
490
Journal of Basic Microbiology 2011, 51, 490 – 498
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Research Paper
Wax ester-like compounds as biosurfactants produced
by Dietzia maris from n-alkane as a sole carbon source
Miyo Nakano
1
, Masaki Kihara
1
, Shunpei Iehata
1
, Reiji Tanaka
1
, Hiroto Maeda
1
and Takeshi Yoshikawa
2
1
Marine Microbiology, Faculty of Bioresources, Mie University, Kurimamachiya-cho, Tsu, Mie, Japan
2
Faculty of Fisheries, Kagoshima University, Arata, Kagoshima-shi, Kagoshima, Japan
The hydrocarbon-degrading bacterium Dietzia maris WR-3 was isolated from a consortium
comprising ammonia-oxidizing and denitrifying bacteria derived from marine sediments. Here,
we examined biosurfactant production by strain WR-3 when cultured using several different
carbon (D-glucose, n-decane, n-hexadecane, motor oil, olive oil, and rapeseed oil) and nitrogen
(NH
4
)
2
SO
4
, NaNO
3
, yeast extract, and polypeptone) sources as growth substrates. Strain WR-3
was able to grow and reduce the surface tension of culture broth to 31±1.0 mN m
–1
when
cultured using n-hexadecane and nitrate ions. The surface-active compounds produced by
strain WR-3 were extracted and analyzed by thin layer chromatography. Moreover, the main
components in the extract were further purified and subjected to gas chromatography/mass
spectrometry (GC/MS). From the analysis, the surface-active compounds were tentatively
identified as wax ester-like compounds, which were synthesized from the degradation process
of n-alkane. The production of surface-active compounds by strain WR-3 promoted attachment
of cells to hydrocarbon droplets via increased cell hydrophobicity, thus allowing enhanced
degradation of water immiscible substrates. As Dietzia spp. can grow and produce wax esters
from the degradation process of hydrocarbons, these marine bacteria are potentially useful for
the bioremediation of hydrocarbon-contaminated environments.
Keywords: Dietzia maris / Biosurfactant / Wax ester / Marine sediment
Received: October 22, 2010; accepted: December 23, 2010
DOI 10.1002/jobm.201000420
Introduction
*
Microbial compounds that exhibit pronounced surface
and emulsifying activities are classified as biosurfac-
tants or bioemulsifiers. These compounds, such as gly-
colipids, lipopeptides/lipoproteins, polysaccharide-pro-
tein complexes, phospholipids, fatty acids, and neutral
lipids, are either secreted extracellularly or incorpo-
rated into the cell membrane by a wide variety of bac-
teria, yeast, and fungi [1]. Such compounds have several
advantages over synthetic surfactants, including high
biodegradability, low toxicity, low irritancy, and com-
patibility with human skin [2]. The most promising
applications of biosurfactants, which include pharma-
Correspondence: Takeshi Yoshikawa, Faculty of Fisheries, Kagoshima
University, 4-50-20, Arata, Kagoshima-shi, Kagoshima 890-0059, Japan
E-mail: yoshi@fish.kagoshima-u.ac.jp
Phone and fax: +81-99-286-4191
ceuticals, textiles, cosmetics, detergents, and livestock
feed [3], are related to environmental protection, such
as the bioremediation of hydrocarbons, organic pollut-
ants, and heavy metal-contaminated sites, and for en-
hanced oil recovery and treatment of oil spills [4].
Most hydrocarbon-degrading bacterial strains produce
biosurfactants of diverse chemical properties and mo-
lecular size [5]. These specialized lipids of diverse
chemical structures play a role in the modification of
medium properties and can therefore enhance the as-
sociation between bacteria and water-insoluble hydro-
carbons.
Members of the bacterial genus Dietzia are distrib-
uted ubiquitously and have been isolated from diverse
environments including fields, deep sea sediments,
soda lakes, soil, fish, and humans [6–8]. Several docu-
mented species of Dietzia are capable of utilizing ali-
phatic hydrocarbons as a sole carbon and energy source

Journal of Basic Microbiology 2011, 51, 490 – 498
Wax ester-like compounds as biosurfactant
491
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
[9, 10]. Therefore, their ability to utilize a wide range of
hydrocarbons suggests that these species have substan-
tial potential for applications in the bioremediation of
hydrocarbons in contaminated environments. To the
best of our knowledge, the isolation and characteriza-
tion of biosurfactants and bioemulsifiers produced by
Dietzia species have not yet been reported. In this study,
we characterized the hydrocarbon-degrading bacterial
strain WR-3 isolated from marine sediment and identi-
fied it as Dietzia maris on the basis of its growth proper-
ties using n-hexadecane as a sole carbon and energy
source. In addition, isolation and identification of a
functional surface-active compound produced by WR-3
was presented.
Materials and methods
Microorganism and phylogenetic analysis
D. maris strain WR-3 used in this study was previously
isolated from a consortium comprising ammonia-
oxidizing and denitrifying bacteria [11]. WR-3 was iden-
tified on the basis of its phylogenetic and physiological
characteristics. Bacterial DNA was extracted as previ-
ously described [12]. The 16S rRNA gene was selectively
amplified by PCR from the genomic DNA of each iso-
late using the universal primer pair EUB-1 and EUB-2
as previously described [12]. The full sequences of
amplified 1.5-kb 16S rRNAs were determined for
both strands with an ABI Prism 3100 Genetic Ana-
lyzer (Perkin-Elmer Applied Biosystems, Weiterstadt,
Germany), and the full sequences were used to
identify closely related genes using the NCBI data
bank’s BLAST search. Sequences were aligned using
Clustal X [13] and a phylogenetic tree was construct-
ed using the Neighbor-Joining method [14]. The partial
16S rRNA sequence of strain WR-3 has been deposit-
ed in the DDBJ database under accession number
AB576128.
Organic carbon as an energy source
The liquid fermentation medium (termed medium DA)
consisted of the following (g l
–1
): NaCl (23.0), KCl (0.7),
MgCl
2
⋅ 6 H
2
O (10.8), MgSO
4
⋅ 7 H
2
O (5.4), CaCl
2
⋅ 2 H
2
O
(1.0), phosphate buffer (1 mM), and 1 ml of a trace min-
eral solution described previously for NiD medium [11].
For experiments in carbon source utilization, carbohy-
drate (50 mM), amino acids (50 mM), and 10% (v/v) hy-
drocarbons were added to the DA medium. As a nitro-
gen source, NH
4
NO
3
(8.0 g l
–1
) was added, and the pH
was adjusted to between 7.5 and 7.8 in all media.
The supplemented DA medium (10 ml) in 15 ml test
tubes was incubated with silico caps in a water bath
shaker (125 rpm) at 25 °C for 10 d. To examine n-alkane
hydrocarbon utilization, cultures were inoculated for
2 weeks.
Nitrogen source determination
The effect of different nitrogen source was studied by
supplementation with one of the four types of nitrogen
sources (g l
–1
) (NH
4
)
2
SO
4
(6.6), NaNO
3
(8.5), yeast extract
(0.5% w/v), and polypeptone (0.5% w/v) added to the DA
medium. As a carbon source, n-hexadecane 2% (v/v) was
used. The supplemented DA medium (10 ml) in 15 ml
test tubes was incubated with silico caps in a water
bath shaker (125 rpm) at 25 °C for 10 d, and surface
tension was measured every 24 h to determine the ni-
trogen source.
Microbial growth and surface tension measurements
The time course of biosurfactant production during the
growth of strain WR-3 was measured in a 15 ml test
tube containing 12 ml DA-BS medium. The DA-BS me-
dium was supplemented with 5 mM phosphate buffer,
NaNO
3
(4.25), and n-hexadecane 2% (v/v) as a sole car-
bon source based on the DA medium. The tubes were
sealed with silico caps and incubated in a water bath
shaker (125 rpm) at 25 °C for 13 d.
The surface tension measurements of culture super-
natant with and without cells were determined using a
tensiometer (Ito Seisakusyo, Tokyo, Japan) according to
the Du Nouy ring method. The measurements with-
out cells were carried out via filtration culture using a
0.2 μm Nucleopore polycarbonate membrane filter
(Whatman International Ltd., Maidstone, England).
Following filtration, the filter with trapped cells was
dried at 80 °C in a hot air oven for 24 h then weighed to
determine the microbial biomass.
Biosurfactant isolation and purification
For bacterial biosurfactant synthesis, batch growth was
performed in 1 l Erlenmeyer flasks containing 300 ml
of DA-BS medium. The hydrophobic layer (foaming
portion) located on the surface of the culture was care-
fully removed by scrapping, and the bacterial cells
were then removed by centrifugation (10,000 g; 4 °C;
30 min). The foaming portion was then extracted twice
with equal volumes of chloroform/methanol (2:1, v/v).
The solvent layer was separated from the aqueous
phase, and the solvent was removed by rotary evapora-
tion at 40 °C under reduced pressure. Filtration was
performed using a syringe filter of 0.2 μm pore size
(25 mm GD/X, Whatman International Ltd) as required.
The crude extract was dissolved in chloroform/me-

492 M.
Nakano
et al.
Journal of Basic Microbiology 2011, 51, 490 – 498
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
thanol and stored at –20 °C until subjected to further
purification.
The crude extract was further purified using a silica
60 column (230–400 mesh; Nacalai Tesque, Kyoto, Ja-
pan). First, the column was equilibrated with chloro-
form and washed with three volumes of the n-hexane
to remove the residual n-hexadecane. Lipids were then
separated with the following solvent systems of increas-
ing polarity: hexane; n-hexane/dichloromethane (9:1,
v/v); n-hexane-chloroform-acetic acid (20:80:0.5, v/v/v);
chloroform-methanol (95:5); chloroform-methanol-
water (85:15:2, v/v/v) and chloroform-methanol-water
(65:25:4, v/v/v), and methanol as previously described
[15]. Each eluate was collected separately, dried under
nitrogen gas, and dissolved in chloroform. The different
column fractions collected were then subjected to
thin layer chromatography (TLC) on a silica gel F
254
TLC
plate (Merck Co. Inc., Damstadt, Germany), which was
then developed using the following solvent systems:
n-hexane-chloroform-acetic acid (80:20:1, v/v/v); chloro-
form-methanol-water (85:15:2, v/v/v); and chloroform-
methanol-water (65:25:4, v/v/v). Simultaneously, elu-
ates were dissolved in distilled water and the surface
tension was measured to detect the presence of surface-
active compounds.
The color-development of the TLC plate was per-
formed with the following spray reagents to reflect the
functional groups of the lipids: 50% sulphuric acid for
n-alkanes; p-anisaldehyde for unsaturated hydrocarbons
and fatty alcohols, and diphenylamine and/or orcinol-
sulfuric acid for glycolipid; ninhydrin for free amino
groups; Dittmer reagent for phospholipids; hydroxyl-
amine-ferric chloride for acylglycerols; and 2′, 7′ di-
chlorofluorescein-aluminium chloride-iron (III) chloride
for free fatty acids [15].
The eluates which displayed surface-active properties
were subjected to further purification using a silica gel
F
254
preparative chromatography (PLC) plate (Merck Co.
Inc., Damstadt, Germany) to isolate the surface-active
compounds, and the obtained lipids were then visual-
ized using 0.001% primuline reagent under 365 nm UV
light. Subsequently, identified lipid spots were scrap-
ed and re-extracted with chloroform/methanol/water
(2:1:1, v/v/v). The eluates were dried under nitrogen
gas, and the resulting extracts were dissolved with
chloroform and stored at –20 °C until needed for fur-
ther analysis. Second dimensional TLC or high-per-
formance TLC (HPTLC) was also performed as needed to
separate the lipids. Isolated compounds were trans-
ferred to PVDF membranes, for the separation and
qualitative determination by mass spectrometric analy-
sis.
Mass spectrometric analysis
of surface active compounds
The mass spectrometric analysis of the surface active
compounds was carried out using a JMS-T100GC (Gas
Chromatograph Time-of-Flight Mass Spectrometer,
Japan Electron Optics Laboratory
(
JEOL) Ltd., Tokyo,
Japan) utilizing electron ionization mass spectrometry
(EI-MS). The heated capillary temperature was 230 °C,
and the ionizing energy was 70 V. Scanning was con-
ducted in the 35–1000 m/z range. The identical 2 μl
sample was coated on the carbon emitter of a JMS-700
Instrument (JEOL Ltd) and subjected to field desorption
mass spectrometry (FD-MS) using a pressure voltage of
8 kV. The scanning was conducted in the 10–2000 m/z
range. Mass Frontier (Ver. 5) was used for molecular
structural determinations.
Emulsification activity, stability testing,
and surface tension measurements
The crude extract was also evaluated for emulsion abil-
ity, according to a previously described method with
minor modifications [16]. The crude extracts were first
dissolved in 2 ml distilled water, mixed with an identi-
cal volume of n-hexadecane in a test tube, vigorously
vortexed for 2 min, and left to stand for 10 min at room
temperature. The emulsion stability was measured
after 24 h and the emulsification index was calculated
by dividing the measured height of the emulsion layer
by the total height of the mixture and multiplying this
value by 100 [17]. The crude extract was dissolved in
distilled water and the eluted fractions were subjected
to surface tension measurements using a tensiometer,
as described above.
Bacterial adhesion to hydrocarbons test
Cell hydrophobicity was measured by the bacterial
adhesion to hydrocarbons (BATH) test according to a
method described by Rosenberg et al. [18], with minor
modifications. Briefly, cells were washed twice and
suspended in a buffer salt solution (g l
–1
, 16.9 K
2
HPO
4
,
7.3 KH
2
PO
4
) to give an OD 600 nm (OD
600
) of –0.5.
Either 100 μl of hydrocarbons (n-decane or n-hexa-
decane) or oil (motor, olive, or rapeseed oil) was
added to the cell suspension (2 ml) in a test tube
(10 × 100 mm), which was then vortex-shaken for
3 min. After shaking, the oil and aqueous phases were
allowed to separate for 1 h at room temperature. The
OD
600
of the separated aqueous phase was then meas-
ured in a spectrophotometer (DU 530, Beckman In-
struments Inc., Fullerton, CA, USA). Hydrophobicity
was expressed as the percentage of cell adherence to
hydrocarbon or oil, and was calculated as follows:
Journal of Basic Microbiology 2011, 51, 490 – 498
Wax ester-like compounds as biosurfactant
493
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
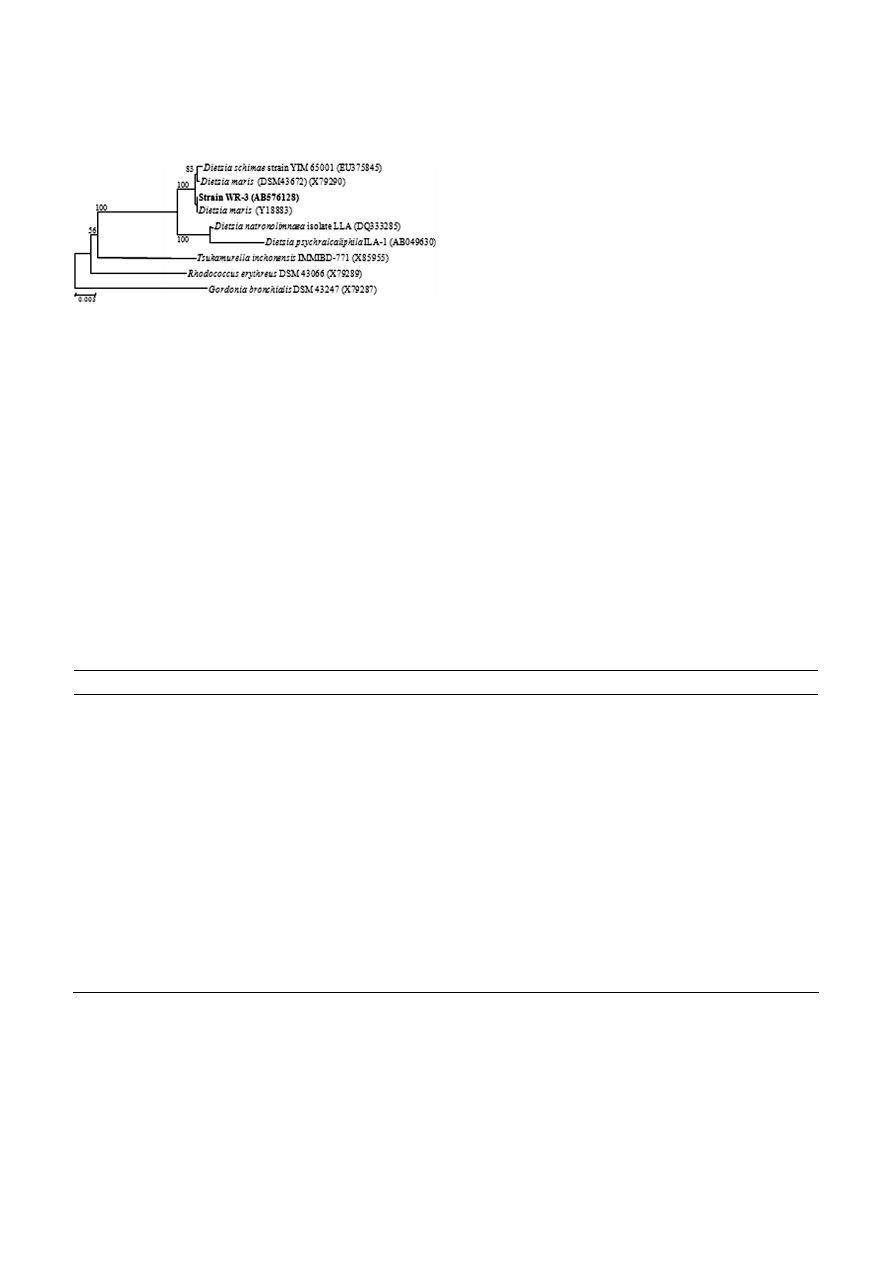
Figure 1. Phylogenetic positions of strain WR-3 and represen-
tatives of other related taxa based on 16S rRNA gene sequences.
The tree was constructed by the neighbor-joining method. The
numbers at the nodes are bootstrap confidence values expected as
percentages of 1,000 replicates, and only values greater than 50
are expressed. Nucleotide sequence database accession numbers
are shown in parentheses.
100 × (1 – OD
600
of the aqueous phase/– OD
600
of the
initial cell suspension).
Results
Characterization of strain WR-3
Phylogenetic analyses using 16S rRNA sequences re-
vealed that strain WR-3 was included in a cluster with
D. maris (Fig. 1). The closest relatives of strain WR-3
were D. maris (1469/1469, 100%, Y18883), D. maris DMS-
43672 (1467/1469, 99%, X79290), D. schimae strain YIM-
65001 (1469/1472, 99%, EU375845), and D. natronolim-
naea strain LLA (1453/1472, 98%, DQ333285).
On nutrient agar plate, strain WR-3, formed dark
orange colonies. The utilization of carbon sources in-
cluding four types of hydrocarbons, by strain WR-3 was
evaluated and compared with other isolated Dietzia
strains (Table 1). In strain WR-3, cell growth was ob-
served only when D-glucose, aspartate, or n-alkane hy-
drocarbons were used as the sole carbon source.
With respect to the utilization of hydrocarbons, type
species D. maris (DSM 43672) was also characterized as
an n-alkane (C
6
to C
17
, C
19
and C
23
) utilizing bacterium
[17]. We determined that strain WR-3 was also able to
utilize n-alkanes (C
10
, C
14
, C
15
, and C
16
), an ability that
was partly shared with other Dietzia sp., such as D. psy-
chralcaliphila [10] and D. cinnamea [27]. In addition, strain
WR-3 observed nitrate reduction but not nitrite reduc-
tion.
Effect of nitrogen and carbon sources
on biosurfactant production
We next examined the effect of several nitrogen
sources on the surface tension of WR-3 culture super-
Table 1. Utilization of sole carbon sources by Dietzia sp. WR-3, and comparison with other Dietzia strains.
Strains
1
1
2
2
3
2
4
2
5
2
6
2
7
2
Sole carbon source
3
D-Glucose + + + + + + +
D-Fructose + + + + + +
D-Mannose + + + + + +
Sucrose
+ + + + + +
Cellobiose + + + + + +
D-Mannitol + + + + + +
Grycerol
+ + + + + +
D-Gluconate
Succinate
L-Glutamate + + + + + +
Aspartate + + + + + + +
L-Serine
+ + + + + +
DL-Alanine + + + + + +
L-Arginine + + +
Hydrocarbon
4
Decane
+ n.d. n.d. n.d.
5
n.d. n.d.
6
n.d.
Tetradecane + n.d. n.d. + n.d. n.d. n.d.
Pentadecane + n.d. n.d. + n.d. n.d. n.d.
Hexadecane + n.d. n.d. + n.d. + n.d.
1
Strains: 1, Dietzia sp. WR-3 in this study; 2, D. maris DSM 43672; 3, D. kunjamensis DSM44907; 4, D. psychralcaliphila DMS 44820;
5, D. natronolimnaea DMS 44860; 6, D. cinnamea DMS 44904; 7, D. papillomatosis DSM 44961.
2
Data from Jones et al. [8].
3
Carbohydrates and amino acids supplements were added at 50 mM each.
4
10% (v/v) hydrocarbon were added as sole carbon source in this study. DA-C medium was used. Positive reaction on growth:
+, variable reaction: ±, negative reaction on growth: –.
5
Data from Yumoto et al. [10].
6
Data from Weid et al. [27].
494 M.
Nakano
et al.
Journal of Basic Microbiology 2011, 51, 490 – 498
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Table 2. Hydrophobicity of cell of Dietzia sp. WR-3 strain under
immiscible substrates.
Substrate Hydrophobicity
(%)
n-decane 89.3
± 1.7
n-hexadecane 81.2
± 5.6
Motor oil (Penzoil 10W-40)
97.9 ± 0.5
Olive oil
n.d.
Rapeseed oil
87.2 ± 1.3
Values are mean of three test tubes ± S.D.
Dietzia sp. WR-3 was incubated aerobically with shaking at
25 °C in DA-BS medium for 12 d.
natants that either contained or lacked cells. In the
cell-containing supernatants, all of the nitrogen sources
resulted in a significant reduction in initial surface
tension from 72 ± 2.0 to 32 ± 1.0 mN m
–1
. However, in
the cell-free supernatants, the surface tension values
displayed greater variation, with the largest reduction
obtained with NaNO
3
(55 ± 2.0 mN m
–1
) followed by
(NH
4
)
2
SO
4
(62 ± 2.5 mN m
–1
), polypeptone (65 ± 2.5 mN m
–1
),
and yeast extract (67 ± 1.5 mN m
–1
). Therefore, NaNO
3
was used as a nitrogen source for further experiments.
As a preliminary experiment, water-miscible and
water-immiscible carbon substrates (D-glucose, n-hexa-
decane, and rapeseed oil) were tested for their capacity
to support biosurfactant production by WR-3. The lar-
gest surface tension reduction was obtained with
n-hexadecane (from 71 ± 1.0 to 31 ± 1.0 mN m
–1
); thus,
n-hexadecane was selected as the sole carbon source for
subsequent experiments.
Cell hydrophobicity and emulsification activity
In the BATH assay, strain WR-3 displayed high affinity
towards all of the immiscible substances, and had
higher affinity for motor oil than n-hexadecane
(Table 2). In addition, the emulsification activity of the
crude extract produced by WR-3 was stable for at least
48 h. Results showed that the biosurfactant produced
via hydrocarbon fermentation could emulsify n-hexa-
decane to the greatest extent, which confirmed its ap-
plicability for the bioremediation of hydrocarbon pollu-
tion. Emulsification enhances the biodegradation of
hydrocarbons by increasing their bioavailability to the
microbes involved.
Biosurfactant production during growth
on n-hexadecane
The growth characteristics and biosurfactant produc-
tion by WR-3 using n-hexadecane as a sole carbon
source were monitored by measuring the total cell
biomass and surface tension of cell-containing and cell-
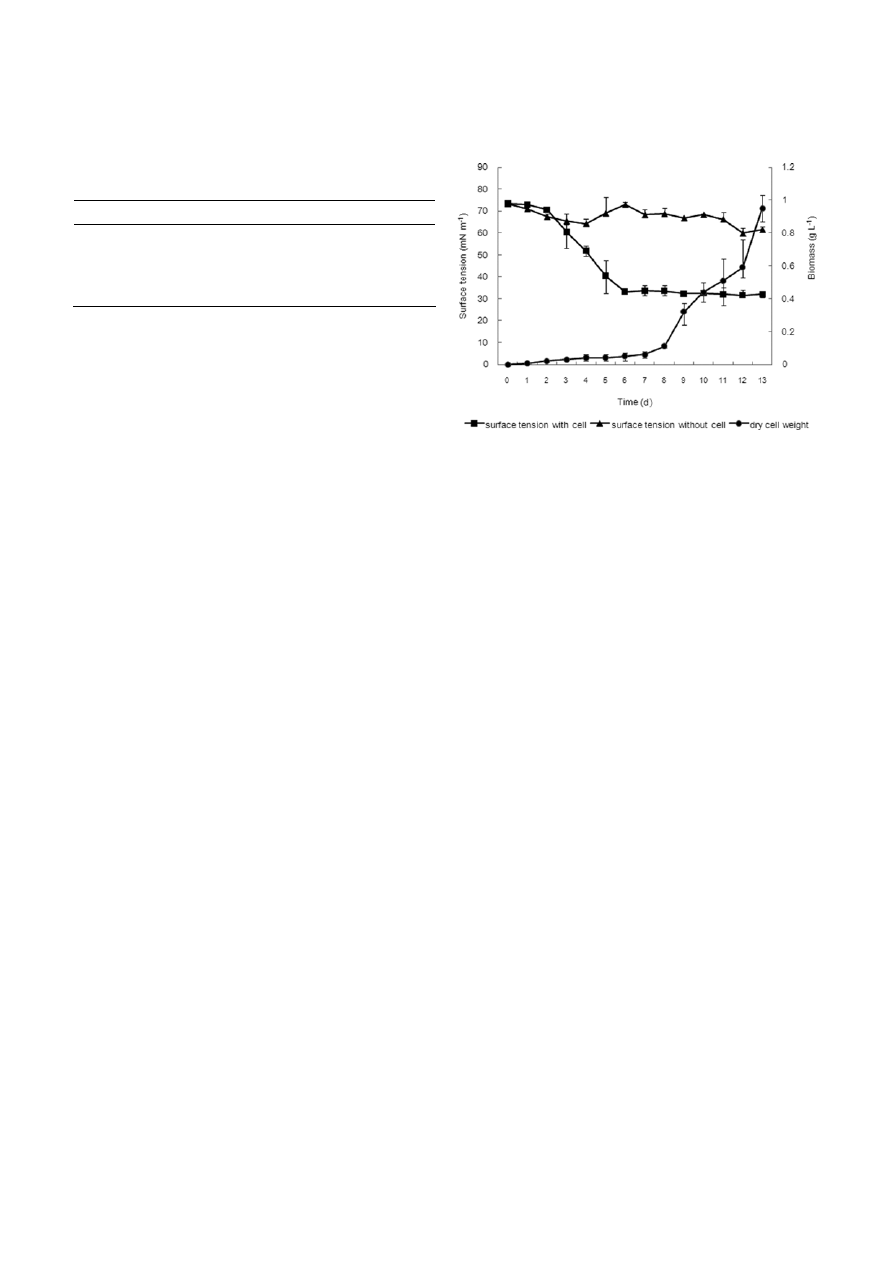
free broths, respectively (Fig. 2). The surface tension of
Figure 2. Total biomass and surface tension of supernatants with
and without cells from cultures of Dietzia sp. WR-3. Strain WR-3
was grown at 25 °C in DA-BS medium supplemented with 2% (v/v)
n-hexadecane as a sole carbon source. The values reported are the
mean ± SD of three measurements.
cell-containing broth decreased after 3 d of cultivation,
reaching 33.1 mN m
–1
after 6 d of cultivation, and then
maintained a constant level. This result may have been
due to the biosurfactant reaching its critical micelle
concentration, beyond which, no further reduction in
surface tension is possible. In contrast, no significant
reduction in surface tension was observed in the cell-
free broth, which varied between 73.3 and 61.5 mN m
–1
throughout the 13 d cultivation period (Fig. 2). The
biosurfactant produced by strain WR-3 appeared to be
either intracellular or cell-associated substances, rather
than extracellular secreted ones. After 12 d fermenta-
tion, a crude biosurfactant (105 ± 21 mg 10 ml
–1
cul-
ture) was obtained from the WR-3 culture. We also
examined the biomass yield after 12 d cultivation,
which was found to increase exponentially after 8 d
and was associated with the amount of foaming sub-
stance, but was not correlated with the decline in sur-
face tension.
Purification of biosurfactants
The biosurfactant was extracted from the hydrophobic
foaming layer of WR-3 cultures after 14 d of growth on
n-hexadecane and was then separated using silica gel
column extraction using different solvent systems. All
of the eluted fractions were developed on TLC plates,
and the composition of the extracts was visualized us-
ing specific spray-type reagents. This analysis revealed
triglycerides, fatty acids, and glycolipids in the extracts
(data not shown); however, they were obtained in
smaller quantities than the compounds eluted from the
n-hexane-chloroform-acetic acid (20:80:0.5, v/v/v) sol-
Journal of Basic Microbiology 2011, 51, 490 – 498
Wax ester-like compounds as biosurfactant
495
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
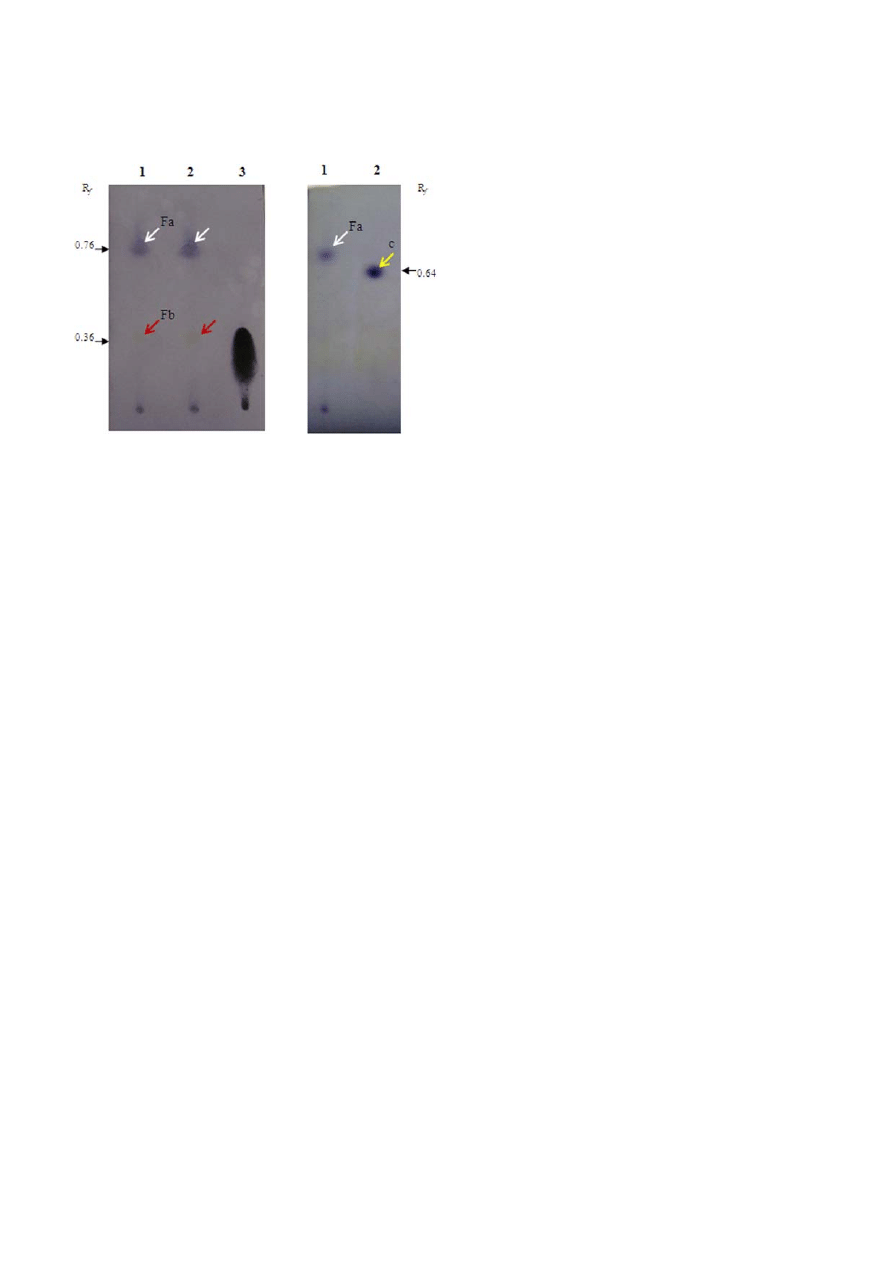
Figure 3. HPTLC analysis of extracted surface-active compounds
produced by Dietzia sp. WR-3. (A) Chromatogram of extracts from
the foaming portion of the culture (lane 1), culture supernatant
(lane 2), and on oleic acid standard (lane 3). The chromatogram
was visualized using p-anisaldehyde, and two spots were identified:
spot Fa (white arrows, R
f
0.76) and spot Fb (red arrows, R
f
0.36),
which was considered to represent free fatty acids. (B) Chroma-
togram of the identical sample from lane 1 in (A) (lane 1) and a hexa-
decyl hexadecanoate standard (lane 2, yellow allow, R
f
0.64). As a
solvent system, hexane:dichloromethane:acetic acid ((80 : 20 : 1)
was used.
vent system. The compound obtained from the eluate
indicated the highest surface-activity. The compounds
in the organic extract were subjected to TLC and visual-
ized with reagents specific for lipids. A brown promi-
nent spot was observed after 50% sulfuric acid solution
staining with an R
f
0.76. Using p-anisaldehyde, a blue
spot with an identical R
f
value was revealed.
Next, the eluate obtained was further purified using
PLC plates to isolate the surface-active compound. The
revealed spots were scraped, re-extracted with chloro-
form/methanol/water (2:1:1, v/v/v), and then subjected
to HPTLC and visualized with p-anisaldehyde (Fig. 3A
and B). This approach allowed the visualization of a
main spot (Fa) and a weak spot (Fb) on the chromato-
gram with R
f
values of 0.76 and 0.36, respectively. Spot
Fb was tentatively identified as a free fatty acid based on
the positive staining with the reagent 2′,7′-dichlorofluo-
rescein-aluminium chloride-iron(Ⅲ) chloride (data not
shown). Additionally, spot Fa was visualized as a non-
polar product, which differed from the staining of 1-hexa-
decyl hexadecanoate used as the standard (Fig. 3B).
Molecular mass and structural information estimated
by gas chromatography/mass spectrometry (GC/MS)
analysis
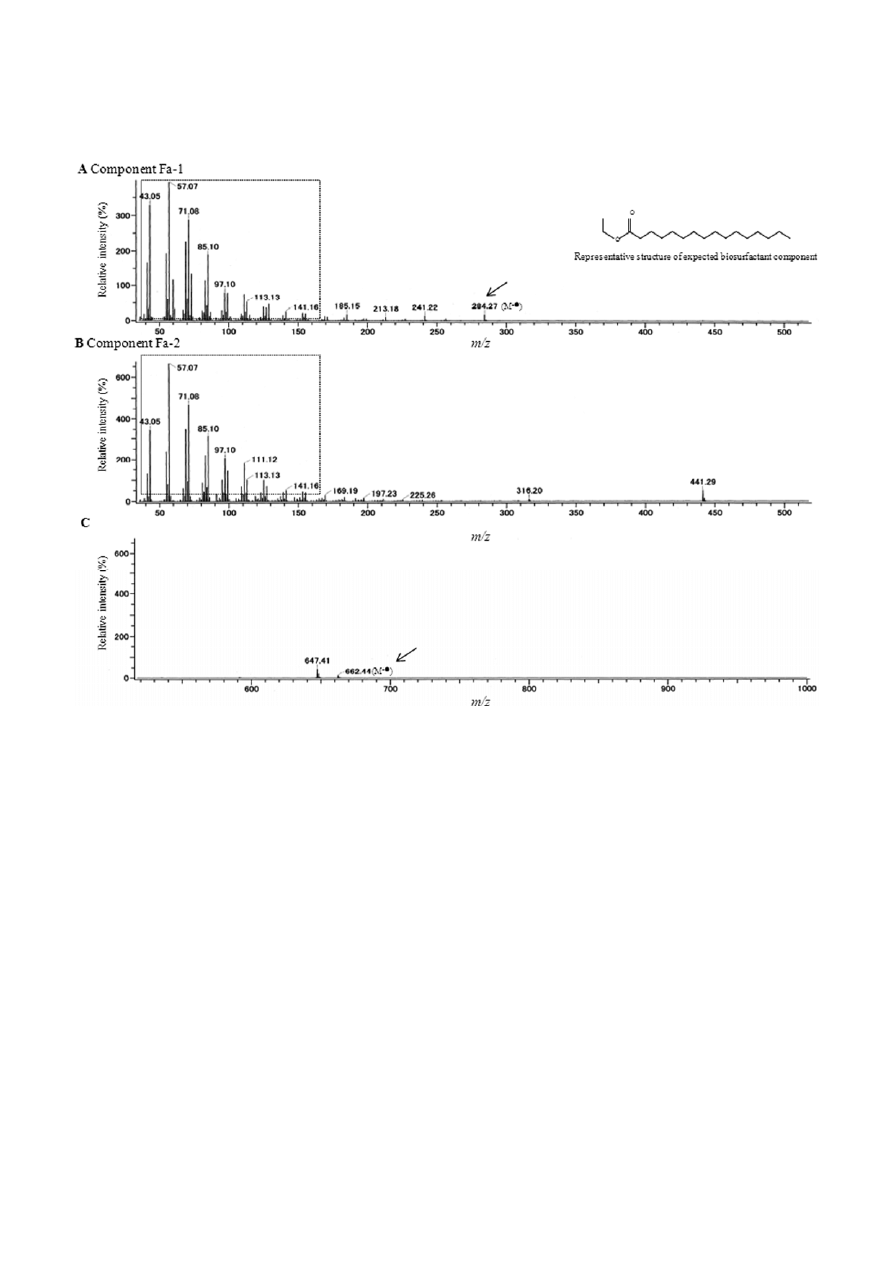
Spot Fa was next subjected to GC/MS analysis, which
revealed that this spot was in fact a mixture of two
species. Two peaks, labeled Fa-1 and Fa-2, were ob-
served at 7 and 12 min, respectively in the TIC chroma-
togram (data not shown). Component Fa-1 showed a
molecular mass at m/z [C
18
H
36
O
2
]
+
= 284.27, and was
estimated to represent a wax ester (Fig. 4A). Interest-
ingly, the chain length of this wax ester did not corre-
spond to the chain length of the supplied n-alkane in
the WR-3 growth medium.
In contrast to Fa-1, component Fa-2 displayed a mo-
lecular mass at m/z =662.44; however, due to the pres-
ence of the weak molecular ion peak (M
+
), we could not
obtain definite information for the structural predic-
tion of this component (Fig. 4B and C). Upon compari-
son of the mass spectrum profiles of components Fa-1
and Fa-2, peaks of identical position and similar inten-
sity were observed at less than 200 m/z (43.05, 57.07,
71.08, 85.10, 97.10, 113.13 and 141.16 m/z) (Fig. 4A and
B), which suggested these two components shared the
same structural composition. The identical peaks were
also detected in the FD-MS spectrum (data not shown),
supporting the obtained the EI-MS data.
Discussion
In carbon source utilization the results contrast greatly
with the other Dietzia sp., which were able to utilize
nearly all examined carbon sources for growth, indicat-
ing that strain WR-3 has a relatively narrow range of
utilizable growth substrates. However, the inability to
utilize D-gluconate or succinate as a solo carbon source
was a characteristic shared among all examined Dietzia
sp.
Strain WR-3 can utilize all tested immiscible sub-
stances as a sole carbon source, which suggested that
the direct contact between strain WR-3 cells and im-
miscible substances is the first step for the meta-
bolization of immiscible compounds. Hydrocarbon-
degrading bacteria increase the contact area between
bacteria and water-insoluble hydrocarbons through the
emulsification of hydrocarbons [19]. Cell hydropho-
bicity is expected to be a significant property in micro-
bial adhesion on surfaces such as hydrophobic sub-
strates [20]. The first step of hydrocarbon degradation
involves the introduction of molecular oxygen into
the molecules by membrane-bound oxygenases [21]. It
has been demonstrated that increased cell hydropho-
bicity promotes the attachment of cells to hydrocarbon
droplets, thus enhancing alkane degradation [22].
Biosurfactants produced by WR-3 may facilitate the
degradation of hydrophobic substrates by increasing
the solubilization and dispersion of these substrates,
A)
B)
496 M.
Nakano
et al.
Journal of Basic Microbiology 2011, 51, 490 – 498
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Figure 4. Electron ionization mass spectra of components Fa-1 (A) and Fa-2 (B and C). Peaks detected in both components Fa-1 and Fa-2
are enclosed within the dotted-line box. The representative structure of expected biosurfactant component Fa-1 was indicated.
and/or by inducing an increase in cell-surface hydro-
phobicity.
The role of biosurfactants can be attributed to the
low water-solubility of n-alkanes which function as
growth substrates. Oxygen is more soluble in the oil
phase than in the aqueous phase, and the low water
solubility of n-alkanes creates an additional complica-
tion for microbial growth: that of mass transfer from
oil into the aqueous phase [23]. This phenomenon may
provide the physiological basis for the production of
bioemulsifiers by strain WR-3 that are associated with
the aerobic degradation of n-alkanes. Such speculation
is supported by cell flocculation on the surface layer of
the strain WR-3 culture medium.
Although D-glucose is a suitable growth substrate for
strain WR-3, it was not utilized in biosurfactant pro-
duction. The utilization of n-hexadecane allowed strain
WR-3 to produce biosurfactant, as indicated by the
lowering of surface tension to 31 ± 1.0 mN m
–1
, while
D-glucose, motor oil, olive oil and rapeseed oil had
no effect on the surface tension from 72 ±1.0 to
68 ± 2.0 mN m
–1
, 46 ± 0.5 to 42 ± 4.0 mN m
–1
, 47 ± 1.0
to 43 ± 2.0 mN m
–1
, and 50 ± 1.0 to 45 ± 3.0 mN m
–1
,
respectively. Meanwhile, n-decane was recorded from
71 ± 2.0 to 40 ± 7.0 mN m
–1
.
In this study, wax ester-like lipophilic compounds
were produced as one of the major surface-active com-
pounds by D. maris WR-3 during fermentation with n-
hexadecane as the sole carbon source. However, due to
the results of the mass spectrometer analysis, the
number of carbon atoms in component Fa-1 was 18,
which did not correspond to the number of carbon
atoms in the n-alkane substrates. Bredemeier et al. [24]
reported that wax ester composition was not dependent
on the chain length of the alkane which seemed to be
regulated in a more complex manner, given that the
dissolved oxygen tension of the cultures influenced the
wax ester composition as well as the supplied alkane.

Journal of Basic Microbiology 2011, 51, 490 – 498
Wax ester-like compounds as biosurfactant
497
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Ishige et al. [25] indicated that β-oxidation of fatty acids
may be part of the metabolic route for the production
of wax esters in Acinetobacter. Furthermore, chain elon-
gation of fatty acids may also be part of the metabolic
route. In addition, wax ester could be derived from
mycolates, which have a straight chain (19:0) and two
double bonds, produced by D. maris [26].
To our knowledge, this is the first report on isolation
and identification of biosurfactant produced by D. maris
isolated from marine sediments. Our analysis revealed
that wax ester-like compounds, produced by D. maris
when grown on n-hexadecane as a sole carbon source,
might play an important role in the degradation pro-
cess of n-alkane. Wax ester-like compounds can play a
role as surface-active emulsifiers, and these hydropho-
bic compounds can enhance the contact between bacte-
ria and water-insoluble hydrocarbons. As Dietzia spp.
can grow and produce wax esters on oil waste sub-
strates as a sole carbon source, these marine bacteria
are potentially useful for the bioremediation in hydro-
carbon-contaminated environments.
Acknowledgements
The present study was performed as part of the “Envi-
ronmental Project on Enclosed Coastal Seas, Ago Bay”,
supported by the Collaboration of Regional Entities for
the Advancement of Technological Excellence (CREATE)
organized by the Japan Science and Technology (JST)
Agency.
References
[1] Desai, J.D., Banat, I.M., 1997. Microbial production of
surfactants and their commercial potential. Mocrobiol.
Mol. Biol. Rev., 61, 47–64.
[2] Banat, I.M., Makkar, R.S., Cameotra, S.S., 2000. Potential
commercial applications of microbial surfactants. Appl.
Microbiol. Biot., 53, 495–508.
[3] Mukherjee, S., Das, P., Sen, R., 2006. Towards commercial
production of microbial surfactants. Trends Biotechnol.,
24, 509–515.
[4] Singh, A., Van Hamme, J.D., Ward, O.P., 2007. Surfactants
in microbiology and biotechnology: part 2. Application
aspects. Biotechnol. Advances, 25, 99–121.
[5] Ron, E.Z., Rosenberg, E., 2002. Biosurfactants and oil bio-
remediation. Curr. Opin. Biotechnol., 13, 249–252.
[6] Colquhoun, J.A., Heald, S.C., Li, L., Tamaoka, J., Kato, C.,
Horikoshi, K., Bull, A.T., 1998. Taxonomy and biotrans-
formation activities of some deep-sea actinomycetes. Ex-
tremophiles, 2, 269–277.
[7] Duckworth, A.W., Grant, S., Grant, W.D., Jones, B.E.,
Meijer, D., 1998. Dietzia natronolimnaios sp. nov., a new
member of the genus Dietzia isolated from an East African
soda lake. Extremophiles, 2, 359–366.
[8] Jones, A.L., Koerner, R.J., Natarajan, S., Perry, J.D., Good-
fellow, M., 2008. Dietzia papillomatosis sp. nov., a novel ac-
tinomycete isolated from the skin of an immunocompe-
tent patient with confluent and reticulated papillo-
matosis. Int. J. Syst. Evol. Microbiol., 58, 68–72.
[9] Rainey, F.A., Klatte, S., Kroppenstedt, R.M., Strackebrandt,
E., 1995. Dietzia, a new genus including Dietzia maris
comb. nov., formerly Rhodococcus maris. Int. J. Syst. Bact.,
45, 32–36.
[10] Yumoto, I., Nakamura, A., Iwata, H., Kojima, K., Kusu-
moto, K., Nodasaka, Y., Matsuyama, H., 2002. Dietzia psy-
chralcaliphila sp. nov., a novel, facultatively psychrophilic
alkaliphili that grows on hydrocarbons. Int. J. Syst. Evol.
Microbiol., 52, 85–90.
[11] Nakano, M., Shimizu, Y., Okumura, H., Sugahara, I., Mae-
da, H., 2008. Construction of a consortium comprising
ammonia-oxidizing bacteria and denitrifying bacteria iso-
lated from marine sediment. Biocont. Sci., 13, 73–89.
[12] Nakano, M., Inagaki, T., Okunishi, S., Tanaka, R., Maeda,
H., 2010. Effect of salinity on denitrification under lim-
ited single carbon source by Marinobacter sp. isolated
marine sediment. J. Basic Microbiol., 50, 285–289.
[13] Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F.,
Higgins, D.G., 1997. The clustal X windows interface: fle-
xible strategies for multiple sequence alignment aided by
quality analysis tools., Nucleic Acids Res., 25, 4876–4882.
[14] Saitou, N., Nei, M., 1987. The neighbor-joining method: a
new method for reconstructing phylogenetic trees. Mol.
Biol. Evol., 4, 406–425.
[15] Kuyukina, M.S., Ivshina, I.B., Philip, K.C., Chiristofi, N.,
Dunbar, S.A., Ritchkova, M.I., 2001. Recovery of Rhodo-
coccus biosurfactants using methyl tertiary-butyl ether
extraction. J. Microbiol. Methods,
46, 149–156.
[16] Das, M., Das, S.K., Mukherjee, R.K., 1998. Surface active
properties of the culture filtrates of a Micrococcus species
grown on n-alkanes and sugars. Biores. Technol., 63, 231–
235.
[17] Cooper, D.G., Goldenberg, B.G., 1987. Surface-active
agents from two Bacillus species. Appl. Environ. Micro-
biol., 53, 224–229.
[18] Rosenberg, M., Gutnick, D., Rosenberg, E., 1980. Adher-
ence of bacteria to hydrocarbons: a simple method for
measuring cell-surface hydrophobicity. FEMS Microbiol.
Lett., 9, 29–33.
[19] Manilla-Perez, E., Lange, A.B., Hetzler, A., Steinbuchel, A.,
2010. Occurrence, production, and export of lipophilic
compounds by hydrocarbonoclasticus marine bacteria
and their potential use to produce bulk chemicals from
hydrocarbons. Appl. Microbiol. Biotechnol., 86, 1693–
1706.
[20] Van Loosdrecht, M.C., Lyklema, J., Norde, W., Schraa, G.,
Zehnder, A.J.B., 1987. The role of bacterial cell wall hy-
drophobicity in adhesion. Appl. Environ. Microbiol., 53,
1893–1897.
[21] Rosenberg, E., 1993. Exploiting microbial growth on
hydrocarbon: new markets. Trends Biotechnol., 11, 419–
424.

498 M.
Nakano
et al.
Journal of Basic Microbiology 2011, 51, 490 – 498
© 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
[22] Zhang, Y., Miller, R.M., 1994. Effect of a Pseudomonas
rhamnolipid biosurfactant on cell hydrophobicity and
biodegradation of octadecane. Appl. Environ. Microbiol.,
60, 2101–2106.
[23] Haferburg, D., Hommel, R., Claus, R., Kleber, H.P., 1986.
Extracellular microbial lipids as biosurfactants. Adv. Bio-
chem. Eng. Biotechnol., 33, 53–93.
[24] Bredemeier, R., Hulsch, R., Metzger, J.O., Berthe-Corti, L.,
2003. Submersed culture production of extracellular wax
esters by the marine bacterium Fundibacter jadensis. Mar.
Biotechnol., 5, 579–583.
[25] Ishige, T., Tani, A., Takabe, K., Kawasaki, K., Sakai. Y.,
Kato, N., 2002. Wax ester production from n-alkane by
Acinetobacter sp. strain M-1: ultrastructure of cellular in-
clusions and role of acyl coenzyme A reductase. Appl. En-
viron. Microbiol., 68, 1192–1195.
[26] Nishiuchi, Y., Baba, T., Yano I., 2000. Mycolic acids from
Rhodococcus, Gordonia, and Dietzia. J. Microbiol. Methods,
40, 1–9.
[27] Weid, I., Marques, J.M., Cunha, C.D., Lippi, R.K. et al.,
2007. Identification and biodegradation potential of a
novel strain of Dietzia cinnamea isolated from a petroleum-
contaminated tropical soil. Syst. Appl. Microbiol., 30,
331–339.
((Funded by
• Collaboration of Regional Entities for the Advance-
ment of Technological Excellence (CREATE) organized
by the Japan Science and Technology (JST) Agency))
Wyszukiwarka
Podobne podstrony:
jobm 201000013
jobm 201000298
jobm 201000191
jobm 201000321
jobm 201000018
jobm 201000214
jobm 201000067
jobm 201000037
jobm 201000074
jobm 201000280
jobm 201000385
jobm 201000198
jobm 201000458
jobm 201000147
jobm 201000520
jobm 201000327
jobm 201000342
jobm 201000364
jobm 201000317
więcej podobnych podstron