiency of rpoS gene, which is a
regulator of a central importance for gene expression in
response to starvation and transition to stationary
phase in many bacteria [46] and for at least seven pro-
teins in B. burgdorferi [47] do not effect sphere formation
under stress conditions. They showed that both the
wild-type and the rpoS knock-out produce cystic forms
with the similar kinetics under the same stimuli.
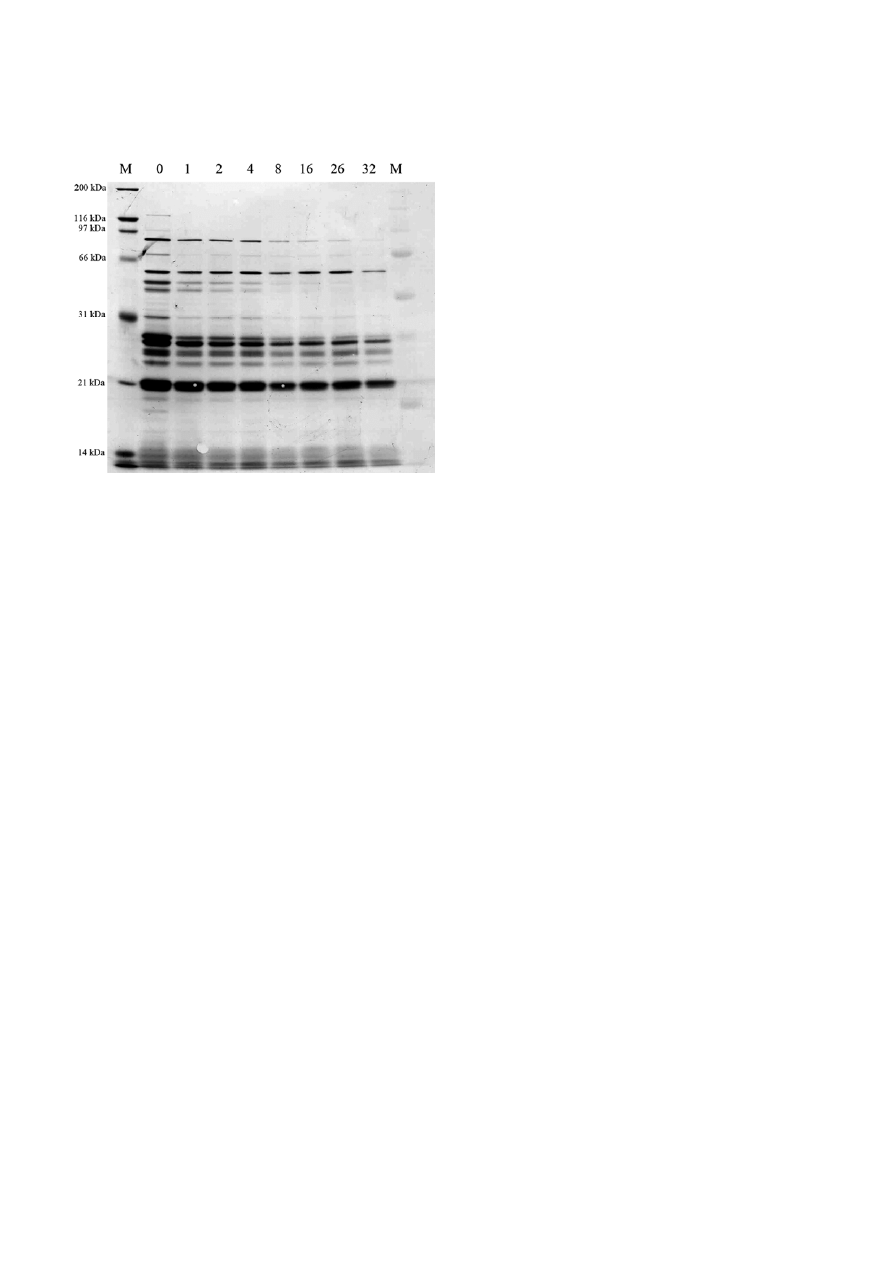
On the other hand, studying the antigenicity of spi-
rochetes and spherical shapes incubated for 1, 2, 4, 8,
16, and 32 d in distilled water with Western blotting
using the serum of an experimentally infected dog with
B. burgdorferi showed that several immunoreactive pro-
teins decreased with prolonged incubation under stress
condition (Fig. 8). No antigenic protein up-regulation
could be detected on these blots. Silver stained SDS gels
confirmed further that protein amounts decreased with
prolonged incubation of B. burgdorferi under unfavor-
able conditions and no up-regulation of proteins in the
extracts of spheres could be detected (data not shown).
The results obtained support other protein analysis
done in our study and are in agreement with the West-
ern blot results of Alban et al. [5] showing that serum
starved cells exhibited many proteins with less reactiv-
ity to sera from infected humans and monkeys with
B. burgdorferi. However, we could not detect up-regu-
lation of any low molecular mass protein as shown on
their blot. As suggested by Murgia and Cinco [8] the
decreased protein amounts with prolonged incubation
time of the spherical cells in the starvation medium
might be due to the low metabolic activity of the
spheres.
S14 Samiya
Al-Robaiy
et al.
Journal of Basic Microbiology 2010, 50, S5 – S17
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Table 1. List of identified proteins (pH = 3 – 10) in borrelia cell extracts using ESI-MS/MS, microsequencing and database comparisons.
Spot Protein name
MW
Score
MS/MS
Matched
peptides
Accession
number (MSDB)
1
dnaK-type molecular chaperone dnaK-2 – Lyme disease spirochete
69233 885
39
E70164
2
heat shock protein – Borrelia garinii 58888
840
33
Q660M1
3
membrane-associated protein p66 – Lyme disease spirochete
68130 551
19
B70175
4
aminopeptidase I (yscI) homolog – Lyme disease spirochete
51468 594
21
E70145
5
enolase homolog – Lyme disease spirochete
47248 686
23
AAC46289
6
oligopeptide permease homolog AV.
61013 37
2
O31303
7
flagellin (flagellar filament 41K core protein flaB – Lyme disease spirochete) 35730 795
49
I40040
8
flagellin (flagellar filament 41K core protein flaB – Lyme disease spirochete) 35730 659
23
I40040
9
flagellin (flagellar filament 41K core protein flaB – Lyme disease spirochete) 35730 211
6
I40040
10
outer membrane porin (oms28) – Lyme disease spirochete
27931 420
12
B70216
11
triose-phosphate isomerase
27756 79
3
AAB53932
12
hypothetical protein BB0238
30360 188
9
F70129
13
L-lactate dehydrogenase (L-LDH)
34823 272
10
Q662S5
14
outer surface protein B
31776 154
5
Q6RH15
15 glyceraldehydes-3-phosphate-dehydrogenase
36232
425 15
A70107
16 glyceraldehydes-3-phosphate-dehydrogenase
36232
703 24
A70107
17 glyceraldehydes-3-phosphate-dehydrogenase
36232
291 12
A70107
18
pyruvate kinase - Lyme disease spirochete
52999 249
10
C70143
19
phosphoglycerate kinase
42319 74
3
AAB53931
20
general stress protein (ctc) – Lyme disease spirochete
24026 300
8
A70198
21
2,3-bisphosphoglycerate dependent phosphoglycerate mutase
30360 88
4
GPM_BORBU
Figure 7. Comparison of SELDI-TOF MS spectra from a reverse phase ProteinChip array showing the indistinguishable spectra in protein ex-
pression between B. burgdorferi spiral-shaped organisms and spheres incubated in water for 3 h as well as 24 h. Protein extracts from the diffe-
rent samples were prepared and applied to the hydrophobic H50 Chip array. The peptide expression profiles are also represented as “gel view”.
Journal of Basic Microbiology 2010, 50, S5 – S17
Metamorphosis of Borrelia burgdorferi organisms S15
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 8. Western blot analysis to determine the antigenicity of
B. burgdorferi spirochetes and spheres after 1, 2, 4, 8, 16, 26 and
32 d of incubation in distilled water. Reduced protein-antibody
reactivity with advanced age of the spheres was observed.
Concluding remarks
In our study we were not able to detect genetic ele-
ments that can be made responsible for shape transfor-
mation. However, we could reveal with electron micro-
scopy that cell Integument, which is a highly dynamic
multilayer structure that supports a multitude of che-
mical and biochemical processes, is integrated in shape
conversion. It begins with membrane budding which is
followed by the folding of the protoplasmatic cylinder
inside the stretched membrane. Cell component analy-
sis showed the indistinguishable spectra of the mem-
brane lipids. The analysis also demonstrated that the
periplasmic flagella, which influence the morphology
of B. burgdorferi, were not involved in this process. An-
other component which is associated with the cyto-
plasmic membrane and thought to be involved in
maintaining cell rigidity and shape is the peptidoglycan
layer, which had been shown to be a component of
B. burgdorferi cell walls [48–50]. The role of this polymer
in sphere formation has not been analyzed in our
study. To clarify if B. burgdorferi shape transformation is
a biochemical process or if it is a result of changes in
mechanical sequence of events merits further analyses.
However, the results obtained showing the intact RNA,
which is necessary for successful reproduction, within
the spheres even after a long starvation period as well
as the decreased antigenicity due to advanced age add
to the evidence that these morphological changes
might represent a strategy of B. burgdorferi to persist
within the infected host.
Acknowledgement
The authors would like to thank N. W. Charon from
the Department of Microbiology and Immunology,
Health Sciences Center, West Virginia University, USA
for allowing us to use the B31 mutant MC-1. We also
thank P. Anda and R. Escudero from Servicio de
Bacteriología, Centro Nacional de Microbiología, Insti-
tuto de Salud Carlos III, Madrid, Spain for providing us
this mutant. We thank Markus M. Simon from Max-
Planck Institute of Immunobiology, Freiburg, Germany
for the monoclonal antibody LA 10 and Amrit Mann
from the Center of Biotechnology and Biomedicine,
Leipzig University, Germany for critical reading of the
manuscript.
References
[1] Salazar, J.C., Pope, C.D., Sellati, T.J., Feder, H.M., Jr. et al.,
2003. Coevolution of markers of innate and adaptive
immunity in skin and peripheral blood of patients with
erythema migrans. J. Immunol., 171, 2660–2670.
[2] Sjowall, J., Carlsson, A., Vaarala, O., Bergstrom, S. et al.,
2005. Innate immune responses in Lyme borreliosis:
enhanced tumour necrosis factor-alpha and interleukin-
12 in asymptomatic individuals in response to live
spirochetes. Clin. Exp. Immunol., 141, 89–98.
[3] Embers, M.E., Ramamoorthy, R., Philipp, M.T., 2004.
Survival strategies of Borrelia burgdorferi, the etiologic
agent of Lyme disease. Microbes. Infect., 6, 312–318.
[4] Zhang, J.R., Hardham, J.M., Barbour, A.G., Norris, S.J.,
1997. Antigenic variation in Lyme disease borreliae by
promiscuous recombination of VMP-like sequence
cassettes. Cell, 89, 275–285.
[5] Alban, P.S., Johnson, P.W., Nelson, D.R., 2000. Serum-
starvation-induced changes in protein synthesis and
morphology of Borrelia burgdorferi. Microbiology, 146,
119–127.
[6] Brorson, Ø., Brorson, S.H., 1997. Transformation of cystic
forms of Borrelia burgdorferi to normal, mobile spirochetes.
Infection, 25, 240–246.
[7] Brorson, Ø., Brorson, S.H., 1998. A rapid method for
generating cystic forms of Borrelia burgdorferi, and their
reversal to mobile spirochetes. APMIS, 106, 1131–1141.
[8] Murgia, R., Cinco, M., 2004. Induction of cystic forms by
different stress conditions in Borrelia burgdorferi. APMIS,
112, 57–62.
[9] Brorson, Ø., Brorson, S.H., Scythes, J., MacAllister, J. et al.,
2009. Destruction of spirochete Borrelia burgdorferi round-
body propagules (RBs) by the antibiotic tigecycline. Proc.
Natl. Acad. Sci. USA, 106, 18656–18661.
[10] Kersten, A., Poitschek, C., Rauch, S., Aberer, E., 1995.
Effects of penicillin, ceftriaxone, and doxycycline on
morphology of Borrelia burgdorferi. Antimicrob. Agents
Chemother., 39, 1127–1133.

S16 Samiya
Al-Robaiy
et al.
Journal of Basic Microbiology 2010, 50, S1 – S17
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
[11] Brorson, Ø., Brorson, S.H., 1998. In vitro conversion of
Borrelia burgdorferi to cystic forms in spinal fluid, and
transformation to mobile spirochetes by incubation in
BSK-H medium. Infection, 26, 144–150.
[12] Gruntar, I., Malovrh, T., Murgia, R., Cinco, M., 2001. Con-
version of Borrelia garinii cystic forms to motile spiro-
chetes in vivo. APMIS, 109, 383–388.
[13] Duray, P.H., Yin, S.R., Ito, Y., Bezrukov, L. et al., 2005.
Invasion of human tissue ex vivo by Borrelia burgdorferi. J.
Infect. Dis., 191, 1747–1754.
[14] Aberer, E., Kersten, A., Klade, H., Poitschek, C. et al., 1996.
Heterogeneity of Borrelia burgdorferi in the skin. Am. J.
Dermatopathol., 18, 571–579.
[15] MacDonald, A.B., 2006. Spirochetal cyst forms in neuro-
degenerative disorders, hiding in plain sight. Med Hypo-
theses, 67, 819–832.
[16] Mursic, V.P., Wanner, G., Reinhardt, S., Wilske, B. et al.,
1996. Formation and cultivation of Borrelia burgdorferi
spheroplast-L-form variants. Infection, 24, 218–226.
[17] Preac-Mursic, V., Weber, K., Pfister, H.-W., Wilske, B.
et al., 1989. Survival of Borrelia burgdorferi in antibioti-
cally treated patients with Lyme borreliosis. Infection, 17,
355–359.
[18] Garon, C.F., Dorward, D.W., Corwin, M.D., 1989. Struc-
tural features of Borrelia burgdorferi – the Lyme disease
spirochete: silver staining for nucleic acids. Scanning
Microsc. Suppl, 3, 109–115.
[19] Raveche, E.S., Schutzer, S.E., Fernandes, H., Bateman, H.
et al., 2005. Evidence of Borrelia autoimmunity-induced
component of Lyme carditis and arthritis. J. Clin.
Microbiol., 43, 850–856.
[20] Motaleb, M.A., Corum, L., Bono, J.L., Elias, A.F. et al., 2000.
Borrelia burgdorferi periplasmic flagella have both skeletal
and motility functions. Proc. Natl. Acad. Sci. USA, 97,
10899–10904.
[21] Barbour, A.G., 1984. Isolation and cultivation of Lyme
disease spirochetes. Yale J. Biol. Med., 57, 521–525.
[22] Kurtti, T.J., Munderloh, U.G., Johnson, R.C., Ahlstrand,
G.G., 1987. Colony formation and morphology in Borrelia
burgdorferi. J. Clin. Microbiol., 25, 2054–2058.
[23] Jobe, D.A., Callister, S.M., Schell, R.F., 1993. Recovery of
Borrelia burgdorferi by filtration. J. Clin. Microbiol., 31,
1896–1898.
[24] Newton, G.L., Unson, M.D., Anderberg, S.J., Aguilera, J.A.
et al., 1999. Characterization of Mycobacterium smegmatis
mutants defective in 1-d-myo-inosityl-2-amino-2-deoxy-
alpha-d-glucopyranoside and mycothiol biosynthesis. Bio-
chem. Biophys. Res. Commun., 255, 239–244.
[25] Blight, E.G., Dyer, W.J., 1959. A rapid method of total
lipid extraction and purification. Can. J. Biochem. Physiol,
37, 911–917.
[26] Schiller, J., Arnhold, J., Benard, S., Müller, M. et al., 1999.
Lipid analysis by matrix-assisted laser desorption and
ionization mass spectrometry: A methodological appro-
ach. Anal. Biochem., 267, 46–56.
[27] Schiller, J., Arnold, K, 2000. Mass spectrometry in struc-
tural biology. In: Encyclopedia of Analytical Chemistry
(Meyers, R.A., ed.). John Wiley & Sons Ltd., Chichester.
p. 559–585.
[28] Schiller, J., Süß, R., Arnhold, J., Fuchs, B. et al., 2004.
Matrix-assisted laser desorption and ionization time-of-
flight (MALDI-TOF) mass spectrometry in lipid and
phospholipid research. Prog Lipid Res, 43, 449–488.
[29] Dihazi, H., Asif, A.R., Agarwal, N.K., Doncheva, Y. et al.,
2005. Proteomic analysis of cellular response to osmotic
stress in thick ascending limb of Henle’s loop (TALH)
cells. Mol. Cell Proteomics., 4, 1445–1458.
[30] Neuhoff, V., Arold, N., Taube, D., Ehrhardt, W., 1988.
Improved staining of proteins in polyacrylamide gels
including isoelectric focusing gels with clear background
at nanogram sensitivity using Coomassie Brilliant Blue
G-250 and R-250. Electrophoresis, 9, 255–262.
[31] Perkins, D.N., Pappin, D.J., Creasy, D.M., Cottrell, J.S.,
1999. Probability-based protein identification by search-
ing sequence databases using mass spectrometry data.
Electrophoresis, 20, 3551–3567.
[32] Laemmli, U.K., 1970. Cleavage of structural proteins dur-
ing the assembly of the head of bacteriophage T4. Nature,
227, 680–685.
[33] Charon, N.W., Goldstein, S.F., Marko, M., Hsieh, C. et al.,
2009. The flat-ribbon configuration of the periplasmic
flagella of Borrelia burgdorferi and its relationship to
motility and morphology. J. Bacteriol., 191, 600–607.
[34] Jeggo, P., 1979. Isolation and characterization of Escheri-
chia coli K-12 mutants unable to induce the adaptive
response to simple alkylating agents. J. Bacteriol., 139,
783–791.
[35] Cariello, N.F., Narayanan, S., Kwanyuen, P., Muth, H. et al.,
1998. A novel bacterial reversion and forward mutation
assay based on green fluorescent protein. Mutat. Res.,
414, 95–105.
[36] Fraser, C.M., Casjens, S., Huang, W.M., Sutton, G.G. et al.,
1997. Genomic sequence of a Lyme disease spirochaete,
Borrelia burgdorferi. Nature, 390, 580–586.
[37] Dowhan, W., 1997. Molecular basis for membrane phos-
pholipid diversity: why are there so many lipids? Annu.
Rev. Biochem., 66, 199–232.
[38] Tang, Y., Hollingsworth, R.I., 1998. Regulation of lipid
synthesis in Bradyrhizobium japonicum: low oxygen concen-
trations trigger phosphatidylinositol biosynthesis. Appl.
Environ. Microbiol., 64, 1963–1966.
[39] Wang, X.G., Scagliotti, J.P., Hu, L.T., 2004. Phospholipid
synthesis in Borrelia burgdorferi: BB0249 and BB0721 en-
code functional phosphatidylcholine synthase and phos-
phatidylglycerolphosphate synthase proteins. Microbiol-
ogy, 150, 391–397.
[40] Hossain, H., Wellensiek, H.J., Geyer, R., Lochnit, G., 2001.
Structural analysis of glycolipids from Borrelia burgdorferi.
Biochimie, 83, 683–692.
[41] Stevenson, B., Schwan, T.G., Rosa, P.A., 1995. Tempera-
ture-related differential expression of antigens in the
Lyme disease spirochete, Borrelia burgdorferi. Infect. Im-
mun., 63, 4535–4539.
[42] Liang, F.T., Nelson, F.K., Fikrig, E., 2002. Molecular adap-
tation of Borrelia burgdorferi in the murine host. J. Exp.
Med., 196, 275–280.
[43] Jungblut, P.R., Grabher, G., Stoffler, G., 1999. Compre-
hensive detection of immunorelevant Borrelia garinii anti-

Journal of Basic Microbiology 2010, 50, S5 – S17
Metamorphosis of Borrelia burgdorferi organisms S17
© 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
gens by two-dimensional electrophoresis. Electrophoresis,
20, 3611–3622.
[44] Barzaghi, D., Isbister, J.D., Lauer, K.P., Born, T.L., 2004.
Use of surface-enhanced laser desorption/ionization –
time of flight to explore bacterial proteomes. Proteomics.,
4, 2624–2628.
[45] Murgia, R., Piazzetta, C., Cinco, M., 2002. Cystic forms of
Borrelia burgdorferi sensu lato: induction, development, and
the role of RpoS. Wien. Klin. Wochenschr., 114, 574–579.
[46] Loewen, P.C., Hengge-Aronis, R., 1994. The role of the
sigma factor sigma S (KatF) in bacterial global regulation.
Annu. Rev. Microbiol., 48, 53–80.
[47] Hubner, A., Yang, X., Nolen, D.M., Popova, T.G. et al.,
2001. Expression of Borrelia burgdorferi OspC and DbpA is
controlled by a RpoN-RpoS regulatory pathway. Proc.
Natl. Acad. Sci. USA, 98, 12724–12729.
[48] Joseph, R., Holt, S.C., Canale-Parola, E., 1973. Peptidogly-
can of free-living anaerobic spirochetes. J. Bacteriol., 115,
426–435.
[49] Beck, G., Benach, J.L., Habicht, G.S., 1990. Isolation, pre-
liminary chemical characterization, and biological activi-
ty of Borrelia burgdorferi peptidoglycan. Biochem. Biophys.
Res. Commun., 167, 89–95.
[50] Izard, J., Renken, C., Hsieh, C.E., Desrosiers, D.C. et al.,
2009. Cryo-electron tomography elucidates the molecular
architecture of Treponema pallidum, the syphilis spiro-
chete. J. Bacteriol., 191, 7566–7580.
Wyszukiwarka
Podobne podstrony:
jobm 201000013
jobm 201000298
jobm 201000191
jobm 201000321
jobm 201000018
jobm 201000214
jobm 201000067
jobm 201000037
jobm 201000280
jobm 201000385
jobm 201000198
jobm 201000458
jobm 201000147
jobm 201000520
jobm 201000327
jobm 201000342
jobm 201000420
jobm 201000364
jobm 201000317
więcej podobnych podstron