
296
Journal of Basic Microbiology 2011, 51, 296 – 303
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Research Paper
Chloride-associated adaptive response in aerobic
methylotrophic dichloromethane-utilising bacteria
Maria L. Torgonskaya
1
, Nina V. Doronina
1
, Edith Hourcade
2
, Yuri A. Trotsenko
1
and Stéphane Vuilleumier
2
1
G.K. Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences,
5 Science Avenue, Pushchino, Moscow region, 142290, Russia
2
Université de Strasbourg, UMR 7156 CNRS, Strasbourg, France
Aerobic methylotrophic bacteria able to grow with dichloromethane (DCM) as the sole carbon
and energy source possess a specific glutathione S-transferase, DCM dehalogenase, which
transforms DCM to formaldehyde, used for biomass and energy production, and hydrochloric
acid, which is excreted. Evidence is presented for chloride-specific responses for three DCM-
degrading bacteria, Methylobacterium extorquens DM4, Methylopila helvetica DM6 and Albibacter
methylovorans DM10. Chloride release into the medium was inhibited by sodium azide and m-
chlorophenylhydrazone, suggesting an energy-dependent process. In contrast, only nigericin
affected chloride excretion in Mb. extorquens DM4 and Mp. helvetica DM6, while valinomycin had
the same effect in A. methylovorans DM10 only. Chloride ions stimulated DCM-dependent induc-
tion of DCM dehalogenase expression for Mp. helvetica DM6 and A. methylovorans DM10, and
shortened the time for onset of chloride release into the medium. Striking chloride-containing
structures were observed by electron microscopy and X-ray microanalysis on the cell surface of
Mp. helvetica DM6 and A. methylovorans DM10 during growth with DCM, and with methanol in
medium supplemented with sodium chloride. Taken together, these data suggest the existence
of both general and specific chloride-associated adaptations in aerobic DCM-degrading bacteria.
Keywords: Dichloromethane / Dehalogenation / Chloride / Salinity / Methylotrophy
Received: July 18, 2010; accepted: October 07, 2010
DOI 10.1002/jobm.201000280
Introduction
*
Dichloromethane (DCM) is a toxic, mutagenic and po-
tentially carcinogenic compound [11, 22]. It is mainly of
anthropogenic origin [13] and widely used as industrial
solvent, degreasing agent and intermediate for chemi-
cal synthesis (www.eurochlor.org). DCM can be used as
sole carbon and energy source by a variety of microor-
ganisms under both aerobic and anaerobic conditions
[26]. Aerobic methylotrophic Gram-negative bacteria
which mineralise DCM are represented by members of
8 different genera of Alpha- and Betaproteobacteria
(Methylobacterium, Hyphomicrobium, Methylopila, Albibacter,
Methylophilus, Methylorhabdus, Paracoccus, Ancylobacter)
and may have serine, ribulose bisphosphate (RuBP) or
Correspondence: Stéphane Vuilleumier, Université de Strasbourg,
UMR 7156 CNRS, 28 rue Goethe, F-67083 Strasbourg, France
E-mail: vuilleumier@unistra.fr
ribulose monophosphate (RuMP) pathways for carbon
assimilation [3, 4, 6, 24]. In all DCM-degrading bacteria
which have been characterised at the molecular level,
dehalogenation of DCM is performed in the cytoplasm
by DCM dehalogenase [17], an enzyme belonging to the
glutathione S-transferase family [27] and encoded by
the dcmA gene [15]. This results in the formation of
formaldehyde and hydrochloric acid. Evidence from
mutant studies [10] as well as failure of the reference
strain Mb. extorquens AM1 to grow with DCM, when
provided with dcmA functionally expressed from a plas-
mid [12], suggest that other proteins and genes are
likely to be involved in growth with DCM. To date,
microbial adaptive mechanisms to DCM remain to be
elucidated, although it is clear that DCM-consuming
bacteria excrete the protons and chloride anions pro-
duced by dehalogenation into the extracellular medium
[5, 7].

Journal of Basic Microbiology 2011, 51, 296 – 303
Chloride-associated adaptive response in dichloromethane-utilising bacteria
297
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
In this work, we explore responses to chloride in the
context of DCM dehalogenation for three DCM-degrad-
ing methylotrophic Alphaproteobacteria, Methylobacte-
rium extorquens DM4 [3, 28], Methylopila helvetica DM6 [3]
and Albibacter methylovorans DM10 [4].
Materials and methods
Chemicals and reagents
All chemicals and reagents were of reagent grade or
better, and obtained from Sigma or Fluka unless indi-
cated otherwise.
Strains and growth conditions
DCM-utilising bacteria Methylobacterium extorquens DM4
(DSMZ 6343, VKM-B-2191), Methylopila helvetica DM6
(DSMZ 6342, VKM-B-2189) and Albibacter methylovorans
DM10 (DSMZ 22840, VKM-B-2236) were grown at 29 °C
on a rotary shaker (180 rpm) in liquid minimal medium
(MM) (pH 7.2) containing (in g ⋅ l
–1
of deionised distilled
water): KH
2
PO
4
– 6.8, (NH
4
)
2
SO
4
– 0.2; MgSO
4
⋅ 7 H
2
O –
0.1 and trace elements (in mg ⋅ l
–1
): Ca(NO
3
)
2
– 25,
FeSO
4
⋅ 7 H
2
O – 0.1, MnSO
4
⋅ 5 H
2
O – 0.1, Na
2
MoO
4
⋅ 2 H
2
O
– 0.025, H
3
BO
3
– 0.01, CuCl
2
⋅ 2 H
2
O – 0.025, ZnSO
4
–
0.03, Na
3
VO
4
⋅ 12 H
2
O – 0.03, CoCl
2
⋅ 6 H
2
O – 0.02, NiCl
2
⋅ 6 H
2
O – 0.009 as described previously [23]. Solid mini-
mal medium MM contained (in g ⋅ l
–1
): K
2
HPO
4
– 1.04,
NaH
2
PO
4
– 0.57, (NH
4
)
2
SO
4
– 0.2; MgSO
4
⋅ 7 H
2
O – 0.1,
agar – 15 and the same trace elements concentrations.
Methanol (20 mM) or DCM (10 mM) as carbon and en-
ergy sources were added after sterilisation. Cultivation
with methanol was performed in 200 ml MM in 750 ml
Erlenmeyer flasks. For cultivation with DCM, 300 ml
glass flasks closed by gas-tight mininert caps (Supel-
co) and containing 25 ml of MM and were used. Ali-
quots of a sterile solution of 5 M NaOH were added
periodically during growth with DCM to neutralise
the medium to pH 7.2. Bacterial growth in liquid cul-
tures was determined by measuring optical density at
600 nm.
Dichloromethane dehalogenase activity
DCM dehalogenase activity was determined by chloride
production in cell suspensions or in cell-free extracts,
and expressed as nmol/min/mg dry biomass or nmol/
min/mg protein, respectively. Experiments were carried
out in triplicate. Biomass was determined basing on a
calibration curve of OD
600
vs. dry weight, obtained from
cell suspensions of different OD
600
in exponential phase
of growth (10 ml) which were filtered through 0.2 μm
filters of known weight and dried for 12 h at 60 °C.
For activity measurements in cell suspensions, ali-
quots of bacterial cultures (1 ml) were pelleted by cen-
trifugation (8,000 g for 5 min), washed twice with
20 mM potassium phosphate buffer (pH 8.0), and resus-
pended in the same buffer at final OD
600
= 1.2. Assays
were carried out in 20 mM potassium buffer (pH 8.0) in
a total volume of 250 μl containing 2 mM glutathione,
2 mM ascorbic acid, and cells (~3–5 mg of dry biomass).
The time course of chloride build-up was measured in
supernatants of these assay solutions by a previously
described method [9].
For activity in cell-free extracts, cells (typically from
10 ml cultures) were harvested and washed as above,
resuspended in 0.5 ml of 20 mM potassium buffer (pH
8.0), and disrupted by 150 W sonication (MSE, U.K.)
using 2 × 30 s pulses at 20 kHz on ice. Cell debris were
removed by centrifugation (15,000 g for 45 min at 4 °C),
and protein concentration in supernatants was deter-
mined using a commercial Bradford reagent (Bio-Rad,
USA) [1], with bovine serum albumin as a standard.
Activity assays were performed with 0.1–0.3 mg of
protein as described above for cell suspensions.
Adaptation to sodium chloride
Bacteria were grown to mid-exponential phase (OD
600
=
0.4) in MM containing 100 mM NaCl with methanol
(20 mM) as the sole carbon and energy source. Cultures
were harvested by centrifugation (6,000 g for 30 min),
washed twice with fresh chloride-free MM, and resus-
pended in the same medium at final OD
600
= 1.2 (~3 mg
cells/ml). Resulting cell suspensions (25 ml) were trans-
ferred to 300 ml glass flasks closed by gas-tight minin-
ert caps (Supelco, USA), supplied with DCM (10 mM)
and incubated at 29 °C on a rotary shaker (140 rpm).
Induction of DCM dehalogenase expression in these
suspensions was estimated by measuring DCM dehalo-
genase activity in cell-free extracts prepared from sam-
ples of cell suspensions taken at different times, as
described above. Chloride release was measured in cell
suspensions of 1 ml aliquots taken at different times, as
described above.
Effects of uncouplers and inhibitors
on chloride production
Cell suspensions (25 ml) of cultures grown to OD
600
= 0.4
and resuspended at the same OD in fresh medium were
placed in 300 ml Erlenmeyer flasks fitted with gas-tight
mininert stoppers, and supplied with DCM (10 mM) and
appropriate amounts of uncoupling agents and inhibi-
tors: valinomycin (100 mM stock in DMSO), nigericin
(100 mM in methanol), m-chlorophenylhydrazone (CCCP,
25 mM in DMSO), sodium azide (2 M in water) or N,N′-

298 M.
L.
Torgonskaya
et al.
Journal of Basic Microbiology 2011, 51, 296 – 303
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
dicyclohexylcarbodiimide (DCCD, 780 mM in DCM). The
resulting suspensions were incubated at 29 °C on a rotary
shaker (180 rpm), and DCM dehalogenation was followed
by measurement of the chloride concentration in the
medium as described above. Chloride release to the su-
pernatant of cell suspensions was determined throughout
the experiment and compared to that of control cell sus-
pensions to which the same volume of the solvent used to
dissolve the inhibitor had been added.
Determination of bacterial viability
Bacterial viability was determined using a spot plating
technique. Cell suspensions obtained from bacterial
cultures were serially diluted (10
2
to 10
6
-fold) and spot-
ted (5 μl) in triplicate onto MM agar plates containing
40 mM methanol. Plates were incubated for 5–7 d at
29 °C, and dilutions with spots containing 5 to 150
colonies were counted.
Electron microscopy
Cultures grown to late-exponential phase (OD
600
= 0.6)
were pelleted by centrifugation, pre-fixed for 2 h at 4 °C
with 2% (w/v) glutaraldehyde in 0.05 M cacodylate buf-
fer (pH 7.2), washed three times with the same buffer,
and additionally fixed in 1% (w/v) OsO
4
for 12 h at
20 °C. After dehydration in a series of alcohols (70–
100%) and absolute acetone, cells were embedded in
Spurr epoxy resin Epon-812 and sectioned with an LKB
2128 Ultratome (Sweden). Ultrathin sections (700–750 Å)
were obtained using a LKB-800A microtome, mounted
on copper grids and double-stained with 2% uranyl
acetate solution in 70% ethanol for 45 min at 37 °C,
followed by 0.2% lead citrate at 20 °C [21]. Thin-sectio-
ned preparations were imaged using a JEM-100B trans-
mission electron microscope (JEOL, Japan) at an operat-
ing voltage of 60 kV.
X-ray microanalysis
Cells were prepared as for electron microscopy except for
the final step of staining which was left out. Cell suspen-
sions were mounted onto Formvar-coated copper grids
and perpendicularly sprayed with carbon. Elemental
composition was analyzed with a JEM-100CXII electron
microscope (JEOL, Japan) fitted with a EM-ASID4D scan-
ning device and a LINK-860 X-ray microanalysis system
with E5423 detector (Link-System, U.K.), at 20,000× mag-
nification with 60 kV operating voltage. Spectra were
processed using standard Link-System software.
Results
Bacteria which metabolise DCM face several challenges.
As a solvent, DCM affects cell membrane integrity. S-
chloromethylglutathione, the reaction intermediate in
the transformation of DCM into formaldehyde by DCM
dehalogenase, was shown to be genotoxic, as it involves
formation of DNA adducts [10, 11]. Dehalogenation is
also acidogenic, being accompanied by intracellular
production of chloride ions, which are usually excreted
into the extracellular medium. This latter aspect of
DCM dehalogenation by methylotrophic bacteria was
addressed here.
Degradation of DCM by methylotrophic bacteria con-
taining the dcmA gene is induced in the presence of
DCM [16]. It was shown here that in Mp. helvetica DM6
and A. methylovorans DM10, but not in Mb. extorquens
DM4, exposure of cultures growing with methanol to
100 mM sodium chloride stimulated DCM-dependent
induction of DCM dehalogenase activity. However, in-
duction of DCM dehalogenase activity was observed
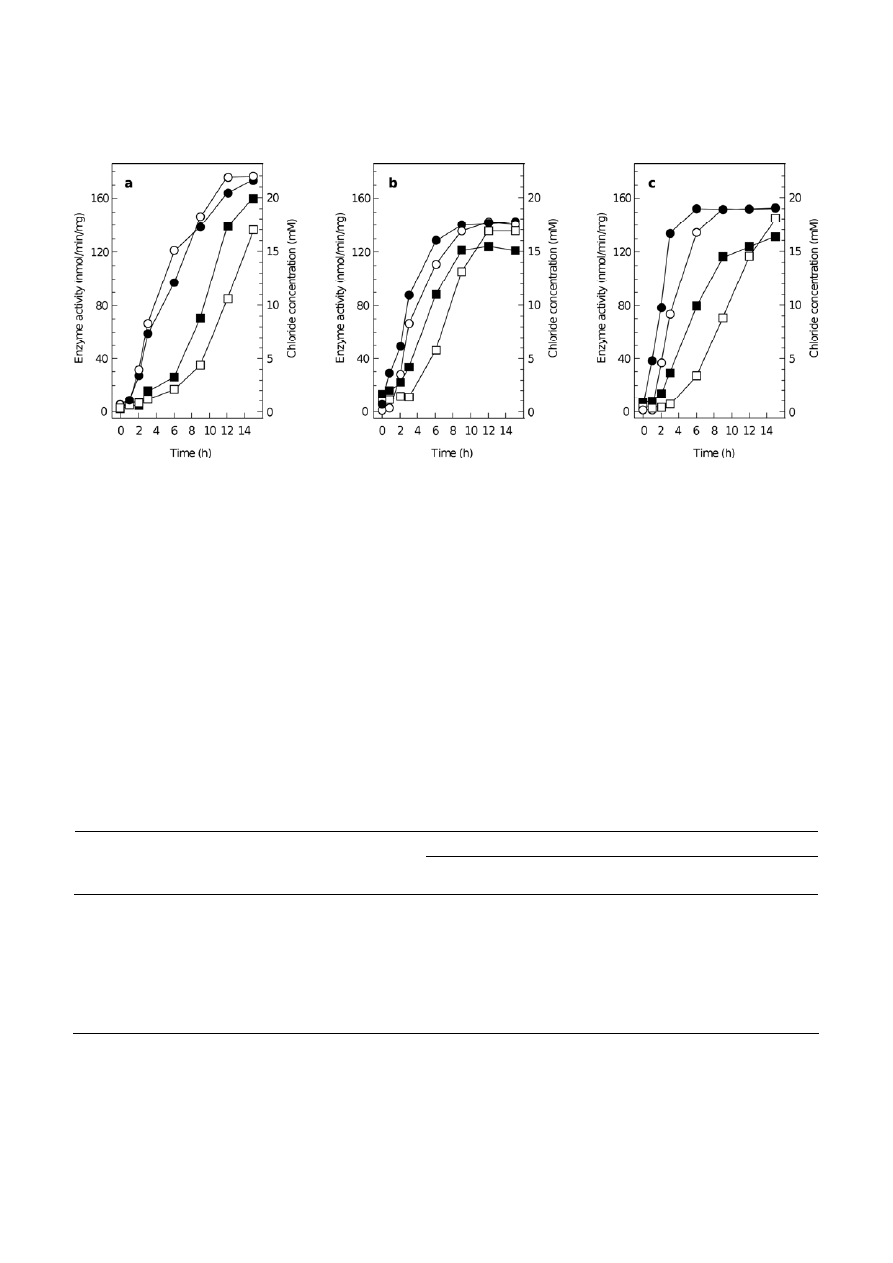
only after addition of DCM (Fig. 1), demonstrating that
in itself, increased salinity was not sufficient to elicit
DCM dehalogenase expression. Also, chloride release
occurred earlier after exposure to DCM if bacteria had
been grown in the presence of 100 mM chloride (Fig. 1).
Production of chloride ions likely requires efficient
chloride efflux against a growing concentration gradi-
ent [5], but it is not yet known whether the well-cha-
racterised bacterial chloride channel [18] participates in
chloride excretion in aerobic methylotrophic bacteria.
Here, the energy dependence of chloride excretion into
the medium was explored as a function of the addition
of different uncoupling agents and electron transport
chain inhibitors in cell suspensions of Mb. extorquens
DM4, Mp. helvetica DM6, and A. methylovorans DM10
grown with DCM (Table 1). Chloride production was
indeed sensitive to the terminal oxidase inhibitor so-
dium azide (0.25 mM). The observed effect was specific,
since this concentration of sodium azide was chosen
because it did not affect DCM dehalogenase activity or
cellular viability for any of the three bacteria investi-
gated (data not shown). The protonophore m-chloro-
phenylhydrazone (CCCP, Table 1) also had an effect,
suggesting that DCM dechlorination may be associated
with a proton-dependent chloride excretion mechanism.
Indeed, addition of the F
1
F
0
-H
+
-ATP-ase inhibitor N,N′-
dicyclohexylcarbodiimide (DCCD) reduced chloride re-
lease in the medium (Table 1), further suggesting that
DCM-degrading bacteria consumed ATP for active trans-
port of chloride. However, contrasting effects were ob-
served for the different bacteria investigated upon addi-
tion of different ionophores. For serine pathway bacte-
ria Mb. extorquens DM4 and Mp. helvetica DM6, nigericin
had a strong effect, while for the facultatively auto-
trophic, RuBP-pathway utilising A. methylovorans DM10,
Journal of Basic Microbiology 2011, 51, 296 – 303
Chloride-associated adaptive response in dichloromethane-utilising bacteria
299
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 1. Dichloromethane dehalogenase expression measured by activity in cell-free extracts (circles) and by chloride concentration in
extracellular medium of 1 ml cell suspensions (squares) in (a) Mb. extorquens DM4, (b) Mp. helvetica DM6 and (c) A. methylovorans DM10.
Dichloromethane was added at t = 0 to cell suspensions of cultures that had been grown with methanol in the presence (filled symbols) or
absence (open symbols) of 100 mM NaCl. Values represent the average of 3 independent experiments which varied by less than 10%.
strong inhibition with valinomycin was observed (Ta-
ble 1). Since all three investigated DCM-degrading bac-
teria are Alphaproteobacteria, the observed effects ap-
peared to correlate less with phylogeny than with the
used pathway for carbon assimilation, as well as with
changes in saturated fatty acid content upon addition
of DCM (decreasing in Mb. extorquens DM4 and Mp. helve-
tica DM6, and increased in A. methylovorans DM10; data
not shown).
Protonophore mobility within membranes may also
be codetermined by differences in cell wall lipid com-
position, and some minor changes were indeed ob-
served upon exposure to DCM (data not shown). Adap-
tation to conditions of higher salt concentration
(100 mM NaCl) may also involve other processes such as
synthesis of osmoprotectants. Many methylotrophs and
methanotrophs are known to be halophilic or halotol-
erant, and feature such specialised adaptations to very
high salt concentrations [14, 25]. However, this does not
apply to the three methylotrophic bacteria investigated
here, whose growth is markedly affected by the pres-
ence of salt in the medium, and which are unable to
grow at concentrations of 3% NaCl in the case of
M. extorquens DM4 [3] and A. methylovorans DM10 [4], and
Table 1. Effect of different inhibitors on chloride ion release from cell suspensions during dehalogenation of DCM.
Chloride release in extracellular medium (% of control)
a
Inhibitor
Mode of action
Conc.
used
(mM)
Mb. extorquens
DM4
Mp. helvetica
DM6
A. methylovorans
DM10
Sodium azide
terminal oxidase inhibitor
0.25
35.1 ± 9.0
60.7 ± 2.8
39.2 ± 2.6
DCCD ATPase
inhibitor 0.5
61.4 ± 4.1
20.5 ± 0.9
15.5 ± 1.0
valinomycin ionophore,
dissipates
Δ
ϕ
,
ΔpH remains constant
0.2
89.4 ± 5.9
99.4 ± 4.5
2.3 ± 0.2
nigericin ionophore,
dissipates
ΔpH,
Δ
ϕ
remains constant
0.2
8.1 ± 0.5
1.2 ± 0.1
96.1 ± 6.4
valinomycin +
nigericin
0.2 each
0.0 ± 0.0
0.0 ± 0.0
1.8 ± 0.1
CCCP protonophore,
uncoupler
0.05
0.3 ± 0.1
0.0 ± 0.0
2.1 ± 0.1
a
Given as percentage of rate value in the absence of inhibitor ± standard error (87.4 ± 2.9 μM/min for Mb. extorquens DM4,
93.7 ± 0.3 μM/min for Mp. helvetica DM6, 90.8 ± 3.2 μM/min for A. methylovorans DM10), for cell suspensions of OD
600
= 0.4, see
Materials and methods). The release rate was determined for the first two hours following addition of DCM. No changes in cell
viability were observed throughout the course of the experiment (times 0–6 h; determined as described under Materials and
Methods, data not shown).
300 M.
L.
Torgonskaya
et al.
Journal of Basic Microbiology 2011, 51, 296 – 303
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
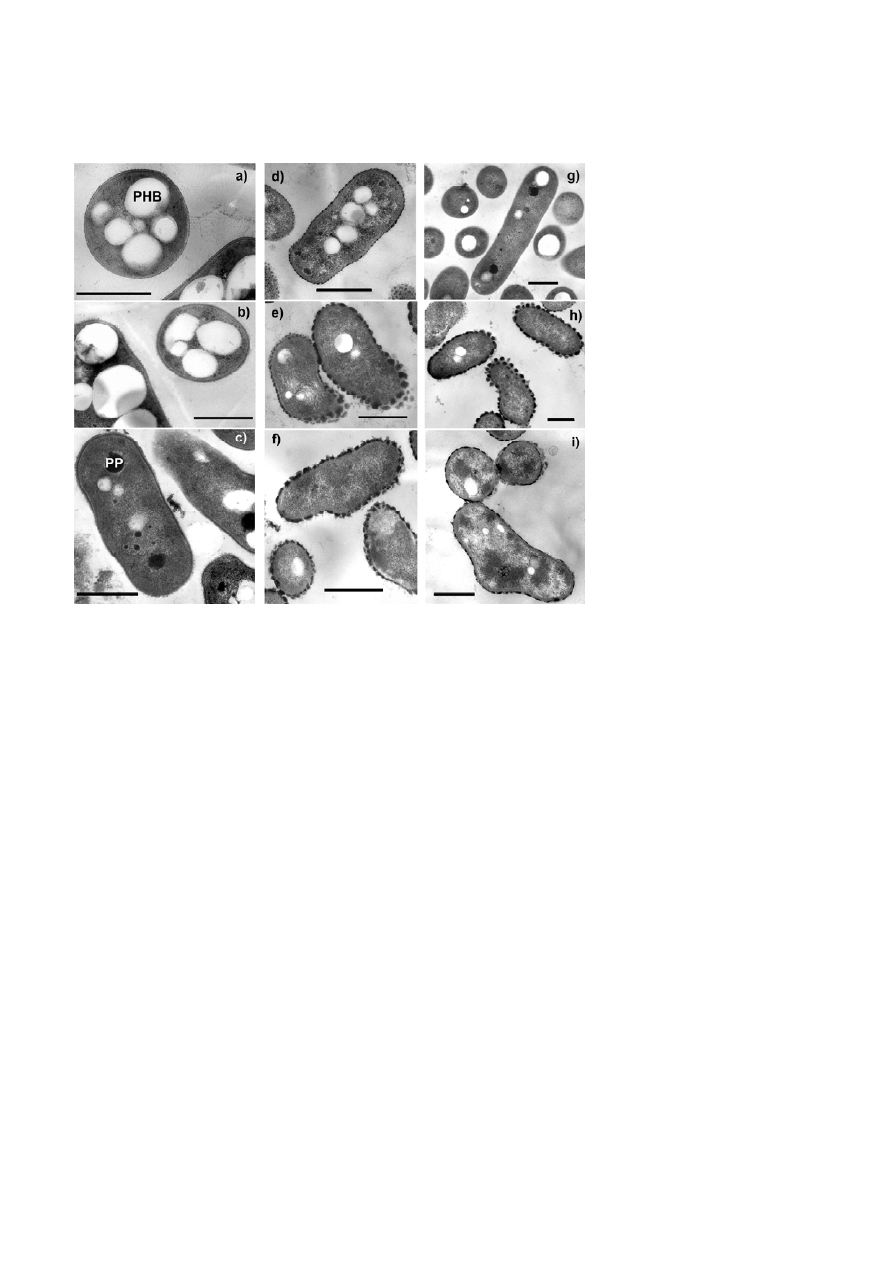
Figure 2. Electron micrographs of ultrathin sections of cells of Mb. extorquens DM4 (a – c), Mp. helvetica DM6 (d – f) and A. methylovorans
DM10 (g – i), from cultures grown with methanol (a, d, g), with methanol in the presence of 100 mM NaCl (b, e, h), and with DCM (c, f, i).
Surface-associated structures are marked by arrows. PHB – polyhydroxybutyrate granules, PP – polyphosphate granules. Bar (all panels),
0.5
μ
m.
above 6% NaCl for Mp. helvetica DM6 [3]. In addition,
DCM-associated differences of membrane lipid compo-
sition and of chloride concentration in the ambient
medium did not significantly affect the volume of pe-
riplasm or cytoplasm, or provoke condensation of cell
wall structures (Fig. 2). Nethertheless, electron micros-
copy analysis revealed unusual cell surface-associated
symmetric ordered structures on Mp. helvetica DM6
and A. methylovorans DM10 during growth with DCM
(Fig. 2f, i), or with methanol in the presence of 100 mM
NaCl (Fig. 2e, h). This was not observed for Mb. extor-
quens DM4, suggesting that the observed structures
may represent a specific type of adaptation that is not
shared between all DCM-degrading bacteria.
An initial characterisation of these cell surface struc-
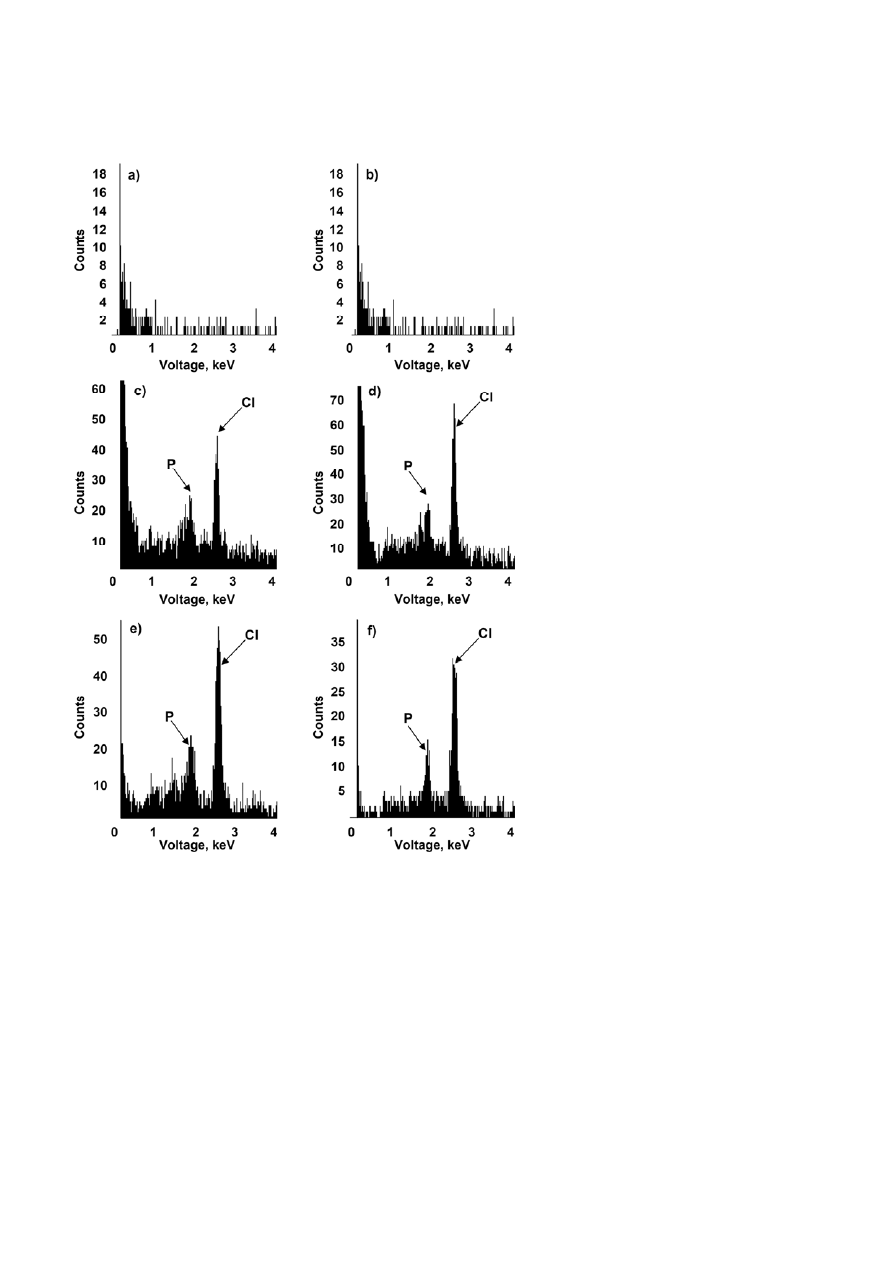
tures was performed by X-ray microanalysis of ultra-
thin cell sections of Mp. helvetica DM6 (Fig. 3). Phospho-
rus and chlorine were the main elements revealed by
this method, and were detected in cultures grown with
DCM (Fig. 3e, f) or with methanol in the presence of
sodium chloride (Fig. 3c, d), but not in cultures grown
with methanol only (Fig. 3a, b). For bacteria grown with
methanol in the presence of NaCl, the ratio of chlorine
to phosphorus peaks appeared to be lower for cyto-
plasmic regions (Fig. 3c) than for cell wall regions
(Fig. 3d), in accordance with the high chloride concen-
tration present in the extracellular medium. For DCM-
grown bacteria, in contrast, the Cl/P ratio was similar
for both cell compartments (Fig. 3e, f), and higher than
that of the cytoplasmic compartment of methanol/NaCl
grown bacteria (Fig. 3c), consistent with the fact that
chloride is generated intracellularly during growth
with DCM.
Discussion
To our knowledge, this is the first report of a positive
effect of chloride, in other words the product of the
DCM dehalogenation reaction, on the onset of DCM
substrate-dependent induction of DCM dehalogenase.
Induction of DCM dehalogenase was followed by activ-
ity rather than through protein or transcript measure-
ments, but it is unlikely that the DCM dehalogenase
protein was already expressed in an enzymatically inac-
tive form in the presence of 100 mM NaCl. One indica-
Journal of Basic Microbiology 2011, 51, 296 – 303
Chloride-associated adaptive response in dichloromethane-utilising bacteria
301
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 3. X-ray microanalysis spectra of ultrathin sections of cytoplasmic compartment (a, c, e) and cell wall (b, d, f) of Mp. helvetica DM6
cells from cultures grown on methanol without NaCl (a, b), on methanol in the presence of 100 mM NaCl (c, d), on DCM without NaCl (e, f).
tion supporting this assumption is that the maximal
DCM dehalogenase activities observed upon prolonged
incubation in the presence of DCM (12–15 h) were the
same for cultures initially grown with or without
100 mM NaCl (Fig. 1). Incidentally, the obtained results
(Fig. 1) further suggest that the process of chloride
excretion is also regulated by salinity, and that chlo-
ride-dependent regulatory events at the transcriptional
level, which have been reported in other contexts in the
past (e.g. [2, 19]), may be involved in dehalogenation
metabolism as well.
Another finding of this work is the observation by X-
ray microanalysis of specific structures on the cell sur-
face of Mp. helvetica DM6 and A. methylovorans DM10 as a
function of the presence of chloride, either added to
the growth medium or produced by dehalogenative
metabolism (Fig. 3). Chloride-dependent formation of
specific structures on cell surfaces was reported previ-
ously for other microorganisms, and was often as-
sumed to be associated with specific adaptative respon-
ses to salinity. For example, a Gram-negative, halophilic
Halobacteroides acetoethylicus strain displayed unusual

302 M.
L.
Torgonskaya
et al.
Journal of Basic Microbiology 2011, 51, 296 – 303
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
crystal-like cell wall structures at high external salinity
[20]. The function of such ion-containing structures is
still unknown, but it has been hypothesised that they
may generate charge density on the cell surface that
decreases the membrane permeability of ions located in
the extracellular medium [8]. According to such a
model, the presence of chloride in cell wall structures
may help in building up a net negative charge on the
outer surface of cells, thereby protecting them against
the potentially detrimental effects of high chloride
concentration in the cell environment. Alternatively,
chloride-containing structures might rather be involved
in chloride extrusion from the cytoplasm during deha-
logenation of DCM. Further work is required to distin-
guish between these two possibilities.
Concluding remarks
This study has confirmed that aerobic DCM-degrading
bacteria, beyond being capable of active transport of
chloride, feature previously undetected specific chlo-
ride-dependent adaptations, including modulation of
DCM dehalogenase activity and the formation of chlo-
ride-containing surface-associated structures. These
novel aspects of bacterial adaptation to dehalogenative
metabolism represent worthwhile topics for future
investigations.
Acknowledgements
Authors are very grateful to Drs. N.E. Suzina (Institute
of Biochemistry and Physiology of Microorganisms RAS,
Pushchino) and V.V. Sorokin (Institute of Microbiology
RAS, Moscow) for help with the electron microscopy
and X-ray microanalysis studies. Work in SV’s labora-
tory is supported by REALISE, the Alsace Research
Network in Environmental Sciences (http://realise.
u-strasbg.fr). This project was funded by the Russian
Foundation of Basic Research (grant 06-04-22000) and
Centre National de la Recherche Scientifique CNRS
(PICS 3380).
Conflict of interest statement
Financial/commercial conflicts of interest: none.
References
[1] Bradford, M.M., 1976. Rapid and sensitive method for
quantitation of microgram quantities of protein utilizing
principle of protein-dye binding. Anal. Biochem., 72,
248–254.
[2] Davis-Kaplan, S.R., Askwith, C.C., Bengtzen, A.C., Radisky,
D., Kaplan, J., 1998. Chloride is an allosteric effector of
copper assembly for the yeast multicopper oxidase Fet3p:
An unexpected role for intracellular chloride channels.
Proc. Natl. Acad. Sci. USA, 95, 13641–13645.
[3] Doronina, N.V., Trotsenko, Y.A., Tourova, T.P., Kuznetsov,
B.B., Leisinger, T., 2000. Methylopila helvetica sp. nov. and
Methylobacterium dichloromethanicum sp. nov. – Novel aero-
bic facultatively methylotrophic bacteria utilizing di-
chloromethane. Syst. Appl. Microbiol., 23, 210–218.
[4] Doronina, N.V., Trotsenko, Y.A., Tourova, T.P., Kuznetsov,
B.B., Leisinger, T., 2001. Albibacter methylovorans gen. nov.,
sp nov., a novel aerobic, facultatively autotrophic and me-
thylotrophic bacterium that utilizes dichloromethane.
Int. J. Syst. Evol. Microbiol., 51, 1051–1058.
[5] Evans, G.J., Ferguson, G.P., Booth, I.R., Vuilleumier, S.,
2000. Growth inhibition of Escherichia coli by dichloro-
methane in cells expressing dichloromethane dehaloge-
nase/glutathione S-transferase. Microbiology, 146, 2967–
2975.
[6] Firsova, J., Doronina, N., Lang, E., Spröer, C., Vuilleumier,
S. et al., 2009. Ancylobacter dichloromethanicus sp. nov. – a
new aerobic facultatively methylotrophic bacterium util-
izing dichloromethane. Syst. Appl. Microbiol., 32, 227–
232.
[7] Firsova, Y.E., Torgonskaya, M.L., Doronina, N.V., Trot-
senko, Y.A., 2005. Effects of DNA-damaging agents on
aerobic methylobacteria capable and incapable of utiliz-
ing dichloromethane. Appl. Biochem. Microbiol., 41,
480–485.
[8] Imhoff, J.F., 1993. Osmotic adaptation in halophilic and
halotolerant microorganisms. In: (Vreeland, R.H. & Hoch-
stein, L.I., eds.), The Biology of Halophilic Bacteria. Boca
Raton: CRC Press, pp. 211–253.
[9] Jörg, G., Bertau, M., 2004. Thiol-tolerant assay for quanti-
tative colorimetric determination of chloride released
from whole-cell biodehalogenations. Anal. Biochem., 328,
22–28.
[10] Kayser, M.F., Stumpp, M.T., Vuilleumier, S., 2000. DNA
polymerase I is essential for growth of Methylobacterium
dichloromethanicum DM4 with dichloromethane. J. Bacte-
riol., 182, 5433–5439.
[11] Kayser, M.F., Vuilleumier, S., 2001. Dehalogenation of
dichloromethane by dichloromethane dehalogenase/glu-
tathione S-transferase leads to the formation of DNA ad-
ducts. J. Bacteriol., 183, 5209–5212.
[12] Kayser, M.F., Ucurum, Z., Vuilleumier, S., 2002. Dichlo-
romethane metabolism and C1 utilization genes in Methy-
lobacterium strains. Microbiology, 148, 1915–1922.
[13] Keene, W.C., Khalil, M.A.K., Erickson, D.J., McCulloch, A.,
Graedel, T.E. et al., 1999. Composite global emissions of
reactive chlorine from anthropogenic and natural sour-
ces: Reactive Chlorine Emissions Inventory. J. Geophys.
Res. Atmos., 104, 8429–8440.
[14] Khmelenina, V.N., Kalyuzhnaya, M.G., Sakharovsky, V.G.,
Suzina, N.E., Trotsenko, Y.A. et al., 1999. Osmoadaptation
in halophilic and alkaliphilic methanotrophs. Arch. Mic-
robiol., 172, 321–329.
[15] La Roche, S.D., Leisinger, T., 1990. Sequence analysis and
expression of the bacterial dichloromethane dehalo-

Journal of Basic Microbiology 2011, 51, 296 – 303
Chloride-associated adaptive response in dichloromethane-utilising bacteria
303
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
genase structural gene, a member of the glutathione S-
transferase supergene family. J. Bacteriol., 172, 164–171.
[16] La Roche, S.D., Leisinger, T., 1991. Identification of dcmR,
the regulatory gene governing expression of dichloro-
methane dehalogenase in Methylobacterium sp. DM4. J. Bac-
teriol., 173, 6714–6721.
[17] Leisinger, T., Bader, R., Hermann, R., Schmid-Appert, M.,
Vuilleumier, S., 1994. Microbes, enzymes and genes in-
volved in dichloromethane utilization. Biodegradation, 5,
237–248.
[18] Mindell, J.A., Maduke, M., 2001. ClC chloride channels.
Genome Biol., 2, article 3003.
[19] Müller, V., Oren, A., 2003. Metabolism of chloride in halo-
philic prokaryotes. Extremophiles, 7, 261–266.
[20] Rengpipat, S., Lowe, S.E., Zeikus, J.G., 1988. Effect of ex-
treme salt concentrations on the physiology and biochem-
istry of Halobacteroides acetoethylicus. J. Bacteriol., 170,
3065–3071.
[21] Reynolds, E.S.T., 1963. The use of lead citrate at high pH
as an electron opaque stain in electron microscopy. J.
Cell. Biol., 17, 208–212.
[22] Starr, T.B., Matanoski, G., Anders, M.W., Andersen, M.E.,
2006. Workshop overview: Reassessment of the cancer
risk of dichloromethane in humans. Toxicol. Sci., 91, 20–
28.
[23] Stucki, G., Gälli, R., Ebersold, H.R., Leisinger, T., 1981.
Dehalogenation of dichloromethane by cell-extracts of
Hyphomicrobium DM2. Arch. Microbiol., 130, 366–371.
[24] Trotsenko, Y.A., Doronina, N.V., 2003. The biology of
methylobacteria capable of degrading halomethanes. Mic-
robiology, 72, 121–131.
[25] Trotsenko, Y.A., Doronina, N.V., Li, T.D., Reshetnikov,
A.S., 2007. Moderately haloalkaliphilic aerobic methylo-
bacteria. Microbiology (Russia), 76, 253–265.
[26] Vuilleumier, S., 2002. Coping with a halogenated one-
carbon diet: aerobic dichloromethane-mineralising bacte-
ria. In: (Reineke, W. and Agathos, S., eds.), Biotechnology
for the Environment, Focus on Biotechnology Series Vol. 3A.
Dordrecht: Kluwer Academic Publishers, pp. 105–131.
[27] Vuilleumier, S., Pagni, M., 2002. Bacterial glutathione S-
transferases: new lessons from bacterial genomes. Appl.
Microbiol. Biotechnol., 58, 138–146.
[28] Vuilleumier, S., Chistoserdova, L., Lee, M.-C., Bringel, F.,
Lajus, A. et al., 2009. Methylobacterium genome sequences: a
reference blueprint to investigate microbial metabolism
of C1 compounds from natural and industrial sources.
PLoS ONE, 4, e5584.
((Funded by
• Russian Foundation of Basic Research; grant number: 06-04-22000
• Centre National de la Recherche Scientifique CNRS; grant number: PICS 3380))
Wyszukiwarka
Podobne podstrony:
jobm 201000013
jobm 201000298
jobm 201000191
jobm 201000321
jobm 201000018
jobm 201000214
jobm 201000067
jobm 201000037
jobm 201000074
jobm 201000385
jobm 201000198
jobm 201000458
jobm 201000147
jobm 201000520
jobm 201000327
jobm 201000342
jobm 201000420
jobm 201000364
jobm 201000317
więcej podobnych podstron